Abstract
Dorsoventral cell fate in the Drosophila embryo is specified by activation of the Toll receptor, leading to a ventral-to-dorsal gradient across nuclei of the NF-κB transcription factor Dorsal. Toll receptor has been investigated genetically, molecularly, and immunohistologically, but much less is known about its dynamics in living embryos. Using live imaging of fluorescent protein chimeras, we find that Toll is recruited from the plasma membrane to Rab5+ early endosomes. The distribution of a constitutively active form of Toll, Toll10b, is shifted from the plasma membrane to early endosomes. Inhibition of endocytosis on the ventral side of the embryo attenuates Toll signaling ventrally and causes Dorsal to accumulate on the dorsal side of the embryo, essentially inverting the dorsal/ventral axis. Conversely, enhancing endocytosis laterally greatly potentiates Toll signaling locally, altering the shape of the Dorsal gradient. Photoactivation and fluorescence recovery after photobleaching studies reveal that Toll exhibits extremely limited lateral diffusion within the plasma membrane, whereas Toll is highly compartmentalized in endosomes. When endocytosis is blocked ventrally, creating an ectopic dorsal signaling center, Toll is preferentially endocytosed at the ectopic signaling center. We propose that Toll signals from an endocytic compartment rather than the plasma membrane. Our studies reveal that endocytosis plays a pivotal role in the spatial regulation of Toll receptor activation and signaling and in the correct shaping of the nuclear Dorsal concentration gradient.
Keywords: Rab5, Toll-like receptor, pattern formation
Toll, the founding member of the Toll-like receptor family of transmembrane receptors, directs the redistribution of Dorsal/NF-κB from the cytoplasm to the nucleus, generating a ventral-to-dorsal concentration gradient across syncytial and cellular blastoderm nuclei. The nuclear concentration of Dorsal specifies the dorsal/ventral (d/v) identity of blastoderm nuclei during nuclear cycles 10–14 by differentially regulating the transcription of zygotic effector genes, which, in turn, interact to direct further downstream patterning (1). An extracellular signal that determines global d/v polarity originates through a protease cascade in the ventral part of the perivitelline space, resulting in the processing of the Spaetzle ligand (2, 3). Many of the components of the embryonic Toll signaling pathway have been identified and characterized, as reviewed by Moussian and Roth (4). These include the Toll Interleukin Receptor domain adaptor MyD88 (5), the death-domain adaptor Tube, and the serine/threonine kinase Pelle (6). The assembly of these proteins into a complex with activated Toll receptor at the plasma membrane has been proposed to lead to the autophosphorylation and activation of Pelle. In a way that is not currently understood, this leads to the simultaneous phosphorylation- and polyubiquitination-directed degradation of the I-κB inhibitor protein Cactus (7, 8) and phosphorylation of the Rel-domain of Dorsal (9). Despite many years of research, the mechanism underlying the shaping and modulation of the Dorsal gradient remains obscure. It has been proposed to depend on the graded generation of Spaetzle ligand (4), lateral diffusion of activated Toll receptor within the plasma membrane, or even the intracellular propagation of activated Pelle (10).
Endocytosis has emerged as a major regulator and, at times, even a prerequisite for important developmental signaling pathways. Internalization and trafficking of the nascent receptor–ligand complex to a particular endosomal compartment can be required for signaling, as in the case of Notch and Unpaired/Domeless signaling (11) (12). In other cases, endocytosis is thought to modulate signaling over large areas indirectly by controlling the shape and extension of morphogen gradients, either by removing the morphogen from the extracellular space or by facilitating its propagation via a transcellular route (13–15).
Here, we use live-imaging techniques in combination with fluorescent protein chimeras to investigate the localization and dynamics of the Toll receptor in vivo. We find that Toll is present at the plasma membrane as well as in Rab5+ early endosomes and that endocytosis of Toll is strongly correlated with active signaling. Microinjection experiments demonstrate that not only is endocytosis required for Toll to signal but it is involved in shaping the Dorsal gradient. Our data also suggest that Toll signaling occurs not from the plasma membrane, as previously believed, but, instead, from endosomes.
Results
Toll Is Present Not Only at the Plasma Membrane but Within an Early Endosomal Compartment.
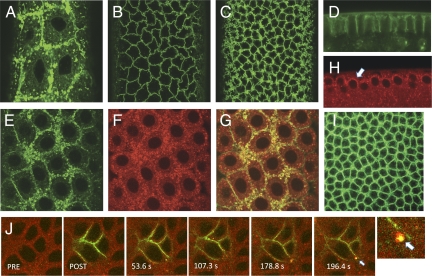
To address the localization and dynamics of the Toll receptor during early embryogenesis, we generated transgenic Drosophila lines expressing a chimeric Toll-GFP fusion protein under GAL4/UAS regulation and crossed them to either nanos-GAL4:VP16 or embryonic tubulin 67c-GAL4:VP16 driver lines. Embryos derived from female flies of either cross exhibited normal viability and development, indicating that fusion of GFP to the carboxy terminus of Toll does not interfere with signaling. Functionality of the chimera is demonstrated by the activity of a Toll10b-GFP chimera, which is described later in this study. Both nanos-GAL4:VP16 and embryonic tubulin 67c-driven Toll-GFP transgenes exhibited the same distribution and localization pattern in embryos. In contrast to the primarily plasma membrane localization observed with antibody staining (Fig. 1I), we find that Toll is highly dynamic over nuclear cycles 10–14 in live imaging (Fig. S1). Although a portion of the Toll-GFP signal is localized to the plasma membrane, as previously reported (16), prominent localization to cytoplasmic particles was observed throughout syncytial and cellular blastoderm stages (Fig. 1 A–D). To determine whether this particulate distribution corresponds to Toll that entered the endocytic pathway, we constructed transgenic lines expressing Rab5, an early endosomal marker, fused to mCherry and driven by the endogenous Rab5 promotor and regulatory elements. The distribution of mCherry-Rab5 in the syncytial and early blastoderm embryo was primarily particulate within the cortical cytoplasm (Fig. 1H), and Rab5+ particles appeared morphologically similar to those observed with Toll-GFP (Fig. 1 F and H). We crossed Toll-GFP with nanos-GAL4:VP16, mCherry-Rab5, and coimaged embryos from female flys expressing both transgenes. Toll-GFP (green) and mCherry-Rab5 (red) show a strong overlap (yellow) within the particulate compartment, indicating that at any given time during blastoderm stages, a significant fraction of Toll resides within the Rab5+ early endosomes (Fig. 1 E–G).
Fig. 1.
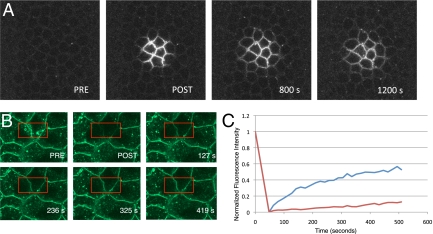
Toll-GFP localizes to an early endosomal compartment in living embryos. (A–C) Surface views of Toll-GFP at the syncytial and cellular blastoderm. Toll localization shifts from predominantly vesicular and cytoplasmic during nuclear cycles 10 (A), 11, and 12 to predominantly plasma membrane in nuclear cycles 13 (B) and 14 (C). (D) Toll-GFP in a side view at nuclear cycle 14. (E–G) Coimaging at nuclear cycle 12 of Toll (green) and mCherry-Rab5 (red) shows significant overlap (yellow in F). (H) mCherry-Rab5 is distributed in particles concentrated in the cortical cytoplasm (arrow) at nuclear cycle 12. (I) Antibody staining of heat-fixed whole-mount embryos at nuclear cycle 14 using anti-Toll antibody showing both plasma membrane and particulate localization. (J) Toll moves from the plasma membrane to early endosomes and selectively partitions to discrete endosomal domains. Toll-paGFP was photoactivated (green) and appears first within the plasma membrane and subsequently in Rab5+ early endosomes (yellow). The last panel shows a higher magnification view of the region around the arrow in the preceding 196.4-s panel. PRE, preinjection; POST, postinjection.
To ask whether Toll trafficks from the plasma membrane to early endosomes, we generated lines expressing Toll-paGFP, a photoactivatable form of Toll, crossed them to a mCherry-Rab5 line, and imaged embryos produced by F1 female flys (17). We photoactivated Toll-paGFP at the surface of the cellular blastoderm embryo and initially observed a plasma membrane distribution (Fig. 1J, POST). Within ∼54 s after photoactivation, Toll appeared within several small Rab5+ particles immediately adjacent to the plasma membrane within the zone of photoactivation. Within 3 min after photoactivation, Toll was observed within larger discrete Rab5+ particles. We reproducibly observed that Toll localized to a patch or discrete island comprising a portion of the surface of the Rab5+ endosome (Fig. 1J, arrow). This patching suggests that Toll selectively partitions from plasma membrane on endocytosis into discrete domains of the endosomal membrane and that these domains are relatively stable over the time course of imaging.
Endocytosis of Toll Is Enhanced in Constitutively Active Toll10b.
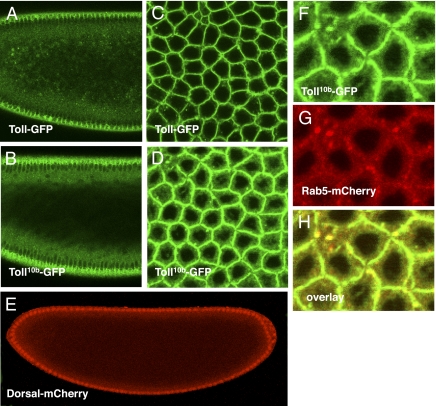
To address the question of whether endocytosis of Toll is correlated with activation of the Toll receptor, we generated transgenic lines expressing UAS-Toll10b-GFP. The Toll10b protein contains a single amino acid change, resulting in a form of Toll that constitutively signals independent of ligand binding (18). To determine whether Toll10b-GFP is functional, we crossed it to a line expressing Dorsal-mCherry under the endogenous Dorsal promotor as well as the α-tubulin 67C-GAL4:VP16 driver. Embryos from mothers expressing Toll10b-GFP under α-tubulin 67C-GAL4:VP16 regulation failed to hatch and were ventralized. Also, in the presence of the Toll10b-GFP transgene, Dorsal-mCherry accumulates to high levels in all blastoderm nuclei, with no residual d/v asymmetry typical of Toll10b ventralization (Fig. 2E). This indicates that the Toll10b-GFP fusion protein is functional and competent of signaling. In contrast to Toll-GFP (Fig. 2 A and C), Toll10b-GFP consistently exhibits a reduced plasma membrane distribution and is shifted toward the particulate distribution (Fig. 2 B and D). Toll10b-GFP also shows a higher diffuse cytoplasmic signal (Fig. 2 B and D). When Toll10b-GFP was coimaged with Rab5, we observed a very strong overlap between Rab5+ particles and Toll10b-GFP (Fig. 2 F–H). Our data indicate a strong correlation between Toll receptor activation and increased residence within Rab5+ early endosomes and suggest that endocytosis might be involved in regulating the activated form of Toll.
Fig. 2.
Constitutively active Toll10b-GFP is shifted from the plasma membrane toward an internal cytoplasmic distribution. (A) Toll-GFP in an anterior sagittal section, nuclear cycle 14. (B) Toll10b-GFP in an anterior sagittal section, nuclear cycle 14. (C) Toll-GFP surface view, nuclear cycle 14. (D) Toll10b-GFP surface view, nuclear cycle 14. (E) Dorsal-mCherry distribution in an early nuclear cycle 14 embryo expressing Toll10b-GFP showing Dorsal protein in all nuclei along the d/v axis. (F–H) Overlap of Toll10b-GFP (green) with mCherry-Rab5 (red) showing that Toll10b is colocalized (yellow) with Rab5.
Endocytosis Is Required for Toll Signaling and the Generation of the WT Dorsal Gradient.
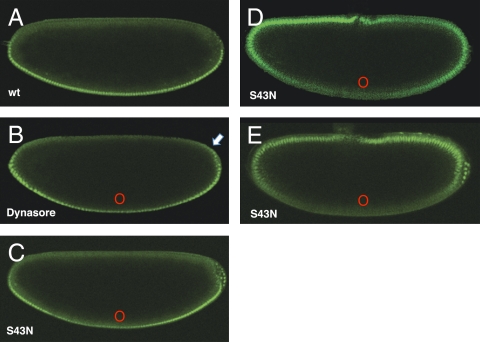
To address the role of endocytosis in embryonic Toll signaling, we locally inhibited entry into the endocytic pathway in embryos expressing Dorsal-GFP at different dorsoventral positions and asked how a block in endocytosis affected Dorsal nuclear accumulation. First (Fig. 3B), we microinjected Dynasore (Sigma), a small-molecule Dynamin inhibitor (19), on the ventral side of the embryo and observed that nuclear Dorsal levels were reduced near the site of injection, suggesting that inhibition of endocytosis ventrally attenuates Toll signaling. Control microinjections of solvent alone showed no effect and appeared as wild type (Fig. 3A). We then synthesized mRNA encoding a dominant negative form of Rab5, Rab5S43N, which is analogous to the mammalian Rab5S34N that has previously been used to block trafficking between the plasma membrane and early endosomes in mammalian cells. Synthetic mRNA was injected at specific positions in Dorsal-GFP–expressing embryos. We expected the effects of these injections to be highly localized; mRNA microinjection in Drosophila embryos has previously been shown to display highly localized effects because RNAs do not diffuse significantly from the point of injection over the short time scale of these experiments (20). When Rab5S43N mRNA is microinjected near the ventral midline, we observe a local reduction of nuclear Dorsal concentration at the site on injection (Fig. 3C). Unexpectedly, 30–45 min postinjection, we also observe a shift of Dorsal nuclear accumulation to dorsolateral positions, where Dorsal normally does not appear within nuclei (Fig. 3B, arrow). Depending on the amount of injected mRNA and the time after injection, the effects of depositing the dominant negative Rab5S43N mRNA near the ventral midpoint range from subtle local attenuation of nuclear Dorsal levels with a slight expansion of the nuclear gradient (Fig. 3C) to complete inversion of the Dorsal gradient (Fig. 3 D and E). In contrast, Rab5S43N mRNA microinjections on the dorsal midline had little, if any, observable effect on the Dorsal gradient. When we observe inversion of the Dorsal gradient, we also observe inversion of the gastrulation movements and migration of the pole cells toward the ventral side of the embryo, indicating true inversion of d/v polarity.
Fig. 3.
Inhibition of endocytosis ventrally reduces nuclear accumulation of Dorsal proximally and can alter the polarity of the Dorsal gradient. Live imaging of transgenic embryos expressing Dorsal-GFP at nuclear cycle 14. (A) Mock-injected control embryo expressing Dorsal-GFP showing the WT (wt) nuclear Dorsal gradient. (B) Embryo microinjected with Dynasore, a dynamin inhibitor, as a small bolus on the ventral midline in which nuclear accumulation of Dorsal is attenuated near the site of injection and slightly expanded dorsally (arrow). (C) Embryo injected with synthetic mRNA encoding Rab5S43N, a dominant negative form of Rab5 on the ventral midline. (D) Embryo injected as in C and allowed to develop for 40 min, illustrating a progressive shifting of nuclear translocation to the dorsal side of the embryo. (E) Rab5S43N ventrally injected embryo showing complete inversion of the Dorsal gradient. In all cases, ventral is down, dorsal is up, and red circles mark the site of injection of drug or synthetic mRNA.
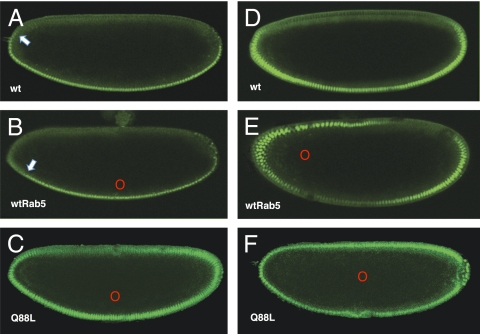
Rab5 concentration has been shown to be rate-limiting for endocytosis (21). To determine how locally increasing endocytosis might affect Toll signaling, we synthesized mRNA encoding both WT Rab5 and Rab5Q88L, the equivalent of mammalian Rab5Q79L, a constitutively active form of Rab5. Microinjection of WT Rab5 mRNA near the ventral midline leads to a slight contraction of the Dorsal gradient (Fig. 4B). However, microinjection of WT Rab5 mRNA at lateral positions leads to potentiation of signaling and relocalization of the signaling center to the site of injection (Fig. 4E). This shift corresponds to repositioning of an existing signaling center rather than the de novo induction of an ectopic signaling center, because microinjections at the dorsal midpoint have no effect. Control microinjections of buffer alone were also performed and showed no effect on the Dorsal nuclear gradient (Fig. 4 A and D). Deposition of the constitutively active Rab5Q88L mRNA in the ventral cytoplasm causes an expansion of the Dorsal gradient (Fig. 4C). However, Rab5Q88L mRNA in the center of the embryo leads to a global expansion of the Dorsal gradient, with Dorsal accumulating in all nuclei across the d/v axis (Fig. 4F). We conclude that endocytosis can be rate-limiting for Toll signaling.
Fig. 4.
(A) Mock-injected control embryo expressing Dorsal-GFP showing the WT (wt) Dorsal gradient. The Dorsal gradient is retracted toward the ventral midline (arrow). (B) Dorsal-GFP–expressing embryo injected near the ventral midline with synthetic mRNA encoding WT Rab5 (red circle). The Dorsal gradient is retracted toward the ventral midline (arrow). (C) Dorsal-GFP–expressing embryo injected ventrolaterally (red circle) with mRNA encoding constitutively active Rab5Q88L. The Dorsal gradient is expanded dorsolaterally but exhibits normal polarity. (D) Mock-injected Dorsal-GFP control embryo viewed at the same relative ventrolateral position as the embryo in E. (E) Dorsal-GFP–expressing embryo injected with synthetic mRNA encoding WT Rab5 axially at 25% egg length (red circle). The Dorsal gradient is shifted away from the ventral midline toward the site of injection only at the anterior of the embryo. (F) Dorsal-GFP–expressing embryo injected centrally (red circle) with synthetic mRNA encoding Rab5Q88L. Dorsal accumulates in all nuclei. In all cases, ventral is down, dorsal is up, red circles mark the site of injection, and embryos are in nuclear cycle 14.
One way in which inhibition of endocytosis could result in a spatial alteration of Toll signaling so extreme as to lead to inversion of the Dorsal gradient could be through the lateral diffusion of activated Toll receptors within the plasma membrane. In such a mechanism, in the absence of endocytosis, activated Toll would diffuse out of the ventral activation zone rather than being spatially restricted through internalization. In this case, Toll would be expected to show a relatively high degree of diffusional mobility in the plasma membrane. To access the diffusional mobility of Toll within the plasma membrane, we performed photoactivation studies with Toll-paGFP. As shown in Fig. 5A, Toll diffuses less than two energid diameters in ≈20 min at 22 °C. We also performed fluorescence recovery after photobleaching (FRAP) experiments in embryos expressing Toll-GFP. As shown in Fig. 5B, before FRAP, Toll-GFP can be detected within the plasma membrane as well as within cytoplasmic particles. After FRAP, it can be seen in the recovery panels that Toll in the plasma membrane recovers by about 50% in ≈7.5 min over one cell diameter, whereas 5-fold less recovery is observed for Toll in the particulate compartment over the same distance. The results show that Toll diffusion within the membrane is highly limited and its mobility within the endocytic pathway is even more restricted (Fig. 5C). The data suggest that diffusion of activated Toll receptor within the plasma membrane is not the mechanism by which inversion of the axis is achieved.
Fig. 5.
Toll-GFP exhibits limited diffusion within the plasma membrane and is compartmentalized within the endocytic pathway. (A) Photoactivation of Toll-paGFP on the plasma membrane surface. Toll within the plasma membrane at nuclear cycle 14 diffuses one to two cell diameters within 20 min at 22 °C. PRE, preinjection; POST, postinjection. (B) FRAP of both the membrane and particulate fractions of Toll (bleach box in red). (C) Fluorescence recovery curves for the plasma membrane fraction (blue) and the particulate fraction (red) illustrate slow recovery for membrane-localized Toll over a distance of one future blastoderm cell. The recovery rate for particulate Toll (red) is 20% of the recovery rate for the plasma membrane fraction.
Inversion of the d/v Axis by Ventral Microinjection of Dominant Negative Rab5 S43N Leads to Selective Accumulation of Toll in Endosomes on the Dorsal Side of the Embryo.
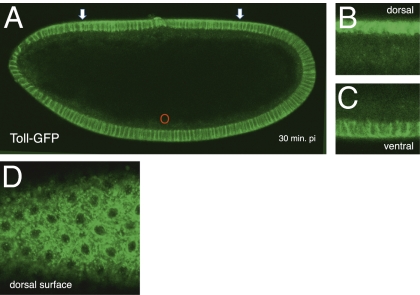
We wished to ask how Toll is localized under the conditions producing complete inversion of the Dorsal gradient and repositioning of the signaling center to the dorsal side of the embryo. To do so, we injected Rab5S43N mRNA ventrally in embryos expressing Toll-GFP. We found that the distribution and levels of Toll near the site of injection do not change (Fig. 6A). Interestingly, we found that dorsally, where the newly created signaling center is located, Toll dramatically shifts from the plasma membrane to a primarily internalized distribution (Fig. 6 B and C). A dorsal surface view of a similarly injected embryo shows that Toll preferentially accumulates at very high levels in particles, with a similar morphology to Rab5+ early endosomes (Fig. 6D). These results demonstrate a correlation of entry of Toll into endosomes with ectopic hyperactivation by dominant negative Rab5 S43N.
Fig. 6.
Inversion of the d/v axis by ventral microinjection of dominant negative Rab5 S43N leads to selective accumulation of Toll in endosomes on the dorsal signaling center. (A) Toll-GFP–expressing embryo injected ventrally with Rab5S43N under conditions that invert the d/v axis. The arrows show a shift in the distribution and accumulation of Toll at the opposite (dorsal) side of the embryo, where a newly created signaling center is located. pi, postinjection. (B) Dorsal view of another embryo injected as in A. (C) Ventral view of the embryo injected as in A. (D) Dorsal surface view of an embryo injected as in A, showing accumulation of Toll in a compartment with morphology characteristic of Rab5+ early endosomes.
Discussion
Endocytosis Is Necessary but Not Sufficient for Toll Signaling.
Our results indicate that endocytosis of Toll has at least two distinct roles in the generation of the Dorsal gradient: (i) Endocytosis is clearly required for the normal signaling of the activated Toll receptor, implying that endosomes may function as a signaling platform, and (ii) endocytosis is involved in the generation and/or shaping of the Dorsal gradient, presumably by restricting the diffusional mobility of one or more ventralizing determinants.
Although a number of signal transduction systems in Drosophila and mammals have been demonstrated to have a requirement for endocytosis, a role for endocytosis in Toll receptor signaling is only now emerging. In mammals, TLR4 first signals from the plasma membrane to activate NF-κB but later switches to the IRF3 activating pathway on ligand-induced internalization and arrival in the early endosomal compartment (22). In Drosophila, the redistribution of Toll from the plasma membrane to an unidentified internal compartment has been observed in larval fat body tissue in response to immune stimulation (23). Recently, Toll signaling in innate immunity has been shown to require components of the endocytic pathway, and Toll was shown to colocalize with components of the endocytic pathway in transfected cells (24). The in vivo experiments presented here extend the involvement of endocytosis to Toll function in embryonic development and suggest that activation-induced Toll internalization and signaling from endosomes is a general property of Toll.
Role of Endocytosis in Shaping the Dorsal Gradient.
The simplest hypothesis to explain the global and long-range effects of inhibiting or potentiating Rab5 activity is that endocytosis regulates Toll signaling by restricting the mobility of one or more ventralizing factors. Although the diffusion of activated Toll within the plasma membrane is one possibility, Toll only diffuses two to three cell diameters within the plasma membrane over the 30 min during which the Dorsal gradient can be inverted. Therefore, membrane diffusion of Toll cannot by itself explain the global shift in the Dorsal nuclear gradient occurring within the observed time frame. Significantly, a physical interaction between the intracellular half of Toll and the cytoskeletal organizer filamin occurs (25), making it possible that Toll's low mobility is attributable to anchoring to the actin cytoskeleton. Another possibility is that Rab5 inhibition induces Toll depletion from the plasma membrane by interfering with receptor recycling, leading to a shift in signaling to lateral and dorsal positions. However, plasma membrane Toll is not locally depleted on the ventral side by injection of dominant negative Rab5 S43N (Fig. 6), and dominant negative Rab11 that would be expected to block receptor recycling does not induce Dorsal gradient inversion. Therefore, interference with receptor recycling is not a probable mechanism. A third possibility is that processed Spaetzle ligand is the ventralizing determinant spatially constrained by endocytosis. Loss of embryonic Toll has been shown to lead to the selective accumulation of the cleaved Spaetzle C-terminal ligand fragment but not the unprocessed form or the N-terminal prodomain (26, 27). The differential stability of C-terminal ligand is consistent with preferential endocytosis of Spaetzle ligand. Another nonexclusive explanation is that endocytosis traps or removes the active forms of one or more of the upstream proteases required for generation of ligand, restricting the zone of activated Spaetzle ligand generation to the ventral side of the embryo (28). We favor the hypothesis that Spaetzle ligand is the ventralizing determinant constrained by endocytosis.
Interpretation of the Polarity-Inducing Effects of Toll.
Classic studies on Toll described that injection of WT cytoplasm into young Toll embryos restored d/v pattern elements in a position-dependent manner (29). No matter where the cytoplasm was injected relative to the d/v axis of the egg, the site of injection defined the most ventral point of the rescued pattern. Toll was the only maternal d/v mutant exhibiting this property in rescue assays. Our results provide a clear mechanistic insight into the observation. First, Toll is very slow to diffuse within the plasma membrane; therefore, it should accumulate in a highly localized region of the plasma membrane near the site of microinjection. Second, in the absence of Toll, and therefore the ability to internalize a Spaetzle/Toll complex, activated Spaetzle could rapidly diffuse within the perivitelline space until it reaches a uniform distribution. On injection of cytoplasm or RNA generating de novo Toll, a localized region of the plasma membrane would accumulate Toll and would then serve as a sink for the freely diffusing Spaetzle ligand. Thus, microinjections would create an ectopic signaling center proximal to the site of injection, where newly synthesized Toll protein would be sequestered. Although we do not have measurements for the mobility of the activated Spaetzle ligand in the perivitelline space, results from FRAP of Spaetzle-GFP indicate that the mobility of bulk Spaetzle approaches the free diffusion rate. If the diffusion behavior of Spaetzle ligand is similar to that of Spaetzle, diffusion of ligand could easily account for the observed “position-defining” properties of Toll in these early studies.
Our results imply that a relatively stable signaling complex assembles within the endocytic pathway. This has two consequences for the spatial and temporal regulation of Dorsal nucleocytoplasmic shuttling and the dynamic modulation of the Dorsal gradient. First, it provides a means of spatially “fixing” a graded extracellular distribution of ligand, and, second, it provides a signaling platform within the endocytic pathway that is physically associated with individual syncytial blastoderm nuclei and their nuclear import and export machinery, enabling adjacent nuclei to maintain discrete nuclear Dorsal concentrations.
Materials and Methods
Transformed Fly Lines.
We have previously generated a series of transformation vectors for the simplified construction of fluorescently tagged transgenes under the regulation of GAL4/UAS or endogenously derived regulatory sequences. These were made by inserting the fluorescent cassettes coding for either EGFP, mCherry, Venus, Cerulean, or paGFP preceded by a unique multiple cloning site into the pUASp vector (30) or into the pCaSpeR-4 vector (31) supplied with the f(s)K10 terminator (these vectors are described elsewhere.)
GAL4-inducible Toll-GFP and Toll-PAGFP were made by inserting the Toll ORF amplified from embryonic cDNA using oligonucleotides Tl4: CTTGTCATCGACACGCGTTCCGCCTACGTCGCTCTGTTTGGC and Tl5: GCCTCGCGGCCGCATGCCACCATGAGTCGACTAAAGGCCGC and inserting them into pUASp-GFP and pUASp-PAGFP, respectively, through NotI and MluI. The Toll10b ORF was generated from the existing Toll constructs through a megaprimer mutagenesis procedure using the oligo Tl10: GGAGATCGCAATGAAATGATGTACGTGAACGCCGAGATGCC to cause the C781Y amino acid substitution and was cloned into the pUASp-GFP vector as above.
Endogenously driven mCherry-Rab5 was constructed as follows. The Rab5 ORF was amplified from genomic DNA using oligonucleotides Rab5-1: TCACTTGCAGCAGTTGTTCG and Rab5-2: CGATGGTACACGCGTGGCGGAGGTGGCATGGCAACCACTCCACGCAG, restricted with MluI, and inserted into the pV0 vector [a derivative of pCaSpeR4 containing an f(s)K10 terminator and a unique multiple cloning site], which had been digested with MluI and StuI. Next, the upstream regulatory region amplified from genomic DNA using oligonucleotides Rab5-3: CGATGGTACACGCGTCATGATCGGTTCGGGTTG and Rab5-4: GTCATCGAGCGGCCGCTAGTTCGATTGCGTTTGCAG was inserted into the resulting construct through NotI and MluI. Finally, the mCherry cassette amplified using oligonucleotides spnmCh-1: CGATGGTACACGCGTGGGGTGAGCAAGGGCGAGG and spnmCh-2: GTGCATCGGACGCGTCTTGTACAGCTCGTCCATGCC was inserted using the MluI site. The resulting transgene expresses mCherry-Rab5 fusion separated by a TRGGGGG linker.
Endogenously driven Dorsal-mCherry was constructed by amplifying a fragment containing the Dorsal coding sequence and the 5 Kb 5′ UAS from the Dorsal-GFP construct described by DeLotto et al. (32) using oligonucleotides D100: ACATTGCATCACTAGTCGTGGATATGGACAGGTTCGATATCTGCAGATCTTCCGAATTGAGGCGCAG and D101: GCATACTGCACTAGTTTCCCGCACCTGGCGGTACTTG, digesting it with SpeI, and cloning it into the pV2-mCherry vector digested similarly.
All constructs were introduced into w1118 flies by standard P-element transformation, generating multiple transgenic lines.
Other Fly Stocks.
Other fly stocks used in this study are P{GAL4::VP16-nos.UTR}, w; P{matα4-GAL-VP16}V37, and P{dl.URS-dl-GFP} as described by DeLotto et al. (32).
In Vitro Transcription and RNA Microinjections.
Rab5 was amplified from genomic DNA using oligonucleotides Rab5-5: CCGACGAATTCTAACCACCATGGCAACCACTCCACGC and Rab5-9: GCGTCGGATCCTCACTTGCAGCAGTTGTTCG. Mutations altering the coding sequence to either N43S or Q88L were introduced through mutagenesis using megaprimers generated with Rab5-5 and Rab5-6: GAAGCGCAGCACCAGTGAGTTCTTGCCCACAGCGGACTC (for N43S) or Rab5-5 and Rab5-8: CTAAGCTGTGGTACCGCTCCAGGCCAGCCGTGTCCCAG (for Q88L). WT and mutant ORFs were cloned into pGEM-4 using EcoRI and BamHI.
In vitro transcription was done using the MEGAscript SP6 In Vitro Transcription Kit (Ambion) according to the manufacturer's instructions. To ensure 5′ capping, the m7G(5′)ppp(5′)G cap analogue (Ambion) was added to the reaction mix in a 5:1 ratio to GTP. RNA was purified by LiCl and ethanol precipitation and resuspended in water.
Dynasore Microinjections.
Dynasore (Sigma) was dissolved in DMSO at a concentration of 10 μg/μL and microinjected as described for the RNAs. Control injections were done with DMSO (Sigma).
Antibody Staining.
Embryos were dechorionized with 50% (vol/vol) sodium hypochlorite, washed, and resuspended in Triton-salt solution (4% (wt/vol) sodium chloride, 0.3% Triton X-100). They were then heated for 30 s at 96 °C, immediately transferred to ice to stop heat fixation, and then transferred to 4% (wt/vol) paraformaldehyde in PBS for 5 min with shaking. Subsequent handling was as previously described by Hashimoto et al. (16). Anti-Toll antibody (toll d-300; Santa Cruz Biotechnology) was used at a dilution of 1:50. Alexa 488-conjugated anti-rabbit antibody was obtained from Molecular Probes.
Image Acquisition.
Imaging was performed on a Zeiss LSM 510 confocal microscope using Zeiss 20× and Zeiss 63× water immersion objectives. Argon (488 nm) and HeNe (543 nm) laser lines were used in imaging to excite GFP and mCherry. Data collection was set up to detect fluorescent emission in the window of 505–530 nm as the GFP signal and above 585 nm as the mCherry signal. Photobleaching was performed using 458-, 488-, and 514-nm argon lines. Photoactivation of paGFP was performed using a 405-nm diode laser. For live imaging, embryos were dechorionized in 2% (wt/vol) sodium hypochlorite for 1–2 h and mounted on siliconized coverslips in PBS. Data analysis was done using native Zeiss LSM 510 software.
Supplementary Material
Acknowledgments
We thank the Novo Nordisk Fund, Lundbeck Fund, and Novo Scholarship Fund for funding and the Carlsberg Fund for fellowship support.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009157107/-/DCSupplemental.
References
- 1.Hong JW, Hendrix DA, Papatsenko D, Levine MS. How the Dorsal gradient works: Insights from postgenome technologies. Proc Natl Acad Sci USA. 2008;105:20072–20076. doi: 10.1073/pnas.0806476105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLotto R, Spierer P. A gene required for the specification of dorsal-ventral pattern in Drosophila appears to encode a serine protease. Nature. 1986;323:688–692. doi: 10.1038/323688a0. [DOI] [PubMed] [Google Scholar]
- 3.DeLotto Y, DeLotto R. Proteolytic processing of the Drosophila Spätzle protein by easter generates a dimeric NGF-like molecule with ventralising activity. Mech Dev. 1998;72:141–148. doi: 10.1016/s0925-4773(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 4.Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo—Shaping and transducing a morphogen gradient. Curr Biol. 2005;15(Suppl):R887–R899. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Charatsi I, Luschnig S, Bartoszewski S, Nüsslein-Volhard C, Moussian B. Krapfen/dMyd88 is required for the establishment of dorsoventral pattern in the Drosophila embryo. Mech Dev. 2003;120:219–226. doi: 10.1016/s0925-4773(02)00410-0. [DOI] [PubMed] [Google Scholar]
- 6.Towb P, Sun H, Wasserman SA. Tube Is an IRAK-4 homolog in a Toll pathway adapted for development and immunity. J Innate Immun. 2009;1:309–321. doi: 10.1159/000200773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reach M, et al. A gradient of cactus protein degradation establishes dorsoventral polarity in the Drosophila embryo. Dev Biol. 1996;180:353–364. doi: 10.1006/dbio.1996.0308. [DOI] [PubMed] [Google Scholar]
- 8.Whalen AM, Steward R. Dissociation of the dorsal-cactus complex and phosphorylation of the dorsal protein correlate with the nuclear localization of dorsal. J Cell Biol. 1993;123:523–534. doi: 10.1083/jcb.123.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drier EA, Huang LH, Steward R. Nuclear import of the Drosophila Rel protein Dorsal is regulated by phosphorylation. Genes Dev. 1999;13:556–568. doi: 10.1101/gad.13.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang AM, Rusch J, Levine M. An anteroposterior Dorsal gradient in the Drosophila embryo. Genes Dev. 1997;11:1963–1973. doi: 10.1101/gad.11.15.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;180:755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devergne O, Ghiglione C, Noselli S. The endocytic control of JAK/STAT signalling in Drosophila. J Cell Sci. 2007;120:3457–3464. doi: 10.1242/jcs.005926. [DOI] [PubMed] [Google Scholar]
- 13.Marois E, Mahmoud A, Eaton S. The endocytic pathway and formation of the Wingless morphogen gradient. Development. 2006;133:307–317. doi: 10.1242/dev.02197. [DOI] [PubMed] [Google Scholar]
- 14.Torroja C, Gorfinkiel N, Guerrero I. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development. 2004;131:2395–2408. doi: 10.1242/dev.01102. [DOI] [PubMed] [Google Scholar]
- 15.Chang WL, et al. The gradient of Gurken, a long-range morphogen, is directly regulated by Cbl-mediated endocytosis. Development. 2008;135:1923–1933. doi: 10.1242/dev.017103. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto C, Gerttula S, Anderson KV. Plasma membrane localization of the Toll protein in the syncytial Drosophila embryo: Importance of transmembrane signaling for dorsal-ventral pattern formation. Development. 1991;111:1021–1028. doi: 10.1242/dev.111.4.1021. [DOI] [PubMed] [Google Scholar]
- 17.Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science. 2002;297:1873–1877. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- 18.Anderson KV, Jürgens G, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: Genetic studies on the role of the Toll gene product. Cell. 1985;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- 19.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Smith CL, DeLotto R. Ventralizing signal determined by protease activation in Drosophila embryogenesis. Nature. 1994;368:548–551. doi: 10.1038/368548a0. [DOI] [PubMed] [Google Scholar]
- 21.Bucci C, et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 22.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettencourt R, Tanji T, Yagi Y, Ip YT. Toll and Toll-9 in Drosophila innate immune response. J Endotoxin Res. 2004;10:261–268. doi: 10.1179/096805104225004897. [DOI] [PubMed] [Google Scholar]
- 24.Huang HRCZ, Chen ZJ, Kunes S, Chang GD, Maniatis T. Endocytic pathway is required for Drosophila Toll innate immune signaling. Proc Natl Acad Sci USA. 2010;107:8322–8327. doi: 10.1073/pnas.1004031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards DN, Towb P, Wasserman SA. An activity-dependent network of interactions links the Rel protein Dorsal with its cytoplasmic regulators. Development. 1997;124:3855–3864. doi: 10.1242/dev.124.19.3855. [DOI] [PubMed] [Google Scholar]
- 26.Morisato D, Anderson KV. The spätzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1994;76:677–688. doi: 10.1016/0092-8674(94)90507-x. [DOI] [PubMed] [Google Scholar]
- 27.Morisato D. Spätzle regulates the shape of the Dorsal gradient in the Drosophila embryo. Development. 2001;128:2309–2319. doi: 10.1242/dev.128.12.2309. [DOI] [PubMed] [Google Scholar]
- 28.Dissing M, Giordano H, DeLotto R. Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J. 2001;20:2387–2393. doi: 10.1093/emboj/20.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson KV, Bokla L, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: The induction of polarity by the Toll gene product. Cell. 1985;42:791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- 30.Rørth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 31.Pirrotta V. Vectors for P-mediated transformation in Drosophila. Biotechnology. 1988;10:437–456. doi: 10.1016/b978-0-409-90042-2.50028-3. [DOI] [PubMed] [Google Scholar]
- 32.DeLotto R, DeLotto Y, Steward R, Lippincott-Schwartz J. Nucleocytoplasmic shuttling mediates the dynamic maintenance of nuclear Dorsal levels during Drosophila embryogenesis. Development. 2007;134:4233–4241. doi: 10.1242/dev.010934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.