Abstract
We have shown that the potent phosphodiesterase-5 (PDE-5) inhibitor sildenafil (Viagra) induces a powerful effect on reduction of infarct size following ischemia/reperfusion injury and improvement of left ventricular dysfunction in the failing heart after myocardial infarction or doxorubicin (DOX) treatment. In the present study, we further investigated the potential effects of sildenafil on improving antitumor efficacy of DOX in prostate cancer. Cotreatment with sildenafil enhanced DOX-induced apoptosis in PC-3 and DU145 prostate cancer cells, which was mediated by enhanced generation of reactive oxygen species, up-regulation of caspase-3 and caspase-9 activities, reduced expression of Bcl-xL, and phosphorylation of Bad. Overexpression of Bcl-xL or dominant negative caspase 9 attenuated the synergistic effect of sildenafil and DOX on prostate cancer cell killing. Furthermore, treatment with sildenafil and DOX in mice bearing prostate tumor xenografts resulted in significant inhibition of tumor growth. The reduced tumor size was associated with amplified apoptotic cell death and increased expression of activated caspase 3. Doppler echocardiography showed that sildenafil treatment ameliorated DOX-induced left ventricular dysfunction. In conclusion, these results provide provocative evidence that sildenafil is both a powerful sensitizer of DOX-induced killing of prostate cancer while providing concurrent cardioprotective benefit.
Keywords: apoptosis, phosphodiesterase-5, reactive oxygen species
Prostate cancer remains among the most frequently diagnosed solid tumors in men and is one of the leading causes of cancer-related deaths in Western countries (1). Treatment options are limited and are associated with significant morbidity and mortality. Doxorubicin [(DOX) Adriamycin] is a broad-spectrum antitumor antibiotic that has been widely used for treatment of several cancers, including breast, ovarian, and prostate cancers (2). The effectiveness of DOX is limited due to its high toxicity and side effects, including myelosuppression, alopecia, acute nausea, vomiting, stomatitis, cumulative cardiotoxicity (3), and strong multidrug resistance response in tumor cells after repeated administration (4).
Sildenafil (Viagra), vardenafil (Levitra), and tadalafil (Cialis) inhibit phosphodiesterase-5 (PDE-5), the predominant enzyme in the corpus cavernosum that plays an essential role in vascular smooth muscle contraction through specific regulation of cGMP (5). We have demonstrated that sildenafil and other PDE-5 inhibitors induce a powerful protective effect against ischemia/reperfusion injury (6–10), DOX-induced cardiomyopathy (11), and myocardial infarction-induced heart failure (12). The cardioprotective effect is attributed to limiting apoptosis and necrosis through several mechanisms. These include enhanced expression of nitric oxide synthase (NOS), particularly the endothelial NOS (eNOS) and inducible NOS (iNOS); activation of protein kinase C and protein kinase G (PKG); phosphorylation of ERK1/2; PKG-dependent phosphorylation of GSK-3β; up-regulation of Bcl-2/Bax; and opening of the mitochondrial KATP channels (6–9, 12–14).
It has been shown that PDE-5 expression is increased in multiple human carcinomas including metastatic breast cancers, colon adenocarcinoma, bladder squamous carcinoma, and lung cancers (15–17), suggesting its potential role in controlling tumor cell growth and death. Sildenafil and vardenafil suppress tumor cell growth and induce caspase-dependent apoptosis in B-cell chronic lymphatic leukemia (18, 19). Another PDE-5 inhibitor, exisulind (sulindac sulfone), and its higher affinity analogs also induce apoptosis and inhibit cell proliferation in colon tumor cells lines by activating PKG and phosphorylation of β-catenin (20). These compounds also inhibit growth and induce apoptosis in several human prostate cancer cell lines and prostate cancer xenografts in nude mice (21–23). A low dose combination of colecoxib, a cyclooxygenase-2 (COX-2) inhibitor, with exisulind prevents prostate carcinogenesis by altering key molecular events (24).
In the present study, we tested whether sildenafil potentiates the antitumor efficacy of DOX in prostate cancer. Our results show that DOX and sildenafil induce a potent antitumor effect in prostate cancer while simultaneously providing a cardioprotective effect.
Results
Sildenafil Potentiates DOX-Induced Killing of Prostate Cancer Cells.
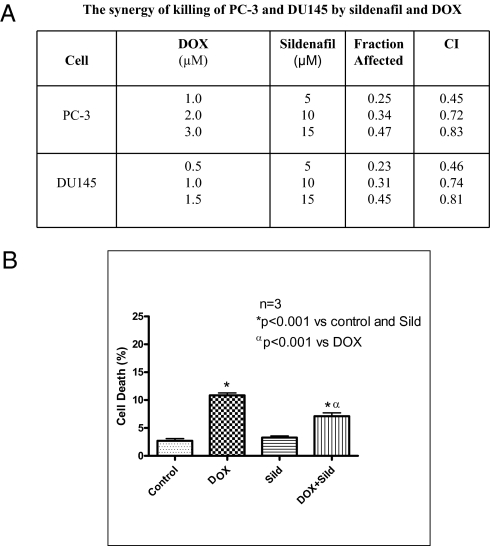
First, we examined the dose-dependent effect of DOX treatment in PC-3 and DU145 cells. Cell growth was reduced in a dose-dependent manner with DOX in both cells (Fig. 1 A and B). Cotreatment with sildenafil resulted in an additive effect on DOX-induced reduction of proliferation (Fig. 1 A and B). Cell killing assessed by trypan blue exclusion assay also confirmed similar additive effect (Fig. 1 C and D). DOX treatment also increased apoptosis as evaluated by TUNEL assays (Fig. 1 E and F). The sildenafil and DOX combination further enhanced apoptosis in PC-3 and DU145 cells, whereas sildenafil alone had no effect. Colony formation assays performed using median dose effect isobologram analysis further corroborated the synergistic effect of sildenafil and DOX in enhancing cell killing (Fig. 2A). In contrast, DOX treatment induced cell death in the normal prostate epithelial cells (PrEC), which was significantly reduced by sildenafil (Fig. 2B). Sildenafil treatment alone had no effect on cell death in the PrEC or prostate cancer cells.
Fig. 1.
Sildenafil (Sild) enhances DOX-induced cell death. Viability of (A) PC-3 and (B) DU145 cells after 72 h of treatment with different concentrations of DOX and/or Sild (10 μM). Red line represents DOX only; green line represents DOX + Sild (10 μM) (*P < 0.001 vs. respective concentration of DOX; n = 6). Cell death assessed by trypan blue exclusion assay after 24 h of treatment of (C) PC-3 with 1.5 μM DOX ± 10 μM Sild and (D) DU145 with 0.5 μM DOX ± 10 μM Sild (*P < 0.001 vs. control and αP < 0.001 vs. DOX; n = 6). Sild enhances DOX-induced apoptosis. Percentage of TUNEL-positive nuclei in (E) PC-3 cells after 72 h of treatment with 1.5 μM DOX ±10 μM Sild and (F) DU145 cells after 72 h of treatment with 0.5 μM DOX ± 10 μM Sild (*P < 0.001 vs. control and αP < 0.001 vs. DOX; n = 3). Results are presented as means ± SE.
Fig. 2.
(A) Table showing the synergy of PC-3 and DU145 cell killing by sildenafil and DOX performed by colony formation assays using median dose effect isobologram analysis. (B) Sildenafil (Sild) does not enhance DOX-induced cell death in normal PrEC. PrEC cell death assessed by trypan blue exclusion assay after 24 h of treatment with DOX (1 μM) with/or without Sild (10 μM) (*P < 0.001 vs. control and Sild and αP < 0.001 vs. DOX; n = 3).
Apoptosis was also quantified using Annexin-V-FITC and propidium iodide (PI) staining followed by flow cytometry analysis. The cells in the subpopulations labeled by staining of Annexin-V-FITC(+)/PI(−) were indicative of early apoptotic cells, whereas those labeled by Annexin-V-FITC(+)/PI(+) were indicative of late apoptotic/necrotic cells. DOX induced apoptosis after 72 h of treatments in PC-3 (7.52%) and DU145 cells (45.01%) compared with control (5.49% in PC-3 and 5.52% in DU145) (Fig. S1 A and B). Cotreatment with sildenafil and DOX increased apoptosis relative to DOX alone in both cell lines (18.71% in PC-3 and 56.82% in DU145 cells) (Fig. S1 A and B). Moreover, the combination treatment increased apoptotic cell death in other cancer cell types including sarcoma OSAC-1 (Fig. S1C), ovarian cancers UCI 101 (Fig. S1D), and A2780 (Fig. S1E).
Sildenafil Enhances DOX-Induced Generation of Reactive Oxygen Species.
Reactive oxygen species (ROS) generation is the key component of antitumor activity of anthracyclines in a variety of tumor cells (25, 26). We tested whether sildenafil enhances cell killing through increased ROS generation that was measured by exposing DOX- and/or sildenafil-treated cells to the indicator dye dichlorodihydrofluorescein diacetate (H2DCFDA). As expected, DOX increased ROS levels in PC-3 and DU145 cells as indicated by positively stained cells (H2DCFDA green fluorescence) (Fig. 3 A and B, and Fig. S2 A and B). However, cells exposed to sildenafil and DOX further boosted ROS generation. In contrast, the sildenafil and DOX combination attenuated DOX-induced ROS generation in the PrEC normal cells (Fig. 4 A and B). Sildenafil alone had no effect on ROS generation in normal or cancer cells. Furthermore, incubation with a putative antioxidant, mercaptopropionyl glycine (MPG), attenuated the enhanced killing effect of sildenafil and DOX combination in DU145 (Fig. S3A) and PC-3 cells (Fig. S3B).
Fig. 3.
Sildenafil (Sild) enhances DOX-induced intracellular ROS generation in prostate cancer cells. (A) H2DCFDA-positive PC-3 cells after 48 h and 72 h of treatment with DOX (1.5 μM) ± Sild (10 μM) (*P < 0.01 vs. other groups after 48-h treatment and αP < 0.001 vs. other groups after 72-h treatment; n = 4). (B) H2DCFDA-positive DU145 cells after 24 h and 48 h of treatment with DOX (0.2 μM) ± Sild (10 μM) (*P < 0.01 vs. other groups after 24-h treatment and αP < 0.001 vs. other groups after 48-h treatment; n = 4).
Fig. 4.
(A and B) Sildenafil (Sild) reduces DOX-induced intracellular ROS generation in PrEC cells. H2DCFDA-positive cells after 24 h of treatment with DOX (1 μM) ± Sild (10 μM) in PrEC cells (*P < 0.01 vs. other groups and αP < 0.001 vs. DOX; n = 4).
Sildenafil and DOX Enhance Intrinsic Pathway of Apoptosis.
DOX increased caspase-3 activity that was further enhanced by cotreatment with both sildenafil and DOX in PC-3 and DU145 cells (Fig. 5A). Bcl-2 expression was diminished in DU145 cells but remained unaltered in PC-3 cells following treatment with sildenafil and DOX (Fig. 5B and Fig. S4A). Similarly, the expression of the antiapoptotic protein Bcl-xL was reduced with sildenafil and DOX compared with individual treatments or control in both cell lines (Fig. S4B). Bad belongs to the proapoptotic members of the Bcl-2 family and forms a complex with Bcl-xL thereby preventing its antiapoptotic effects. Phosphorylation of Bad impairs its binding to Bcl-xL and therefore abrogates Bad's proapoptotic effects (27). DOX reduced Bad phosphorylation and sildenafil and DOX further decreased Bad phosphorylation (Fig. 5B and Fig. S4C). DOX induced the proapoptotic protein Bax, which was further enhanced by cotreatement with sildenafil in PC-3 cells (Fig. 5B and Fig. S4D). Overexpression of Bcl-xL inhibited cell death with sildenafil and DOX compared with DOX alone (Fig. 5D).
Fig. 5.
Sildenafil (Sild) enhances DOX-induced activation of caspase 3/7 and caspase 9. Cells were treated with DOX (1.5 μM for PC-3 and 0.5 μM for DU145) ± 10 μM Sild for 72 h. (A) Caspase 3/7 activity in PC-3 and DU145 cells (*P < 0.001 vs. control and αP < 0.001 vs. DOX; n = 6). (B) Representative immunoblots for Bcl-2, Bcl-xL, Bax, pBad, Bad, and Actin from PC-3 and DU145 cell lysates after 48 h of treatment with DOX and/or Sild. (C) Capase-9 activity in DU145 cells (*P < 0.001 vs. control and αP < 0.001 vs. DOX; n = 4). (D) DU145 cells were infected with Adeno-Bcl-xL and Adeno-empty vector (CMV) for 24 h. (Inset) Bcl-xL overexpression. Bar diagram shows cell death after 24 h of treatment with 0.5 μM DOX ± 10 μM Sild (*P < 0.05 vs. DOX-CMV; n = 4). (E) DU145 cells were infected with Adeno-dnCaspase9 and Adeno-empty vector (CMV) for 24 h. (Inset) Procaspase-9 overexpression. Bar chart represents cell death following overexpression of dnCaspase 9 or empty vector 24 h after treatment with 0.5 μM DOX ± 10 μM Sild (*P < 0.05 vs. DOX-CMV; n = 4).
Caspase-9 activity was unchanged with sildenafil although it increased after DOX treatment (Fig. 5C). Caspase-9 activity was further increased with sildenafil and DOX treatment compared with DOX alone. Overexpression of dominant negative caspase 9 (dnCasp9) attenuated the synergistic effect of sildenafil and DOX on cell death compared with cells infected with empty vector (Fig. 5E).
Sildenafil Potentiates DOX-Induced Inhibition of Prostate Tumor Xenograft Growth.
Treatment of nude mice carrying PC-3 flank tumors with DOX (1.5 mg/kg, i.p.) reduced tumor volume (Fig. 6A). Sildenafil (5 mg/kg, i.p.) cotreatment potentiated DOX-induced tumor volume reduction (Fig. 6A). The ratio of tumor weight to body weight was also reduced with sildenafil cotreatment (Fig. 6B). Similar results were obtained when sildenafil (10 mg/kg) was administered daily by oral gavage and DOX (3 mg/kg, i.p.) was injected twice per week for 3 wk. (Fig. S5 A and B).
Fig. 6.
Sildenafil (Sild) potentiates DOX-induced inhibition of prostate tumor xenograft growth. Tumor weight and body weight were measured after 18 d of treatment with DOX (1.5 mg/kg, i.p.) and/or Sild (5 mg/kg, i.p.). (A) Tumor growth inhibition (*P < 0.01 vs. control and αP < 0.001 vs. other; n = 8). (B) Ratio of tumor weight and body weight (*P < 0.05 vs. control and Sild and αP < 0.001 vs. other; n = 8). Sildenafil enhances DOX-induced apoptosis in tumors. (C) Bar diagram showing TUNEL-positive cells (*P < 0.001 vs. control and αP < 0.001 vs. DOX; n = 3). Results are reported as means ± SE.
DOX treatment increased apoptosis in tumors that was further amplified by cotreatment with sildenafil (Fig. 6C and Fig. S6A). Sildenafil alone had no effect on apoptosis in tumors. Immunohistochemistry demonstrated that the active form of caspase 3 was induced in tumors from sildenafil- and DOX-treated mice compared with DOX-treated or nontreated control mice (Fig. 7A). These data further support in vitro results that show a similar trend of increased expression of activated caspase 3 (Fig. 5A) in prostate cancer cells.
Fig. 7.
Sildenafil enhances activity of caspase 3 in tumor. (A) Representative images of the immunohistochemical staining for Alexa 488 labeled cleaved caspase 3 in tumors. (Top) Cleaved caspase 3 (green fluorescence), (Middle) nuclei staining with DAPI, and (Bottom) overlay of both types of staining. Cardiac function was assessed by Doppler echocardiography of mice treated with Sild (10 mg/kg by oral gavage) everyday and DOX (3 mg/kg i.p.) twice per week. (B) LVFS and (C) LVEF (*P < 0.05 vs. control and Sild and **P < 0.01 vs. DOX; n = 8). Results are reported as means ± SE.
Sildenafil Ameliorates DOX-Induced Cardiac Dysfunction.
Cardiac function in mice was assessed by performing echocardiography. A slight increase in left ventricular end diastolic diameter (LVEDD) and left ventricular end systolic diameter (LVESD) were observed with DOX (Fig. S6 B and C). Fractional shortening (LVFS) and ejection fraction (LVEF) declined in DOX-treated mice (Fig. 7 B and C). Sildenafil cotreatment with DOX improved LVFS and LVEF compared with the DOX-treated group (P < 0.05) (Fig. 7 B and C). No differences in heart rate were observed between control, DOX, and DOX and sildenafil groups (Fig. S6D). Sildenafil-treated animals showed lower heart rates compared with other groups (P < 0.01; n = 8) (Fig. S6D). These data suggest that changes in LVFS or LVEF were independent of heart rate.
Discussion
The high incidence of recurrence and metastasis, as well as the refractory nature of the malignancy to chemotherapy, make hormone refractory prostate cancer one of the most challenging malignancies for therapeutic drug combination studies (28). Surgical resection of the prostate also causes significant risk of erectile dysfunction due to trauma sustained by the cavernosal nerve (29). PDE-5 inhibitors have been shown to improve erectile function in men postradical prostatectomy (30–32). We have established a powerful cardioprotective effect of PDE-5 inhibitors in animal models (33). Moreover, sildenafil improved DOX-induced left ventricular (LV) dysfunction and cardiomyocyte apoptosis (11). In the present study, we provide evidence that sildenafil potentiates DOX-induced killing of androgen-independent human prostate cancer cells in vitro and in vivo. Moreover, sildenafil attenuated DOX-induced cardiac dysfunction in mice bearing prostate tumors. These results suggest that sildenafil may represent a therapeutic approach to improve DOX efficacy in prostate cancer while simultaneously reducing the risk of cardiomyopathy. Our data also show that the sildenafil and DOX combination enhanced the killing of ovarian cancer and sarcoma cells, suggesting a potential utility of sildenafil in chemosensitization in multiple malignancies.
Mitochondrial ROS is the key component of antitumor activity of DOX in tumor cells (25, 26). In the present study, we observed higher levels of intracellular ROS in PC-3 and DU145 cells after treatment with DOX and sildenafil compared with DOX alone. In contrast, however, sildenafil and DOX treatment decreased ROS production in normal cells. Similar to these results, sulindac, a potent anticancer drug, selectively enhanced killing of cancer cells exposed to oxidizing agents via production of ROS (34). It has been suggested that the basic difference in mitochondrial respiration between normal and cancer cells makes cancer cells more sensitive to oxidative stress (35, 36). Exactly how sildenafil sensitizes cancer cells to amplify DOX-mediated ROS generation is not clear but needs to be investigated. Interestingly, low levels of sulindac also induced delayed preconditioning response against ischemia/reperfusion injury in the heart through up-regulation of putative effectors of cardioprotection including iNOS and HSP27 (37). In this respect, it appears that sildenafil is very similar to sulindac in enhancing the antitumor effect while providing cardioprotection at the same time.
Because resistance to apoptosis is one of the hallmarks of cancer, we further investigated the mechanisms of cell death induced by sildenafil and DOX. Apoptosis is regulated at points within the intrinsic pathway by pro- and antiapoptotic proteins, which include members of the Bcl-2 family together with mitochondria, cytochrome c, and caspases (38). The regulation occurs by the balance of intrinsic protein levels and/or their localization within intracellular compartments (39). In the present study, the increased apoptosis by sildenafil and DOX was associated with enhanced expression of proapoptotic proteins Bad and Bax and suppression of Bcl-2 and Bcl-xL. Also, this cotreatment regimen dephosphorylated Bad, which may enhance Bad heterodimerization with Bcl-xL thereby promoting DOX-induced apoptosis. The ectopic overexpression of Bcl-xL in DU145 cells suppressed the lethality of sildenafil with DOX compared with DOX alone, suggesting that down-regulation of Bcl-xL played a significant role in the synergistic interactions between these therapeutic agents. Sildenafil- and DOX-induced cell killing was also associated with increased caspase-3 and caspase-9 activity. Overexpression of dominant negative procaspase 9 in DU145 cells blocked the enhanced cell killing by combined treatment with sildenafil and DOX compared with DOX alone.
Our results show that sildenafil and DOX treatment also caused significant inhibition of tumor growth and enhanced caspase-3 activity as well as apoptosis. The sildenafil and DOX combination also ameliorated DOX-induced cardiac dysfunction, which is consistent with our previous study showing improved LV function with sildenafil in DOX-treated mice (11). In these studies, sildenafil reduced cardiomyocyte apoptosis, maintained mitochondrial membrane potential, preserved myofibrillar integrity, and prevented electrocardiogram ST interval prolongation after DOX treatment.
Similar to other chemotherapeutic agents, the clinical use of DOX is hampered by its cardiotoxic effects (40). It impairs the clinical response and survival of patients (41). Therefore, rendering cancer cells more sensitive to DOX while improving cardiac function would be an efficient approach to enhance its therapeutic effect. Our results suggest a potential utility of sildenafil in enhancing the antitumor efficacy of DOX while attenuating its cardiotoxic effect in prostate cancer. Clinical studies are warranted to fully define the importance of combined treatment with DOX and sildenafil as therapeutic tool in prostate cancer patients.
Materials and Methods
Cell Growth and Death Assay.
Cell proliferation and metabolically active PC-3 and DU145 cells were measured by CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega Corp.) according to manufacturer's protocol. The percentage of cell death was measured by trypan blue staining.
Apoptosis Assay.
Cell apoptosis was analyzed by TUNEL staining using ApopTag Peroxidase In Situ Apoptosis Detection kit (Chemicon International Company). Apoptosis in tumor section was analyzed using In Situ Cell Death Detection kit, TMR red (Roche Diagnostics).
Measurement of ROS.
Following 24 and 48 h of treatment with DOX and/or sildenafil, cells were incubated with 50 μM of H2DCFDA (Molecular Probes) in growth medium for 30 min at 37 °C. Cells were rinsed with PBS, and ROS levels were visualized by fluorescence microscope.
Caspase-3 and -9 Activity Assay.
Cell viability was measured using CellTiter-Fluor viability assay kit (Promega Corp.) in a 96-well plate. Caspase-3/7 and -9 activities were measured in treated cells using Caspase-Glo 3/7 assay and Caspase-Glo-9 assay kits (Promega Corp.) according to manufacturer's protocol.
In Vivo Tumor Study.
Tumors were generated in Athymic male BALB/cAnNCr-nu/nu mice from National Cancer Institute Developmental Therapeutic Program by s.c. injection of PC-3 cells (5 × 106 cells) with 50-μL matrigel matrices (BD Bioscience). Tumors were permitted to grow to a volume of ∼200 mm3 over the following 2 wk. The animals received i.p. injections of saline (for control), DOX (1.5 mg/kg) alone, sildenafil (5 mg/kg) alone, or DOX (1.5 mg/kg) and sildenafil (5 mg/kg) everyday, 5 d/wk (Fig. S7). Tumor sizes were measured twice weekly. Tumor volume was calculated by ab2/2 where “a” and “b” are the long and short axes of tumor. The animal protocol was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Doppler Ecocardiography.
Cardiac function in nude mice with tumor xenografts was monitored by Doppler echocardiography using the Vevo770 imaging system (VisualSonics) as previously reported (12).
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL51045, HL59469, and HL79424 (to R.C.K); P01-CA104177, R01-CA108325, and R01-DK52825 (to P.D.); and Mid-Atlantic Affiliate Beginning Grant-in-Aid 0765273U (to A.D.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006965107/-/DCSupplemental.
References
- 1.Leonetti C, et al. Therapeutic integration of c-myc and bcl-2 antisense molecules with docetaxel in a preclinical model of hormone-refractory prostate cancer. Prostate. 2007;67:1475–1485. doi: 10.1002/pros.20636. [DOI] [PubMed] [Google Scholar]
- 2.Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: Mechanism and modulation. Mol Cell Biochem. 2000;207:77–86. doi: 10.1023/a:1007094214460. [DOI] [PubMed] [Google Scholar]
- 3.Rivera E. Liposomal anthracyclines in metastatic breast cancer: Clinical update. Oncologist. 2003;8(Suppl 2):3–9. doi: 10.1634/theoncologist.8-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 4.Shen F, et al. Quantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cells. J Pharmacol Exp Ther. 2008;324:95–102. doi: 10.1124/jpet.107.127704. [DOI] [PubMed] [Google Scholar]
- 5.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 6.Das A, Ockaili R, Salloum F, Kukreja RC. Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol. 2004;286:H1455–H1460. doi: 10.1152/ajpheart.01040.2003. [DOI] [PubMed] [Google Scholar]
- 7.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 8.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283:H1263–H1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- 9.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–597. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 10.Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC. Vardenafil: A novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial K(ATP) channels in rabbits. J Mol Cell Cardiol. 2006;40:405–411. doi: 10.1016/j.yjmcc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 12.Salloum FN, et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–H1406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 13.Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem. 2008;283:29572–29585. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2009;296:H1236–H1243. doi: 10.1152/ajpheart.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piazza GA, et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61:3961–3968. [PubMed] [Google Scholar]
- 16.Pusztai L, et al. Phase I and II study of exisulind in combination with capecitabine in patients with metastatic breast cancer. J Clin Oncol. 2003;21:3454–3461. doi: 10.1200/JCO.2003.02.114. [DOI] [PubMed] [Google Scholar]
- 17.Whitehead CM, et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–488. [PubMed] [Google Scholar]
- 18.Sarfati M, et al. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2003;101:265–269. doi: 10.1182/blood-2002-01-0075. [DOI] [PubMed] [Google Scholar]
- 19.Zhu B, Vemavarapu L, Thompson WJ, Strada SJ. Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. J Cell Biochem. 2005;94:336–350. doi: 10.1002/jcb.20286. [DOI] [PubMed] [Google Scholar]
- 20.Li H, et al. Pro-apoptotic actions of exisulind and CP461 in SW480 colon tumor cells involve beta-catenin and cyclin D1 down-regulation. Biochem Pharmacol. 2002;64:1325–1336. doi: 10.1016/s0006-2952(02)01345-x. [DOI] [PubMed] [Google Scholar]
- 21.Goluboff ET, et al. Exisulind (sulindac sulfone) suppresses growth of human prostate cancer in a nude mouse xenograft model by increasing apoptosis. Urology. 1999;53:440–445. doi: 10.1016/s0090-4295(98)00513-5. [DOI] [PubMed] [Google Scholar]
- 22.Lim JT, et al. Sulindac derivatives inhibit growth and induce apoptosis in human prostate cancer cell lines. Biochem Pharmacol. 1999;58:1097–1107. doi: 10.1016/s0006-2952(99)00200-2. [DOI] [PubMed] [Google Scholar]
- 23.Lim JT, Piazza GA, Pamukcu R, Thompson WJ, Weinstein IB. Exisulind and related compounds inhibit expression and function of the androgen receptor in human prostate cancer cells. Clin Cancer Res. 2003;9:4972–4982. [PubMed] [Google Scholar]
- 24.Narayanan BA, et al. Exisulind in combination with celecoxib modulates epidermal growth factor receptor, cyclooxygenase-2, and cyclin D1 against prostate carcinogenesis: In vivo evidence. Clin Cancer Res. 2007;13:5965–5973. doi: 10.1158/1078-0432.CCR-07-0744. [DOI] [PubMed] [Google Scholar]
- 25.Mizutani H, Tada-Oikawa S, Hiraku Y, Kojima M, Kawanishi S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439–1453. doi: 10.1016/j.lfs.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 26.Tsang WP, Chau SP, Kong SK, Fung KP, Kwok TT. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life Sci. 2003;73:2047–2058. doi: 10.1016/s0024-3205(03)00566-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang HG, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 28.Pinto AC, Moreira JN, Simões S. Ciprofloxacin sensitizes hormone-refractory prostate cancer cell lines to doxorubicin and docetaxel treatment on a schedule-dependent manner. Cancer Chemother Pharmacol. 2009;64:445–454. doi: 10.1007/s00280-008-0892-6. [DOI] [PubMed] [Google Scholar]
- 29.Rambhatla A, Kovanecz I, Ferrini M, Gonzalez-Cadavid NF, Rajfer J. Rationale for phosphodiesterase 5 inhibitor use post-radical prostatectomy: Experimental and clinical review. Int J Impot Res. 2008;20:30–34. doi: 10.1038/sj.ijir.3901588. [DOI] [PubMed] [Google Scholar]
- 30.Mydlo JH, Viterbo R, Crispen P. Use of combined intracorporal injection and a phosphodiesterase-5 inhibitor therapy for men with a suboptimal response to sildenafil and/or vardenafil monotherapy after radical retropubic prostatectomy. BJU Int. 2005;95:843–846. doi: 10.1111/j.1464-410X.2005.05413.x. [DOI] [PubMed] [Google Scholar]
- 31.Ohebshalom M, Parker M, Guhring P, Mulhall JP. The efficacy of sildenafil citrate following radiation therapy for prostate cancer: Temporal considerations. J Urol. 2005;174:258–262. doi: 10.1097/01.ju.0000164286.47518.1e. discussion 262. [DOI] [PubMed] [Google Scholar]
- 32.Teloken PE, Ohebshalom M, Mohideen N, Mulhall JP. Analysis of the impact of androgen deprivation therapy on sildenafil citrate response following radiation therapy for prostate cancer. J Urol. 2007;178:2521–2525. doi: 10.1016/j.juro.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Kukreja RC, et al. Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascul Pharmacol. 2005;42:219–232. doi: 10.1016/j.vph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Resnick L, Rabinovitz H, Binninger D, Marchetti M, Weissbach H. Topical sulindac combined with hydrogen peroxide in the treatment of actinic keratoses. J Drugs Dermatol. 2009;8:29–32. [PubMed] [Google Scholar]
- 35.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moench I, Prentice H, Rickaway Z, Weissbach H. Sulindac confers high level ischemic protection to the heart through late preconditioning mechanisms. Proc Natl Acad Sci USA. 2009;106:19611–19616. doi: 10.1073/pnas.0911046106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 39.Kelly MM, Hoel BD, Voelkel-Johnson C. Doxorubicin pretreatment sensitizes prostate cancer cell lines to TRAIL induced apoptosis which correlates with the loss of c-FLIP expression. Cancer Biol Ther. 2002;1:520–527. doi: 10.4161/cbt.1.5.169. [DOI] [PubMed] [Google Scholar]
- 40.Bast A, Kaiserová H, den Hartog GJ, Haenen GR, van der Vijgh WJ. Protectors against doxorubicin-induced cardiotoxicity: Flavonoids. Cell Biol Toxicol. 2007;23:39–47. doi: 10.1007/s10565-006-0139-4. [DOI] [PubMed] [Google Scholar]
- 41.Bryant J, et al. Use of cardiac markers to assess the toxic effects of anthracyclines given to children with cancer: A systematic review. Eur J Cancer. 2007;43:1959–1966. doi: 10.1016/j.ejca.2007.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.