Abstract
Long-term memory relies on modulation of synaptic connections in response to experience. This plasticity involves trafficking of AMPA receptors (AMPAR) and alteration of spine morphology. Arc, a gene induced by synaptic activity, mediates the endocytosis of AMPA receptors and is required for both long-term and homeostatic plasticity. We found that Arc increases spine density and regulates spine morphology by increasing the proportion of thin spines. Furthermore, Arc specifically reduces surface GluR1 internalization at thin spines, and Arc mutants that fail to facilitate AMPAR endocytosis do not increase the proportion of thin spines, suggesting that Arc-mediated AMPAR endocytosis facilitates alterations in spine morphology. Thus, by linking spine morphology with AMPAR endocytosis, Arc balances synaptic downscaling with increased structural plasticity. Supporting this, loss of Arc in vivo leads to a significant decrease in the proportion of thin spines and an epileptic-like network hyperexcitability.
Keywords: plasticity, seizure, AMPA receptors
Formation of long-term memory relies on a neuron's ability to modulate synaptic connections in response to input it receives. This plasticity requires coordinated activity-dependent synthesis of specific mRNAs and proteins that facilitate molecular and structural changes at the synapse. Excitatory synapses are located at dendritic spines that receive glutamatergic presynaptic inputs (1). Spines form a variety of shapes and sizes that correlate with their synaptic strength, motility, and structural plasticity (2). For example, thin spines with small synapses are nicknamed “learning spines” because they are highly motile and likely to change shape in response to activity, whereas stubby and mushroom spines, also known as “memory spines,” are less motile and more stable (2–4).
How a neuron regulates the morphology of spines in response to activity remains unclear. Although much research has focused on cytoskeletal remodeling within spines, activity-dependent endosomal recycling also plays an important role in regulating spine size (5). Specifically, exocytosis of GluR1 after long-term potentiation (LTP) is required to maintain spine enlargement (6). Although long-term depression (LTD) leads to both AMPA receptor (AMPAR) internalization and reduction in spine size (7–9), it is not known whether endocytosis of AMPARs is required for the reduction in spine size.
An activity-regulated cytoskeleton-associated protein (Arc) is an ideal candidate for regulating spine morphology. Its expression is tightly regulated by neuronal activity (10, 11), and its RNA and protein are localized to dendrites and spines after activity (12–15). Furthermore, Arc induction is required for late LTP and memory consolidation (16–18), as well as LTP-induced cofilin phosphorylation and F-actin stabilization (17). Finally, Arc facilitates endocytosis of AMPARs through its interaction with endocytic proteins endophilin 3 and dynamin 2 (19, 20) and, in doing so, is critical for LTD (21, 22) and homeostatic plasticity (23).
Here, we investigated the role of Arc in regulating spine morphology. We report that Arc significantly increases spine density and the proportion of thin “plastic” spines. Furthermore, an Arc mutant unable to interact with endophilin 3 did not alter spine morphology, suggesting that Arc plays a novel role in linking activity-dependent receptor endocytosis with reduction in spine size. This coordinated mechanism ultimately increases the potential for plasticity through addition of thin spines and decreases synaptic efficacy by reducing surface GluR1. Such changes in spine morphology fit well with the net effect of homeostatic plasticity: stabilization of activity while maintaining relative changes in synaptic strength. Supporting this, Arc−/− mice had fewer thin spines and more mushroom spines, as well as aberrant spontaneous cortical network discharge activity, highly associated with epilepsy.
Results
Arc Expression Alters Spine Morphology to Favor Thin Spines and Filopodia.
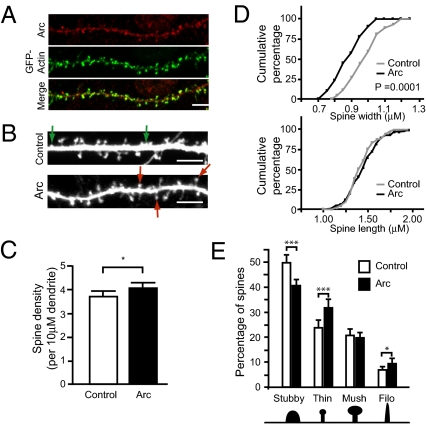
To determine whether Arc is sufficient to alter spine density and/or morphology, we sought to mimic the strong induction of Arc after activity by exogenously expressing it in mature [18–19 d in vitro (DIV)], medium density, primary hippocampal cultures. In this system, exogenously expressed Arc localizes to dendritic spines and colocalizes with actin enriched in spines (Fig. 1A), similar to Arc in vivo (14, 15). Changes in spine morphology and density were visualized by using GFP as a morphology and transfection marker. Fixed neurons expressing GFP and cotransfected with Arc or a control vector were imaged by confocal microscopy (Fig. 1B).
Fig. 1.
Arc expression increases spine density and alters spine morphology. (A) Arc (red) localizes to dendritic spines and colocalizes with GFP-actin (green). (Scale bar, 5 μm.) (B–E) Medium density hippocampal neurons at 18–19 DIV were transfected with GFP and Arc or an empty vector and imaged 36–48 h after transfection. Examples of thin and stubby spines are marked by red and green arrows, respectively. (Scale bars, 5 μm.) (C) Arc expression increases spine density. *P < 0.05. (D) Cumulative frequency plots of spine width and length. Arc significantly decreases spine width but does not affect length. Kolmogorov-Smirnov test, P < 0.0001. (E) Arc significantly increases the percentage of thin spines (red arrows in B) and filopodia, and decreases the percentage of stubby spines (green arrows in B). t test, ***P < 0.0005, *P < 0.05. Error bars represent 95% CI. More than 2,500 spines from 64 to 65 dendrites on 12–18 neurons from three separate experiments were analyzed per condition. Measurements were averaged per dendrite. Mush, mushroom; Phyllo, filopodia.
Neurons overexpressing Arc showed a small but significant increase in spine density (Fig. 1C), and the spines were significantly thinner than those of neurons transfected with GFP alone (Fig. 1D). No change in spine length was observed. To determine whether Arc expression altered the distribution of spine type, we categorized spines into stubby, thin, mushroom, and filopodia (Material and Methods) and calculated the percentage of each spine type per dendrite. We found that Arc overexpression increased the percentage of thin spines and filopodia and decreased the percentage of stubby spines (Fig. 1E). The percentage of mushroom spines was unaffected.
Arc Specifically Alters GluR1 Surface Localization at Thin Spines.
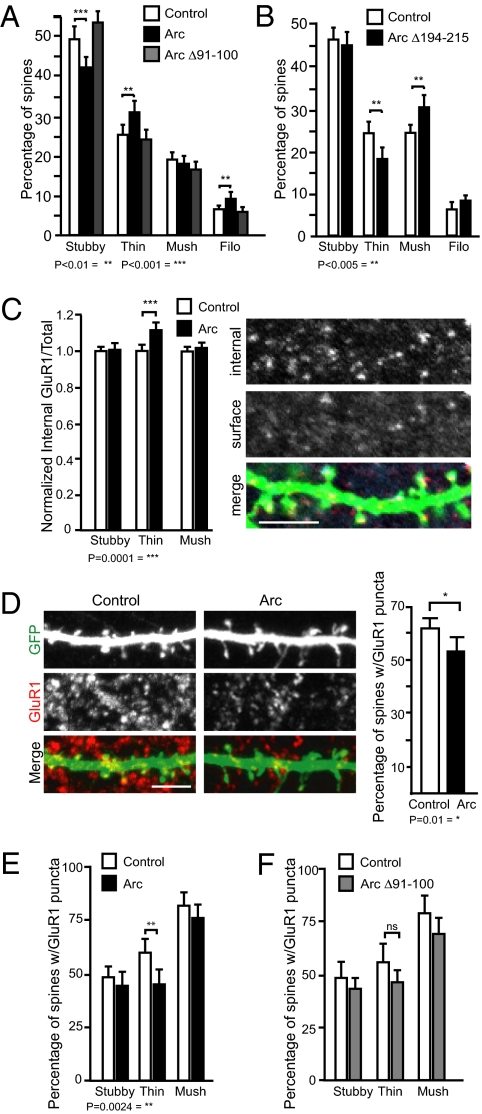
Previous work showed that Arc regulates AMPA receptor endocytosis through its interaction with endophilin 3 and dynamin 2 (19, 20). Given this, we wondered whether the effect of Arc on spine morphology depended on the ability of Arc to mediate AMPAR endocytosis. To test this, we used a mutant of Arc: Arc Δ91–100. Amino acids 91–100 of Arc interact with endophilin 3, a component of the clathrin-coated vesicle endocytosis machinery, and Arc Δ91–100 localizes to dendrites (Fig. S1) but fails to induce AMPAR endocytosis (19). If Arc-mediated AMPAR endocytosis is required for the alterations in spine morphology, overexpression of Arc Δ91–100 should not affect spine morphology. Indeed, unlike Arc, overexpressing Arc Δ91–100 resulted in the same distribution of spine morphologies as control (Fig. 2A).
Fig. 2.
Arc-mediated GluR1 endocytosis is required for alterations in spine morphology. (A) Deletion of endophilin interaction domain in Arc (Arc Δ91–100) blocks its ability to regulate spine morphology. One-way ANOVA with post hoc Tukey test, F(3.244)= 13.07stubby, 6.536 thin, 0.7452mushroom, 5.304filopodia; **P < 0.01, ***P < 0.001. More than 1,500 spines from 40 dendrites over three experiments per condition were analyzed. (B) Deletion of the dynamin interaction domain alters effect of Arc on spine morphology. Expression of Arc Δ194–215 decreases percentage of thin spines and increases percentage of mushroom spines. t test, **P < 0.005. More than 2,000 spines from 45 dendrites were analyzed in two experiments per condition. Error bars represent 95% CI. (C) Arc expression leads to an increase in the ratio of internal-to-total GluR1 at thin spines. t test, ***P = 0.0001. More than 100 spines of each type over three experiments per condition were analyzed. An example of internal and external surface staining is shown to the right. (D) Arc expression decreases the percentage of spines with surface GluR1. t test, *P = 0.01 (E) Surface GluR1 is specifically decreased in thin spines. t test, **P = 0.0024. More than 3,500 spines from 26 to 27 cells were analyzed per condition over five experiments. (F) Arc Δ91–100 expression does not reduce GluR1 surface expression at thin spines. (Scale bar, 5 μm.)
Because any given deletion can have unwanted effects, we tested another version of Arc with a completely different deletion (Arc Δ195–214). Arc Δ195–214 does not bind dynamin and thus cannot mediate AMPAR endocytosis. Overexpression of Arc Δ195–214 had the opposite effect of Arc overexpression. It significantly decreased the percentage of thin spines and increased the percentage of mushroom spines, compared with control (Fig. 2B and Fig. S1). One possible explanation for the decrease in thin spines is that this mutant could be acting as a dominant negative, blocking the effects of endogenous Arc in maintaining thin spines. Importantly, in two independent examples, a deletion in Arc that disrupts its ability to mediate AMPAR endocytosis also disrupts the ability of Arc to promote thin spine formation, suggesting these functions of Arc are linked.
Nevertheless, from these experiments alone, it remained possible that these deletions might also abrogate interactions between Arc and other unknown proteins that regulate spine structure independently of AMPAR endocytosis. So, we directly tested whether Arc expression would affect internalization of AMPARs at specific spine types. If Arc links surface expression of AMPARs with spine morphology, we hypothesized that Arc expression would specifically reduce surface AMPARs at thin spines. We performed an antibody-feeding assay (Material and Methods) and quantified the degree of GluR1 internalization at each spine type in live neurons expressing Arc or a control vector. Arc significantly and specifically increased GluR1 endocytosis at thin spines (Fig. 2C). Furthermore, surface staining of GluR1 on transfected cultured hippocampal neurons showed that Arc overexpression reduced the percentage of spines with surface GluR1 puncta, and this reduction in GluR1 puncta was specific to thin spines (Fig. 2 D and E and Fig. S2). Surface staining of GluR2 was not reduced in any spine type (Fig. S3). Finally, Arc Δ91–100 did not alter GluR1 surface expression at thin spines (Fig. 2F). These data support a role for Arc in regulating spine morphology through AMPAR endocytosis.
Arc−/− Mice Have Decreased Spine Density and Increased Spine Width.
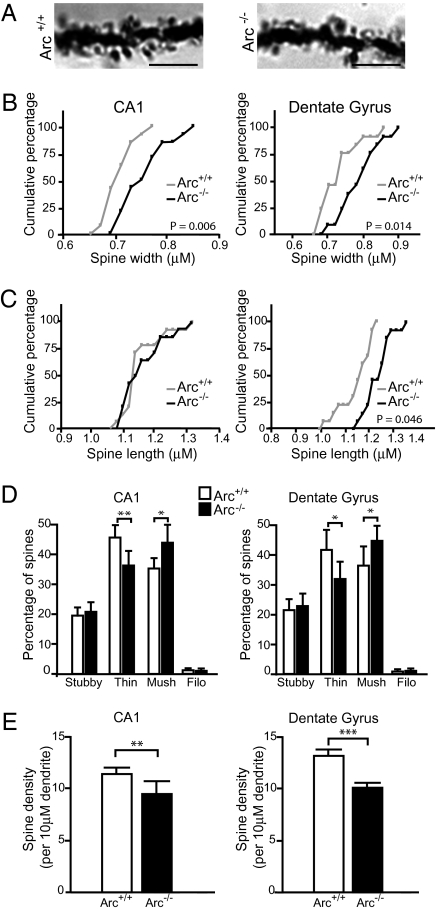
To confirm both the specificity and the in vivo significance of the above findings, we analyzed spine densities and morphologies from brains of adult mice lacking Arc (24) (Fig. 3A). In agreement with our primary culture data, morphometric analysis of Golgi–Cox stains confirmed that both CA1 pyramidal neurons and DG cells lacking Arc had increased spine width and decreased proportion of thin spines (Fig. 3 B–D). Furthermore, Arc−/− mice also had significantly lower spine densities in both CA1 and DG cells (Fig. 3E). Loss of Arc in vivo did not alter the proportion of stubby spines, as was observed in vitro, but rather increased the percentage of mushroom spines (Fig. 3D). This difference could reflect the different spine distribution patterns observed in vivo and in cultured hippocampal neurons, or differences in neuronal activity between the two systems. Hippocampal neurons in vivo had more mushroom spines and fewer stubby spines than in culture (Fig. 1E and Fig. 3D).
Fig. 3.
Arc−/− mice have decreased spine density and increased spine width. (A) Example of dentate granule Golgi staining from 3-mo-old Arc+/+ and Arc−/− mice. (Scale bars, 5 μm.) Stacks (10-μm) were imaged, and individual spines were measured in their appropriate focal plane. (B and C) Cumulative frequency plots of spine width and length. Arc−/− mice have increased spine width in both CA1 and DG cells (P = 0.006, P = 0.014, respectively). Spine length was also increased in DG cells of Arc−/− mice. P values were determined using Kolmogorov–Smirnov test. (D) Loss of Arc in vivo decreases percentage of thin spines (t test, CA1: P < 0.005, DG: P < 0.05) and increases percentage of mushroom spines (t test, CA1: P < 0.05, DG: P < 0.05). (E) Loss of Arc significantly decreases spine density in CA1 pyramidal cells (t test, P = 0.0053) and DG cells (t test, P < 0.0005). Fourteen dendrites from three animals per genotype were analyzed. Error bars represent 95% CI.
Arc−/− Mice Exhibit Aberrant NPY Expression and Network Hyperexcitability.
Arc is thought to mediate homeostatic plasticity through endocytosis of AMPARs. Specifically, after strong bouts of synaptic activity, Arc induction facilitates downward scaling of synapses by reducing surface GluR1 levels (23). Our data support this model by demonstrating that Arc expression reduces the size of dendritic spines and specifically reduces GluR1 surface expression at thin spines. Homeostatic plasticity is believed to be important for regulating network activity in response to excessive neuronal discharge, such as a seizure. Loss of such a negative feedback loop could lead to an epileptic-like state and associated spine loss (25). Indeed, several other activity-induced genes, whose protein products localize to dendrites and affect AMPAR endocytosis or spine morphology, have been implicated in regulating network excitability (26–28).
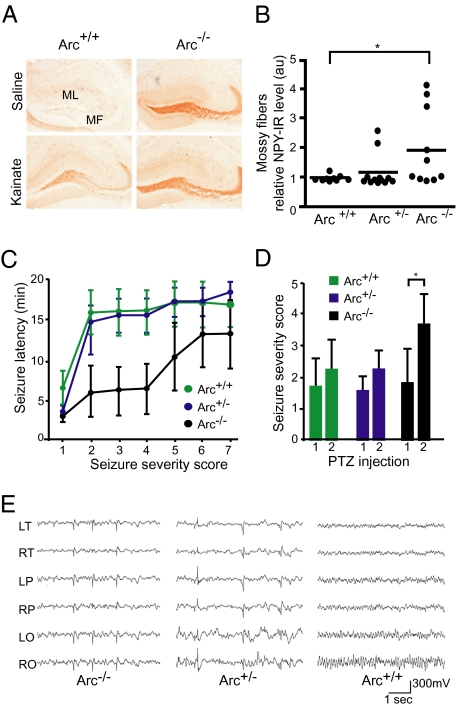
To examine this hypothesis, we asked whether mice lacking Arc were more susceptible to convulsant drugs. Mice were given an i.p. injection of either kainate or saline to induce seizure activity and chronic epilepsy and were examined for granule cell NPY expression. In both human and animal models of chronic hippocampal epilepsy, neuropeptide Y (NPY) expression is transiently increased and ectopically expressed in mossy fibers, the axons of DG cells (29, 30). To our surprise, saline-injected Arc−/− mice showed strong NPY expression (Fig. 4A), suggesting that the hippocampal network in these mice is hyperexcitable even under normal conditions. We found a dose-dependent effect of Arc expression on ectopic NPY expression. Five of 10 Arc−/− mice (50%) showed aberrant NPY expression, and interestingly, two of 12 Arc+/− mice (16%) also showed aberrant NPY expression (Fig. 4B). None of the WT mice showed aberrant NPY expression. Aberrant neuronal activity or epileptic activity has also been associated with calcium dysregulation and depletion of the calcium buffer calbindin-D28K in the dentate gyrus of rats (31) and humans (32, 33). Arc−/− mice also had lower dentate gyrus calbindin levels than controls (Fig. S4). Furthermore, levels of calbindin negatively correlated with levels of NPY in the mossy fibers and molecular layer of the DG (Fig. S4). These data indicate that loss of Arc expression in vivo leads to significant alterations in protein expression indicative of aberrant neuronal overexcitation.
Fig. 4.
Increased seizure susceptibility and EEG epileptiform activity in Arc−/− mice. (A) Mice 4–6 mo old were injected i.p. with saline or kainate (17 mg/kg) and analyzed 5 d later. Brain sections were stained for NPY with immunoperoxidase. Arc+/+ (WT) and Arc−/− mice injected with kainate exhibit expected aberrant NPY expression in mossy fibers. This abnormal NPY pattern was also found in saline-treated Arc−/− mice. MF, mossy fibers; ML, DG molecular layer. (B) Quantification of mossy fiber NPY levels in saline-treated mice. au, arbitrary units. One-way ANOVA with post hoc Tukey test, F(2,29) = 3.652; *P < 0.05. (C) Arc−/− mice exhibited shorter seizure latency than either Arc +/− or Arc+/+ (WT) mice (one-way ANOVA: F(2,18) = 5.297; P < 0.05). (D) Arc−/− mice had significantly more severe seizures after two consecutive PTZ doses of 30 mg/kg 6 h apart. Paired t test, *P < 0.05. (E) Chronic EEG recordings reveal frequent generalized cortical interictal spike discharges in Arc−/− and Arc+/− mice. EEG recordings of Arc+/+ (WT) littermate mice revealed normal background cortical activity with no abnormal discharges. Calibration 1 s, 300 mV, electrode montage. LO, left occipital; LP, left parietal; LT, left temporal; RO, right occipital; RP, right parietal; RT, right temporal.
Next, we determined whether Arc−/− mice are more susceptible to seizures in response to systemic challenge with pentylenetetrazol (PTZ). On the first day, mice were given a single dose of 30 mg/kg of PTZ intraperitoneally and observed for 20 min. No difference in latency or seizure severity was observed after this first dose of PTZ. On the following day, the same mice were given two consecutive doses of 30 mg/kg PTZ, 6 h apart. After the final dose of PTZ, Arc−/− mice showed significantly shorter seizure latencies and increased seizure severity (Fig. 4 C and D) than both Arc+/− and WT mice.
To more directly test whether Arc−/− mice display spontaneous aberrant neuronal activity, we performed prolonged cortical EEG monitoring of nine adult (aged 7–8 mo) mice (5 Arc−/−, 2 Arc+/−, and 2 Arc+/+) over 1 mo with a digital video–EEG system (SI Material and Methods). The background cortical activity recorded in freely behaving Arc+/− and Arc−/− mice showed a frequent (3–181 spikes/h), generalized pattern of sharp, synchronous epileptiform discharges (which were never seen in WT littermates) without concurrent behavioral manifestations (Fig. 4E). Aberrant network discharges were observed in all mutant genotypes, and although there was a wide range of discharge frequencies, no significant difference was observed in mean discharge frequencies between Arc−/− (71/h ± 80) and Arc+/− (69/h ± 70) mice. Despite the abundant abnormal cortical hyperexcitability, no spontaneous cortical seizures were seen during the recording period. However, we did observe one Arc+/− mouse having a convulsive seizure under normal housing conditions. As expected, 2 h after the onset of the seizure, protein expression of c-Fos, a commonly used marker of synaptic activity, was highly up-regulated in the hippocampus of this animal (Fig. S5).
Discussion
In this study, we demonstrated that Arc is important for regulating dendritic spine morphology in vitro and in vivo. Furthermore, our data suggest that Arc links structural and functional plasticity by altering spine morphology through AMPAR endocytosis. Arc expression balances reduction in synaptic strength (20) with increased structural plasticity by increasing the proportion of thin spines. In agreement with this finding, we show that loss of Arc in vivo leads to network hyperexcitability, supporting the role of Arc in homeostatic plasticity and demonstrating Arc's importance in preventing aberrant network activity, such as epilepsy.
Arc Modulates Spine Morphology.
Dendritic spine size is a critical determinant of activity-dependent plasticity. Specifically, thinner spines, nicknamed “learning spines” are more motile, are more transient, and have greater capacity to enlarge and stabilize after LTP. Large spines, nicknamed “memory spines,” are stable and less likely to change structure in response to activity (34–36). By increasing the proportion of learning spines, Arc expression may enhance a neuron's ability to form new synapses and to respond to changes in activity. Recent studies in brain slices and in vivo demonstrate that synaptic activity leads to selective spine turnover by stabilizing active spines and replacing inactive spines with new ones (37). Similar to what we have observed with Arc overexpression, these new spines are often thin, suggesting that Arc expression facilitates this selective turnover. Age-related reduction in thin spines has also been observed in rhesus monkeys, with cognitive performance inversely proportional to thin spine volume (38). Although synaptic activity does not affect Arc-mediated AMPAR endocytosis (20), activity could regulate the location of Arc's effects. Thus, it would be useful to investigate Arc-mediated changes in spine morphology in the presence or absence of synaptic activity.
Further investigation of the mechanism of Arc's effect on spine morphology showed that versions of Arc incompetent for endocytosis are incapable of increasing the proportion of thin spines. Thus, endosomal receptor recycling may bidirectionally affect spine morphology and synaptic strength. Specifically, an Arc mutant unable interact with endophilin 3 had no affect on spine morphology, and Arc expression specifically increased GluR1 internalization and decreased surface expression at thin spines. These findings highlight Arc as a coregulator of spine morphology and synaptic transmission. Surface expression of AMPARs is tightly linked to spine size; large spines contain many AMPARs, and thin spines contain few AMPARs. Local exocytosis of recycling endosomes has been implicated as a mechanism for activity-dependent spine enlargement (5), and insertion of GluR1 into synapses is required for stable spine enlargement after LTP (6). The specificity of Arc-mediated GluR1 internalization to thin spines suggests that GluR1 endocytosis could mechanistically play a role in reducing the size of spine heads. Alternatively, GluR1 recycling may be important for maintaining thin spines, rather than reducing size. It is also possible that these two functions are distinct. Future studies that follow Arc-expressing or Arc-lacking spines over time will be useful in teasing these mechanisms apart.
Although deleting Arc's endophilin-binding domain (Arc Δ91–100) blocked Arc-mediated increases in thin spines, deleting the dynamin-binding domain (Arc Δ195–214) led to fewer thin spines and more mushroom spines than in controls. In the Arc–endophilin–dynamin complex, dynamin is thought to interact with endophilin's SH3 domain (19). One possible explanation for the Arc Δ195–214 effect on mushroom spines is that removal of dynamin frees the endophilin SH3 domain to bind other proteins. One such SH3 binding partner is the actin-nucleating factor neural-Wiskott-Aldrich syndrome protein (N-WASP), which could enlarge spines through Arp2/3 activation.
Arc reduces surface expression of both GluR1 and GluR2 (19, 20). However, in our culture system, GluR2 surface expression was not specifically reduced in thin spines, suggesting that Arc-mediated endocytosis of GluR1, not GluR2, regulates spine morphology.
Arc and Synaptic Plasticity.
Arc has been implicated in LTD and LTP plasticity paradigms as well as homeostatic plasticity (18, 21–23). How Arc facilitates such opposing forms of plasticity has largely remained unanswered. Our data support the role of Arc in LTD through endocytosis of AMPARs and reduction of spine size. However, it is still unclear how Arc facilitates LTP maintenance. Later phases of LTP are associated with new spine formation (37). Messaoudi et al. (17) showed that knockdown of Arc 2 h after high-frequency stimulation blocks LTP-induced F-actin polymerization and induces dephosphorylation of hyperphosphorylated cofilin, a regulator of actin polymerization, indicating that Arc facilitates actin reorganization at spines. We demonstrated that Arc expression in cultures increases spine density and that loss of Arc in vivo decreases spine density.
Our observation of decreased spine density in Arc−/− mice apparently conflicts with a previous study (18), which reported no significant difference in spine density due to Arc loss. In our study, spines from 14 dendrites from three mice per genotype were imaged after Golgi staining of whole brains, whereas Plath et al. imaged three biocytin-injected CA1 pyramidal neurons from knockout hippocampal slices. A statistically significant decrease in spine density might have been observed by Plath et al. if more spines had been measured or if whole brains had been used (39). The mice we used showed memory deficits similar to those used by Plath et al. (Fig. S6); however, differences in genetic background might also be relevant.
The role of Arc in homeostatic plasticity may also be critical for LTP expression and memory consolidation. Arc-mediated changes in spine morphology and receptor content could act to prevent saturation of LTP. In support, unrestrained epileptiform activity can prevent LTP expression (28) and interfere with memory consolidation (40–42). However, transient hippocampal reduction of Arc in WT mice blocks late LTP and memory consolidation. This indicates that the deficits in late LTP and memory consolidation in the Arc−/− mice are likely directly due to Arc loss rather than long-term compensatory network alterations.
Is Arc-Mediated Homeostasis Antiepileptogenic?
Seizure activity is characterized by highly synchronized, high-frequency activation of neurons caused by the imbalance of excitatory and inhibitory circuits. These abnormally high levels of activity often result in long-lasting synaptic changes and excitotoxicity that can increase hyperexcitability in the system and cause the recurrent seizure activity that defines epilepsy. Seizures alter the expression of many genes whose downstream products change neuronal function and synaptic efficacy. In fact, Arc was originally discovered as a gene highly induced by seizures (12). Genes up-regulated after seizure may also act in a negative feedback loop, preventing further activity and inhibiting epileptogenesis.
We found that genetic disruption of Arc expression leads to histological alterations observed in epileptic models. We suggest that loss of Arc reduces homeostatic capacity, leading to network hyperexcitability and ultimately epilepsy. However, only 50% of Arc−/− mice exhibited molecular alterations characteristic of severe epilepsy. The likely explanation for this is that seizures are infrequent in the mutant mice. In fact, despite the presence of relatively abundant hypersynchronous discharges, we witnessed no spontaneous seizures during the prolonged EEG monitoring period. However, one Arc+/− mouse had a convulsive seizure, and several Arc+/− mice developed aberrant NPY expression. As in previous reports (24), Arc expression is reduced by only 30% in Arc+/− mice (Fig. S7). One explanation for the higher expression is the increased neural network activity observed in these mice. Given these findings, memory and plasticity phenotypes in both knockout and heterozygote mice should be re-evaluated in the context of this underlying hyperexcitability.
Recent work in murine models of Alzheimer's disease shows that hAPP-J20 mice also have aberrant excitatory neuronal activity and exhibit similar alterations in NPY and calbindin expression (43). Furthermore, hAPP-J20 mice display significantly lower levels of Arc in the DG than nontransgenics (44), and lower levels of immediate early genes, such as c-fos and Arc, are tightly coupled to cognitive deficits (45). In these mice, levels of Arc also correlate extremely well with NPY alterations, calbindin expression, and seizure severity. Like Arc−/− mice, murine models of Alzheimer's disease have decreased spine density, impaired LTP and LTD (46, 47), and memory deficits. Such correlations raise the provocative possibility that lower basal Arc expression in hAPP-J20 mice plays a critical role, mediating their epileptic activity and memory deficits.
In conclusion, we show that Arc is critical in regulating spine and synapse morphology. By integrating AMPAR endocytosis with spine size, Arc balances the downscaling of synapses with increased morphological plasticity. We suggest that this dual role allows Arc to facilitate both homeostatic and Hebbian plasticity. Understanding how hypersynchronous network activity affects Hebbian plasticity and memory consolidation in Arc−/− mice should give additional insight into the relationship between homeostatic scaling, LTP, and LTD.
Materials and Methods
Mice.
We studied 4- to 9-mo-old Arc-d2EGFP knock-in mice (24) (C57/BL6 strain), which contain d2EGFP, followed by a Neo cassette inserted after the Arc ATG start codon. For harvesting of brain tissue, mice were deeply anesthetized and flush perfused transcardially with phosphate buffer. Hemibrains were drop-fixed in 4% phosphate-buffered paraformaldehyde. All experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Immunostaining.
For Arc staining, a polyclonal antibody was made. Recombinant Arc protein for immunization was expressed and purified as previously described (12). Rabbit immunization and antibody purification was performed through Invitrogen. For surface AMPAR staining, transfected neurons were incubated with N-terminal GluR1 antibody (rabbit, Calbiochem; 1:40) or N-terminal GluR2 antibody (mouse, Chemicon; 1:300) for 45 min and then fixed with 4% PFA/4% sucrose. Coverslips were blocked in PBS with 3% donkey serum and 3% BSA for 1 h and incubated with fluorescent secondary antibody (Alexa donkey 647, 1:200) for 1 h. For synapsin I (rabbit, Chemicon; 1:3,000) staining, neurons were fixed and permeabilized in 0.1% Triton X-100 in PBS for 15 min. Cells were blocked as above and incubated with primary antibody overnight at 4 °C, followed by fluorescent secondary antibody incubation and mounting.
Antibody-Feeding Assay.
Transfected hippocampal neurons at 20–21 DIV were incubated with GluR1 antibody (rabbit, Calbiochem; 1:40) for 10 min. Any unbound antibody was then removed with two washes of Neurobasal (Invitrogen) medium, and the neurons were incubated an additional 15 min in this medium. The neurons were fixed with 4% PFA/4% sucrose and incubated with saturating Alexa donkey 555 secondary antibody (1:100) for 1 h to stain surface GluR1. The neurons were then permeabilized with 0.5% Triton X-100 in PBS for 15 min, followed by incubation with Alexa donkey 647 to stain internalized GluR1.
Microscopy and Image Analysis.
Images of primary hippocampal cultures were acquired with a LSM510 confocal microscope system (Zeiss) and a 63× oil immersion lens (1,024 × 1,024 pixels). Each image consisted of a stack of images taken through the z-plane of the cell. Confocal microscope settings were kept the same for all scans in each experiment. Healthy, pyramidal-like neurons expressing the cotransfection and morphology marker GFP were chosen randomly for quantification from three coverslips from three to four independent experiments for each construct. Spines on primary and secondary dendrites (>75% of dendrites were secondary with no significant difference between conditions) were analyzed and no tertiary dendrites were included. For spine size, the maximal length and head width were measured manually with Metamorph (Universal Imaging). Each spine was categorized as having or not having a neck. Spines with necks were separated into thin and mushroom spines based on head width. Spines with heads less than the average width (1 μm for GFP images, 0.75 μm for Golgi staining) were categorized as thin, and those with heads greater than the average width were categorized as mushroom. Filopodia were protrusions greater than 1.5 μm in length without a neck. The investigator was blinded to experimental conditions during both image acquisition and morphometric analysis for both culture and Golgi-stained tissue analysis. Golgi-stained sections were imaged under brightfield illumination on a Nikon Eclipse TE2000-E microscope with a 60× oil immersion objective. Next, 10-μm spaced Z stacks were collected, and spine length, and width were measured in the appropriate focal plane as above using Metamorph. For internalization assays, regions were drawn around individual spine heads and the ratio of internalized over total (surface + internalized) average GluR1 fluorescence intensity was calculated for each spine. Ratios were normalized to the average control ratio for each spine type within experiments.
Supplementary Material
Acknowledgments
Rat Arc cDNA was provided by Dr. J. F. Guzowski (University of California, Irvine, CA). GFP-actin expression plasmid was a gift from Yukiko Goda (University College London, London, United Kingdom). We thank Susumu Tonegawa (Massachusetts Institute of Technology, Boston) for providing the Arc-d2EGFP mice. GW1-rArc was constructed by Vikram Rao. Behavioral testing was performed in the Gladstone Institute Behavioral Core by Tracy Hamto and Nino Devidze. We also thank Kathryn Bradley for additional EEG recordings. We thank Gary Howard and Stephen Ordway for editorial assistance and Kelley Nelson for administrative assistance. This work was supported by Grants 2R01 NS0390746 and 2R01 NS045191 from the National Institute of Neurological Disorders and Stroke (to S.F.), Grant 2P01 AG022074 from the National Institute on Aging and by the Keck Foundation (to J.N. and S.F.), and NS29709 and IDDRC HD024064 (to J.L.N.). The National Institutes of Health/National Institute of General Medical Sciences University of California at San Francisco Medical Scientist Training Program supported C.L.P. Additional support was provided by the Epilepsy Foundation of America (C.L.P.). The animal care facility was partly supported by National Institutes of Health Extramural Research Facilities Improvement Program Project C06 RR018928.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006546107/-/DCSupplemental.
References
- 1.Hering H, Sheng M. Dendritic spines: Structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 2.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Park M, et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Nägerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Rao VR, et al. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci. 2006;9:887–895. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- 11.Pintchovski SA, Peebles CL, Kim HJ, Verdin E, Finkbeiner S. The serum response factor and a putative novel transcription factor regulate expression of the immediate-early gene Arc/Arg3.1 in neurons. J Neurosci. 2009;29:1525–1537. doi: 10.1523/JNEUROSCI.5575-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyford GL, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 13.Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 14.Moga DE, et al. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez JJ, et al. Long-term potentiation in the rat dentate gyrus is associated with enhanced Arc/Arg3.1 protein expression in spines, dendrites and glia. Eur J Neurosci. 2005;21:2384–2396. doi: 10.1111/j.1460-9568.2005.04068.x. [DOI] [PubMed] [Google Scholar]
- 16.Guzowski JF, et al. Inhibition of activity-dependent Arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messaoudi E, et al. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plath N, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury S, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park S, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shepherd JD, et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang KH, et al. In vivo two-photon imaging reveals a role of Arc in enhancing orientation specificity in visual cortex. Cell. 2006;126:389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Swann JW, Al-Noori S, Jiang M, Lee CL. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000;10:617–625. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Klugmann M, et al. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Potschka H, et al. Kindling-induced overexpression of Homer 1A and its functional implications for epileptogenesis. Eur J Neurosci. 2002;16:2157–2165. doi: 10.1046/j.1460-9568.2002.02265.x. [DOI] [PubMed] [Google Scholar]
- 28.Seeburg DP, Sheng M. Activity-induced Polo-like kinase 2 is required for homeostatic plasticity of hippocampal neurons during epileptiform activity. J Neurosci. 2008;28:6583–6591. doi: 10.1523/JNEUROSCI.1853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Prog Brain Res. 2007;163:285–297. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- 30.Nadler JV, Tu B, Timofeeva O, Jiao Y, Herzog H. Neuropeptide Y in the recurrent mossy fiber pathway. Peptides. 2007;28:357–364. doi: 10.1016/j.peptides.2006.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter DS, Harrison AJ, Falenski KW, Blair RE, DeLorenzo RJ. Long-term decrease in calbindin-D28K expression in the hippocampus of epileptic rats following pilocarpine-induced status epilepticus. Epilepsy Res. 2008;79:213–223. doi: 10.1016/j.eplepsyres.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selke K, Müller A, Kukley M, Schramm J, Dietrich D. Firing pattern and calbindin-D28k content of human epileptic granule cells. Brain Res. 2006;1120:191–201. doi: 10.1016/j.brainres.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 33.Nägerl UV, et al. Surviving granule cells of the sclerotic human hippocampus have reduced Ca(2+) influx because of a loss of calbindin-D(28k) in temporal lobe epilepsy. J Neurosci. 2000;20:1831–1836. doi: 10.1523/JNEUROSCI.20-05-01831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 35.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 37.De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- 38.Dumitriu D, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirov SA, Petrak LJ, Fiala JC, Harris KM. Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neuroscience. 2004;127:69–80. doi: 10.1016/j.neuroscience.2004.04.053. [DOI] [PubMed] [Google Scholar]
- 40.Lopes da Silva FH, Gorter JA, Wadman WJ. Kindling of the hippocampus induces spatial memory deficits in the rat. Neurosci Lett. 1986;63:115–120. doi: 10.1016/0304-3940(86)90046-7. [DOI] [PubMed] [Google Scholar]
- 41.Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8:504–515. doi: 10.1016/j.yebeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Kotloski R, Lynch M, Lauersdorf S, Sutula T. Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. Prog Brain Res. 2002;135:95–110. doi: 10.1016/S0079-6123(02)35010-6. [DOI] [PubMed] [Google Scholar]
- 43.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palop JJ, et al. Vulnerability of dentate granule cells to disruption of Arc expression in human amyloid precursor protein transgenic mice. J Neurosci. 2005;25:9686–9693. doi: 10.1523/JNEUROSCI.2829-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palop JJ, et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobsen JS, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.