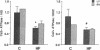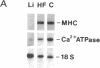Abstract
A decrease in the myocardial level of the mRNA encoding the Ca2(+)-ATPase of the sarcoplasmic reticulum (SR) has been recently reported during experimental cardiac hypertrophy and failure. To determine if such a deficit occurs in human end-stage heart failure, we compared the SR Ca2(+)-ATPase mRNA levels in left (LV) and right ventricular (RV) specimens from 13 patients undergoing cardiac transplantation (6 idiopathic dilated cardiomyopathies; 4 coronary artery diseases with myocardial infarctions; 3 diverse etiologies) with control heart samples using a rat cardiac SR Ca2(+)-ATPase cDNA probe. We observed a marked decrease in the mRNA for the Ca2(+)-ATPase relative to both the 18S ribosomal RNA and the myosin heavy chain mRNA in LV specimens of patients with heart failure compared to controls (-48%, P less than 0.01 and -47%, P less than 0.05, respectively). The LV ratio of Ca2(+)-ATPase mRNA to 18S RNA positively correlated with cardiac index (P less than 0.02). The RV ratio correlated negatively with systolic, diastolic and mean pulmonary arterial pressures (P less than 0.02, P less than 0.02, and P less than 0.01, respectively). We suggest that a decrease of the SR Ca2(+)-ATPase mRNA in the myocardium plays an important role in alterations of Ca2+ movements and myocardial relaxation reported during human end-stage heart failure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikfalvi A., Enouf J., Bredoux R., Lompre A., Dupuy E., Bourdeau N., Levy-Toledano S., Tobelem G. Evidence of endoplasmic reticulum-related Ca2+ ATPase in human microvascular endothelial cells. Exp Cell Res. 1989 Sep;184(1):28–36. doi: 10.1016/0014-4827(89)90360-1. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Das P. K., Sharma G. P. Subcellular basis of cardiac contractile failure. J Mol Cell Cardiol. 1978 Apr;10(4):363–385. doi: 10.1016/0022-2828(78)90384-x. [DOI] [PubMed] [Google Scholar]
- Gunteski-Hamblin A. M., Greeb J., Shull G. E. A novel Ca2+ pump expressed in brain, kidney, and stomach is encoded by an alternative transcript of the slow-twitch muscle sarcoplasmic reticulum Ca-ATPase gene. Identification of cDNAs encoding Ca2+ and other cation-transporting ATPases using an oligonucleotide probe derived from the ATP-binding site. J Biol Chem. 1988 Oct 15;263(29):15032–15040. [PubMed] [Google Scholar]
- Gwathmey J. K., Copelas L., MacKinnon R., Schoen F. J., Feldman M. D., Grossman W., Morgan J. P. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987 Jul;61(1):70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Morgan J. P. Altered calcium handling in experimental pressure-overload hypertrophy in the ferret. Circ Res. 1985 Dec;57(6):836–843. doi: 10.1161/01.res.57.6.836. [DOI] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Ito Y., Suko J., Chidsey C. A. Intracellular calcium and myocardial contractility. V. Calcium uptake of sarcoplasmic reticulum fractions in hypertrophied and failing rabbit hearts. J Mol Cell Cardiol. 1974 Jun;6(3):237–247. doi: 10.1016/0022-2828(74)90053-4. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurabayashi M., Shibazaki Y., Takaku F., Yazaki Y. Molecular cloning and characterization of a Ca2+ + Mg2+-dependent adenosine triphosphatase from rat cardiac sarcoplasmic reticulum. Regulation of its expression by pressure overload and developmental stage. J Clin Invest. 1989 Apr;83(4):1102–1108. doi: 10.1172/JCI113989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczak B., Zarain-Herzberg A., Brandl C. J., Ingles C. J., Green N. M., MacLennan D. H. Structure of the rabbit fast-twitch skeletal muscle Ca2+-ATPase gene. J Biol Chem. 1988 Apr 5;263(10):4813–4819. [PubMed] [Google Scholar]
- Lompre A. M., de la Bastie D., Boheler K. R., Schwartz K. Characterization and expression of the rat heart sarcoplasmic reticulum Ca2+-ATPase mRNA. FEBS Lett. 1989 May 22;249(1):35–41. doi: 10.1016/0014-5793(89)80010-9. [DOI] [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- Mahdavi V., Periasamy M., Nadal-Ginard B. Molecular characterization of two myosin heavy chain genes expressed in the adult heart. Nature. 1982 Jun 24;297(5868):659–664. doi: 10.1038/297659a0. [DOI] [PubMed] [Google Scholar]
- Mendez R. E., Pfeffer J. M., Ortola F. V., Bloch K. D., Anderson S., Seidman J. G., Brenner B. M. Atrial natriuretic peptide transcription, storage, and release in rats with myocardial infarction. Am J Physiol. 1987 Dec;253(6 Pt 2):H1449–H1455. doi: 10.1152/ajpheart.1987.253.6.H1449. [DOI] [PubMed] [Google Scholar]
- Nagai R., Zarain-Herzberg A., Brandl C. J., Fujii J., Tada M., MacLennan D. H., Alpert N. R., Periasamy M. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2966–2970. doi: 10.1073/pnas.86.8.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani E. D., Alousi A. A., Grant A. M., Older T. M., Dziuban S. W., Jr, Allen P. D. Changes in myofibrillar content and Mg-ATPase activity in ventricular tissues from patients with heart failure caused by coronary artery disease, cardiomyopathy, or mitral valve insufficiency. Circ Res. 1988 Aug;63(2):380–385. doi: 10.1161/01.res.63.2.380. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B., Schwartz K., Apstein C. S. Decreased contractility after myocardial hypertrophy: cardiac failure or successful adaptation? Am J Cardiol. 1984 Aug 1;54(3):437–440. doi: 10.1016/0002-9149(84)90212-1. [DOI] [PubMed] [Google Scholar]
- de la Bastie D., Moalic J. M., Bercovici J., Bouveret P., Schwartz K., Swynghedauw B. Messenger RNA content and complexity in normal and overloaded rat heart. Eur J Clin Invest. 1987 Jun;17(3):194–201. doi: 10.1111/j.1365-2362.1987.tb01235.x. [DOI] [PubMed] [Google Scholar]
- de la Bastie D., Wisnewsky C., Schwartz K., Lompré A. M. (Ca2+ + Mg2+)-dependent ATPase mRNA from smooth muscle sarcoplasmic reticulum differs from that in cardiac and fast skeletal muscles. FEBS Lett. 1988 Feb 29;229(1):45–48. doi: 10.1016/0014-5793(88)80794-4. [DOI] [PubMed] [Google Scholar]