Abstract
Salicylic acid (SA) is a defense hormone required for both local and systemic acquired resistance (SAR) in plants. Pathogen infections induce SA synthesis through up-regulating the expression of Isochorismate Synthase 1 (ICS1), which encodes a key enzyme in SA production. Here we report that both SAR Deficient 1 (SARD1) and CBP60g are key regulators for ICS1 induction and SA synthesis. Whereas knocking out SARD1 compromises basal resistance and SAR, overexpression of SARD1 constitutively activates defense responses. In the sard1-1 cbp60g-1 double mutant, pathogen-induced ICS1 up-regulation and SA synthesis are blocked in both local and systemic leaves, resulting in compromised basal resistance and loss of SAR. Electrophoretic mobility shift assays showed that SARD1 and CBP60g represent a plant-specific family of DNA-binding proteins. Both proteins are recruited to the promoter of ICS1 in response to pathogen infections, suggesting that they control SA synthesis by regulating ICS1 at the transcriptional level.
Keywords: plant immunity, SAR Deficient 1, Isochorismate Synthase 1, CBP60g
Systemic acquired resistance (SAR) is a secondary immune response in the distal parts of plants activated by local defense responses. SAR is long-lasting and effective against a broad spectrum of pathogens, including fungi, bacteria, and viruses (1). Traditionally, SAR is induced by incompatible pathogens that cause localized cell death. Tissue necrosis at inoculation sites is not required for SAR activation, however (2).
Salicylic acid (SA) is a phytohormone that plays a central role in defense signaling (3). It is required for both basal defense and SAR. Early studies showed that pathogen infections lead to increased SA levels in both local and distal parts of plants (4–6). Whereas application of exogenous SA or SA analogs induces resistance to pathogens (7–9), degradation of SA by transforming plants with the bacterial salicylate hydroxylase gene NahG blocks SA accumulation and SAR (10). SA activates defense responses through its downstream components NPR1 (11) and three redundant transcription factors, TGA2, TGA5, and TGA6 (12). Increased SA levels induce redox changes and result in reduction of NPR1 to a monomeric form that accumulates in the nucleus to activate defense gene expression (13).
In Arabidopsis, mutations in SID2 and EDS5 block pathogen-induced SA synthesis and result in defects in SAR as well as basal resistance (14). SID2 encodes Isochorismate Synthase 1 (ICS1), a key enzyme in pathogen-induced SA biosynthesis (15). ETHYLENE INSENSITIVE3 (EIN3) and EIN3-LIKE1 have been reported to negatively regulate SA synthesis through repression of ICS1 expression (16). How the ICS1 expression is positively regulated during pathogen infection remains to be determined. Because up-regulation of SA biosynthesis is critical to the activation of basal resistance and SAR, identification of upstream regulatory components required for the induction of SA biosynthesis genes such as ICS1 is essential for understanding SA-mediated defense responses. Here we report our discovery of two members of a plant-specific family of transcription factors that regulate the induction of ICS1 and the accumulation of SA on pathogen infection.
Results
Development of an SAR Assay.
In traditional SAR assays for Arabidopsis, local leaves are infiltrated with avirulent bacteria to induce SAR, and distal leaves are later challenged with a virulent bacterial pathogen. Bacterial growth in the distal leaves is quantified to determine whether SAR indiction occurred. Because quantifying bacterial growth for a large number of plants is tedious and bacterial growth varies depending on the growth conditions, traditional SAR assays are unsuitable for large-scale screening of SAR-deficient mutants.
To address this problem, we tested whether SAR can be induced against the oomycete pathogen Hyaloperonospora arabidopsidis Noco2 (H.a. Noco2) after infiltration with Pseudomonas syringae p.v. maculicola (P.s.m.) ES4326. We found that infiltrating local leaves with a low dose of P.s.m. ES4326 (OD600 = 0.001) consistently induced SAR against H.a. Noco2 (Fig. 1A). We then tested a selection of known SAR mutants using this assay. As shown in Fig. 1B, SAR was severely compromised in all mutants tested, including sid2-1, eds5-3, npr1-1, eds1-2 (Col), and pad4-1. Because this assay is easy to perform and provides consistent results, it is feasible for application in a large number of plants.
Fig. 1.
Growth of H.a. Noco2 on the distal leaves of WT and SAR-deficient mutants. (A) Induction of SAR by infiltrating two primary leaves of 3-wk-old plants with different concentrations (OD600) of P.s.m. ES4326. (B) Testing of SAR response in known SAR-deficient mutants. Two days after two primary leaves were infiltrated with P.s.m. ES4326 (OD600 = 0.001) or 10 mM MgCl2 (mock), plants were sprayed with H.a. Noco2 spores at a concentration of 5 × 104 spores per mL of water. Infection was scored 7 d later by counting the number of conidiophores on the distal leaves. A total of 15 plants were scored for each treatment. Disease rating scores are as follows: 0, no conidiophores on the plants; 1, one leaf infected with no more than five conidiophores; 2, one leaf infected with more than five conidiophores; 3, two leaves infected, but with no more than five conidiophores on each infected leaf; 4, two leaves infected with more than five conidiophores on each infected leaf; 5, more than two leaves infected with more than five conidiophores.
SARD1 Is Required for SAR.
To identify genes required for SAR, we assayed T-DNA insertion mutants of ∼200 genes induced by P.s.m. ES4326 for loss of the SAR phenotype. These genes and their corresponding T-DNA mutants have been described previously (17). Among them, only At1g73805 was found to be required for SAR. Both SALK_138476 and SALK_052422 contain T-DNAs in the second intron of At1g73805 (Fig. 2A), which disrupt expression of the gene (Fig. 2B). On primary induction with P.s.m. ES4326, WT plants developed systemic resistance to H.a. Noco2. In contrast, SAR was reduced in both mutants and was lost in the npr1-1 control plants (Fig. 2C). Given the SAR-deficient phenotypes, we designated At1g73805 as SAR Deficient 1 (SARD1), SALK_138476 as sard1-1, and SALK_052422 as sard1-2. As shown in Fig. S1 A and B, P.s.m. ES4326 induced the expression of SARD1 in both local and systemic leaves.
Fig. 2.
Analysis of sard1 knockout mutants. (A) Positions of T-DNA insertions within SARD1. sard1-1, SALK_138476; sard1-2, SALK_052422. Lines indicate introns, and black boxes are exons. (B) RT-PCR analysis of SARD1 expression in sard1-1 and sard1-2. (C) Growth of H.a. Noco2 on systemic leaves of the indicated genotypes. Three-wk-old plants were first infiltrated with P.s.m. ES4326 (OD600 = 0.001) or 10 mM MgCl2 on two primary leaves and sprayed with H.a. Noco2 spores 2 d later. At 7 d postinoculation, infections were scored as described in Fig. 1. (D) Growth of P.s.m. ES4326 on systemic leaves of the indicated genotypes. Two leaves from each plant were infiltrated with P.s.m. ES4326 AvrB (OD600 = 0.02) or 10 mM MgCl2 2 d before P.s.m. ES4326 infection (OD600 = 0.001) on distal leaves. The bacterial titers were measured by taking leaf discs within the inoculated area. Error bars represent 95% confidence limits of log-transformed data from four replicates.
We then tested whether SAR can be induced in the sard1 mutants in a traditional SAR assay. As shown in Fig. 2D, P.s.m. ES4326 carrying AvrB induced systemic resistance to P.s.m. ES4326 in the WT plants, whereas SAR responses were partly compromised in sard1-1 and sard1-2. Taken together, these results indicate that SARD1 functions as a positive regulator of SAR.
Overexpression of SARD1 Leads to Enhanced Resistance to Pathogens.
When SARD1 with or without a C-terminal HA tag was expressed under the control of its native promoter in WT plants, about one-quarter of T1 transgenic plants were dwarfed, suggesting possible activation of defense responses in these plants. Two representative SARD1-HA lines were characterized in detail. Lines 1 and 2 expressed SARD1-HA at different levels, with higher expression in line 1 than in line 2 (Fig. 3A). As shown in Fig. S1E, line 1 was smaller than WT and line 2, and senesced early. No spontaneous lesion formation was observed in these lines. The greater expression of SARD1-HA in line 1 was confirmed by Western blot analysis (Fig. S1F). Analysis of defense marker genes PR1 (Fig. 3B) and PR2 (Fig. 3C) showed that both lines exhibit constitutive expression of the PR genes, with greater expression of PR1 and PR2 in line 1. Furthermore, line 1 displayed strongly enhanced resistance to H.a. Noco2 (Fig. 3D). Enhanced resistance to P.s.m. ES4326 was also observed in line 1 (Fig. S1G), suggesting that overexpression of SARD1 leads to enhanced resistance to pathogens. Analysis of SA levels showed that greater accumulation of both free and total SA in line 1 compared with WT and line 2 (Fig. 3 E and F).
Fig. 3.
Overexpression of SARD1 leads to constitutive activation of defense responses and increased SA levels. (A) SARD1 expression in two independent SARD1-HA transgenic lines. (B and C) Expression of PR1 (B) and PR2 (C) in the indicated genotypes. (D) Growth of H.a. Noco2 on the indicated genotypes. The plants were sprayed with H.a. Noco2 spores at a concentration of 5 × 104 spores/mL of water. Infection was scored at 7 d postinoculation. (E and F) Free SA (E) and total SA (F) levels in the indicated genotypes.
Mutations in SARD1 and CBP60g Have Additive Effects on SAR.
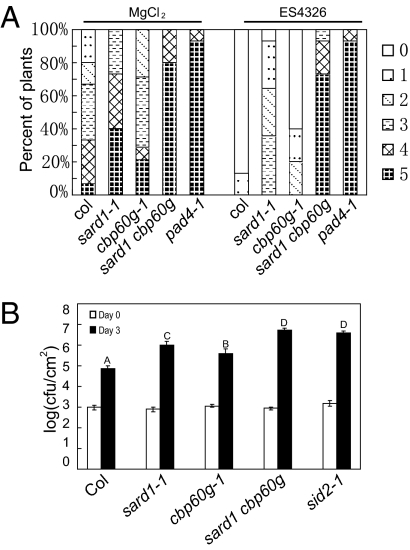
SARD1 belongs to a plant-specific protein family previously termed ACBP60 (Fig. S2) (18). We found that knocking out another member of the ACBP60 family, CBP60g, has a small but reproducible effect on SAR. Like SARD1, CBP60g is also induced by P.s.m. ES4326 in both local and systemic leaves (Fig. S1 C and D). Because SARD1 and CBP60g belong to the same protein family, we generated the sard1-1 cbp60g-1 double mutant to test whether these proteins have additive effects on SAR. The double mutant displayed WT morphology (Fig. S3A). As shown in Fig. 4A , systemic resistance to H.a. Noco2 induced by P.s.m. ES4326 was further impaired in the double mutant. In addition, systemic resistance to P.s.m. ES4326 induced by P.s.m. ES4326 avrB was also lost in the double mutant (Fig. S3B). These data suggest that SARD1 and CBP60g have overlapping functions or function in two independent pathways in the regulation of SAR.
Fig. 4.
Loss of SAR and basal resistance in sard1-1 cbp60g-1. (A) Growth of H.a. Noco2 on systemic leaves of the indicated genotypes. Primary infection with P.s.m. ES4326, secondary inoculation with H.a. Noco2, and scoring of the infection were performed as shown in Fig. 2C. (B) Growth of P.s.m. ES4326 on the indicated genotypes. Leaves of 5-wk-old plants were infiltrated with a bacterial suspension at OD600 = 0.0002. Bacterial titers were measured on day 0 and day 3. The values presented are averages of six replicates ± SD. Statistically significant differences among the samples are labeled with different letters (P < 0.01).
To test whether SARD1 and CBP60g are also required for basal defense, we inoculated the single and double mutants with P.s.m. ES4326. As shown in Fig. 4B, compared with WT, sard 1-1 supported about 10-fold more bacterial growth, whereas cbp60g-1 exhibited only slightly greater bacterial growth. Bacterial growth was much greater in sard1-1 cbp60g-1 than in the single mutants, suggesting that SARD1 and CBP60 also play additive roles in basal defense.
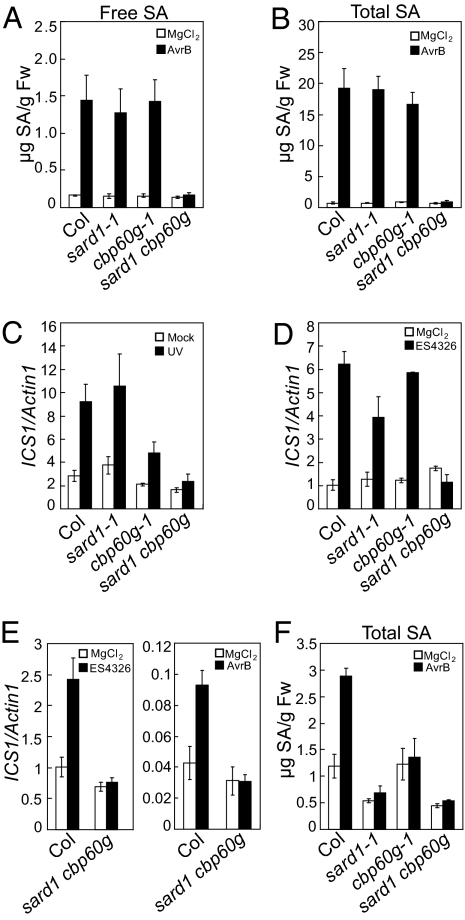
SARD1 and CBP60g are required for SA accumulation and induction of ICS1 during pathogen infection. The loss of SAR phenotype in sard1-1 cbp60g-1 prompted us to test whether SA biosynthesis is affected in the mutant plants. As shown in Fig. 5 A and B, SA levels were similar in the WT and the mutants before induction. After induction by P.s.m. ES4326 avrB, SA levels increased in the WT and the single mutants, but not in the double mutant, indicating blockage of pathogen-induced SA accumulation in the double mutant.
Fig. 5.
ICS1 induction and SA accumulation are blocked in sard1-1 cbp60g-1. (A and B) Induction of free SA (A) and total SA (B) synthesis by P.s.m. ES4326 AvrB. Plants were infiltrated with P.s.m. ES4326 AvrB (OD600= 0.2), and the inoculated leaves were collected 48 h later for SA extraction. (C) Induction of ICS1 by UV-B in the indicated genotypes. Plants were treated by UV-B for 15 min, and samples were taken 24 h postirradiation. (D and E) Induction of ICS1 in local (D) and systemic leaves (E) of the indicated genotypes. Plants were infiltrated with P.s.m. ES4326 (OD600= 0.001) or P.s.m. ES4326 avrB (OD600= 0.01). Samples were taken 48 h postinoculation. (F) SA accumulation in the systemic leaves after local infections. Three leaves of 4-wk-old plants were infiltrated with P.s.m. ES4326 avrB (OD600= 0.01), and the distal leaves were collected 48 h later for total SA extraction.
In plants, pathogen-induced SA is synthesized from chorismate by ICS1. ICS1 expression is induced by both biotic and abiotic stresses. To test whether induction of ICS1 is affected in sard1-1 cbp60g-1, we analyzed the expression of ICS1 before and after induction by UV-B irradiation or bacterial infection. As shown in Fig. 5 C and D, induction of ICS1 was blocked in the double mutant, suggesting that the reduced SA synthesis in the double mutant is caused by loss of ICS1 induction. Further analysis revealed that induction of ICS1 and SA synthesis by P.s.m. ES4326 avrB in the systemic leaves was also blocked in the double mutant (Fig. 5 E and F).
SARD1 Is Targeted to the Promoter of ICS1 After Pathogen Infection.
To determine the subcellular localization of SARD1, we generated transgenic plants expressing the SARD1-GFP fusion protein under its own promoter. Some of the transgenic lines exhibited small stature like the SARD1-HA overexpression lines described earlier (Fig. S4A), suggesting that SARD1-GFP functions similar to SARD1. SARD1-GFP was found to localize in the nucleus of leaf pavement cells after infiltration with P.s.m. ES4326 (Fig. 6A). Without induction, no green fluorescence was observed in the SARD1-GFP transgenic lines, likely due to low levels of the protein. Localization of SARD1 to the nucleus was further confirmed by fractionation and Western blot analysis of protein extracts from SARD1-HA transgenic plants (Fig. S4B).
Fig. 6.
ChIP analysis of recruitment of SARD1 to the promoter of ICS1. (A) GFP fluorescence in leaf pavement cells of SARD1-GFP transgenic plants expressing SARD1-GFP under its native promoter. Leaves were infiltrated with P.s.m. ES4326 (OD600= 0.001) 24 h before being examined by confocal microscopy. Cell walls were stained with 5 mg/mL of propidium iodine. (B) P.s.m. ES4326–induced recruitment of SARD1 to the promoter of ICS1. (C) Locations of the PCR fragments. (D) Enrichment of SARD1-bound fragments of ICS1 promoter after UV-B treatment. (E) Enrichment of SARD1-bound fragments of ICS1 promoter after induction by P.s.m. ES4326. Plants were irradiated by UV-B for 15 min or infiltrated with P.s.m. ES4326 (OD600= 0.001), and samples were taken 24 h later for ChIP analysis. ChIP was performed with an anti-HA antibody (AB) as described previously (24). Quantitative PCR was carried out using the immunoprecipitated DNA as a template. SARD1-GFP transgenic plants were used as negative controls in B.
The requirement for SARD1 and CBP60 for the expression of ICS1 and the nuclear localization of SARD1 prompted us to test whether these proteins are recruited to the promoter of ICS1. We carried out chromatin immunoprecipitation (ChIP) on SARD1-HA transgenic plants using anti-HA antibody, and used real-time PCR on the immunoprecipitated DNA to test whether genomic DNA in the promoter region of ICS1 was enriched by ChIP. Without induction, no enrichment was observed. Induction by P.s.m. ES4326 or UV-B led to enrichment of DNA around the ICS1 promoter (Fig. 6B and Fig. S4C), suggesting that SARD1 is targeted to the ICS1 promoter. ChIP analysis with CBP60g-HA transgenic plants showed that CBP60g was also recruited to the ICS1 promoter after induction by P.s.m. ES4326 (Fig. S4D).
To identify the region to which SARD1 is targeted on the ICS1 promoter, we used 16 pairs of primers (Table S1) designed to amplify a group of overlapping DNA fragments covering the region from 2,351 bp upstream to 396 bp downstream of the translation start site of ICS1 (Fig. 6C). As shown in Fig. 6D, DNA fragment 7 exhibited the greatest enrichment by ChIP compared with the other fragments, suggesting that SARD1 is targeted to this region after induction with UV-B. Similar results were obtained from ChIP analysis performed on SARD1-HA transgenic plants treated with P.s.m. ES4326 (Fig. 6E).
SARD1 and CBP60g Are DNA-Binding Proteins.
Five of the eight ACBP60 proteins were originally shown to bind calmodulin (CaM) (18). The CaM-binding motif is located at the C terminus of the proteins, but this motif is absent in SARD1 and CBP60g. A fragment of 76 amino acids at the N terminus of CBP60g was recently shown to bind CaM (19). To determine whether SARD1 is capable of binding CaM, we expressed full-length SARD1 and a fragment of SARD1 corresponding to the first 76 amino acids at the N terminus of CBP60g with a GST tag. As shown in Fig. S5, the N-terminal fragment of CBP60g bound to CaM, but no CaM-binding activity was detected with either the full-length or the truncated SARD1 protein. In CBP60g, amino acid Val-29 is required for the binding of CBP60g to CaM (19), and this residue is not conserved in SARD1 (Fig. S2), consistent with our finding that the N-terminal fragment of SARD1 was not able to bind CaM.
The proteins of the ACBP60 family all contain a highly conserved domain in their central region (Fig. S2). The function of this domain is unknown. Because SARD1 is localized to the nucleus and targeted to the promoter of ICS1, we tested whether it binds to DNA directly. Three overlapping fragments of SARD1 with C-terminal His-tags were expressed in Escherichia coli and purified using Ni-NTA columns. Electrophoretic mobility shift assays (EMSA) were subsequently carried out using the recombinant SARD1 proteins and DNA fragment 7 from the ChIP-PCR analysis (Fig. 6C). As shown in Fig. 7A, a mobility shift was observed when the 32P-labeled DNA fragment was preincubated with the N-terminal half (aa 1–214), but not with the C-terminal half (aa 215–451), of SARD1. In addition to the indicated mobility shift, another shifted band close to the top of the gel was seen. Analysis of the SARD1 protein by gel filtration indicated that a large fraction of the protein was in an oligomerized form. The shifted band close to the top of the gel might represent oligomerized protein bound to the probe. Similar mobility shifts were observed when the DNA fragment was preincubated with the central region (aa 149–270) of SARD1. Binding of the SARD1 fragments to the labeled probe can be completed using an excess of unlabeled probe but not poly(dIdC) in the reaction, indicating that the binding that we observed is specific. These data suggest that SARD1 is a DNA-binding protein, and that the DNA-binding motif is in the region of aa 149–214.
Fig. 7.
EMSA analysis for binding of SARD1 to a DNA fragment from the ICS1 promoter (A) and a working model for regulation of SA synthesis by SARD1 and CBP60g (B). (A) DNA fragment 7 from the ChIP-PCR analysis (Fig. 6) was end-labeled with [32P]ATP. Then 0.1 pmol labeled DNA was incubated with 1.25 μg of E. coli–expressed truncated SARD1 proteins as indicated (lanes 3–9) or a control protein (the kinase domain of SOBIR1) preparation (lane 1), in the absence (lanes 1, 3, 5, and 7) or the presence (lanes 4, 6, 8, and 9) of unlabeled DNA fragment 7 as a cold competitor. The sample in lane 2 contains only the labeled probe. The 20× and 10× contain 20- and 10-fold more unlabeled probes than labeled probes, respectively. (B) A proposed model of two defense pathways for pathogen-induced SA synthesis. One pathway leads to induction of SARD1 and subsequent recruitment of SARD1 to the promoter of ICS1 and activation of its transcription and SA synthesis. The other pathway leads to Ca2+ influx, which results in binding of CaM to CBP60g and recruitment of CBP60g to the promoter of ICS1 and activation of the transcription of ICS1 and SA synthesis.
To test whether CBP60g is also able to bind to DNA, we expressed the corresponding middle domain (aa 148–263) of CBP60g with a C-terminal His-tag in E. coli. The CBP60g148–263 protein was purified using Ni-NTA columns and used in EMSA. As shown in Fig. S6A, CBP60g148–263 was able to bind DNA fragment 7 from the ICS1 promoter, suggesting that CBP60g is also a DNA-binding protein.
To determine whether binding of CBP60g and SARD1 to DNA is sequence-specific, we synthesized 33 overlapping oligonucleotide probes (Table S2) covering the region of DNA fragment 7, and tested these probes for their relative binding affinities to CBP60g148–263. One of the oligonucleotide probes (oligo-15; gaaattttgg) displayed relatively high affinity to the protein compared with other probes. As shown in Fig. S6B, binding of oligo-15 to CBP60g148–263 can be competed by an excess of unlabeled oligo-15, but not by another oligonucleotide probe, oligo-8. In addition, binding of oligo-15 to the central domain of SARD1 can be efficiently competed by excess of unlabeled oligo-15, but not by oligo-8 (Fig. S6C). These data suggest that CBP60g and SARD1 are sequence-specific DNA-binding proteins.
Discussion
SA is one of the most important signal molecules for plant defense. Pathogen-induced SA synthesis and accumulation are required for both local resistance and SAR. How plants regulate SA biosynthesis is a fundamental question in plant immunity. Using a reverse genetic approach, we identified SARD1 as a key regulator of both SAR and basal defense. In the sard1 cbp60g double mutant, pathogen-induced SA synthesis in both local and systemic leaves is completely blocked, leading to severely compromised local and systemic resistance. Our data demonstrate that SARD1 and CBP60g are crucial regulators for the induction of SA synthesis by pathogens.
Both SAR and local resistance are partially compromised in sard1 and cbp60g single mutants. The compromised SAR likely results from reduced SA accumulation in the systemic leaves. The cause of the reduced local resistance is less clear. Whereas local SA levels are comparable in WT and the single mutants after infection by the avirulent pathogen P.s.m. ES4326 avrB, whether induction of SA synthesis by the virulent P.s.m. ES4326 is affected in the single mutants remains to be determined. It is possible that the compromised local resistance also results from reduced SA accumulation. Alternatively, reduced local resistance could be caused by a loss of induction of other genes regulated by SARD1 and CBP60g. Identifying additional target genes of SARD1 and CBP60g by ChIP sequencing will provide insight into the roles of SARD1 and CBP60g in local resistance.
SARD1 belongs to a protein family with unknown biochemical functions. One of the members, CBP60g, has previously been shown to contribute to MAMP-induced SA synthesis (19), but how CBP60g affects MAMP-induced SA synthesis remains unknown. We have shown that both SARD1 and CBP60g are recruited to the ICS1 promoter in response to pathogen infections, suggesting that they directly regulate ICS1 expression and SA synthesis. SARD1 is targeted to a 181-bp region on the ICS1 promoter. This region contains a predicted W-box and a MYB recognition site. Using EMSA, we demonstrated that SARD1 and CBP60g are DNA-binding proteins. Both proteins preferentially bind the oligonucleotide probe oligo15 (gaaattttgg), which contains no known cis-acting element. Bioinformatic analysis using the microarray database at the Arabidopsis Resource Center showed that the “aatttt” motif on oligo15 is statistically overrepresented in the promoters of genes induced by flg22 or Pseudomonas syringae pv. Tomato (P.s.t.) DC3000 avrRpm1 (P < 10−5).
Binding of SARD1 and CBP60g to DNA is facilitated through the highly conserved central region of the proteins, which exhibits no sequence similarity to other known DNA-binding proteins, suggesting that they represent a plant-specific family of transcription factors. Because not all members in the ACBP family have CaM-binding activity but all share the central DNA-binding domain, we suggest renaming this protein family the SARD1 transcription factor family.
SARD1 shares only 39% identity with CBP60g at the amino acid level. The expression of both SARD1 and CBP60g is up-regulated by pathogen infections. Whereas overexpression of SARD1 leads to constitutive defense responses, similar activation of defense responses was not observed in transgenic plants overexpressing CBP60g. In CBP60g and SARD1, the middle domains that contain the DNA-binding activity are highly conserved, but sequences at the N- and C-termini are quite diverged. The N-terminal domains of CBP60g and SARD1 appear to have different functions. Whereas CBP60g binds to CaM through its N-terminal domain (19), SARD1 is not able to bind CaM. Activation of defense responses by overexpression of SARD1, but not of CBP60g, suggests that CBP60g, but not SARD1, requires activation by CaM.
Our data suggest that SA synthesis is activated through two parallel pathways (Fig. 7B), one dependent on SARD1 and the other dependent on CBP60g. Whereas the activity of CBP60g is most likely modulated by Ca2+, activation SARD1 at the transcription level by upstream regulators is sufficient to trigger downstream defense responses. One might ask why plants need two parallel pathways to activate SA synthesis. These two pathways might have evolved to respond to different stimuli. Another possibility is that plants use individually controlled pathways to fine-tune the timing and magnitude of SA synthesis for better control of SA-mediated defense responses.
In summary, we discovered two members of a plant-specific transcription factor family that regulate the expression of ICS1 and SA synthesis. Separate dissection of the SARD1- and CBP60g-dependent pathways might provide a more complete picture of how pathogen infections activate SA synthesis and suggest new strategies for engineering crop plants with improved pathogen resistance.
Materials and Methods
Plant Materials.
The sid2-1, eds5-3, npr1-1, eds1-2, and pad4-1 mutants used have been described previously (14, 20–22). Seeds of sard1-1 (SALK_138476), sard1-2 (SALK_052422), and cbp60g-1 (SALK_023199) were obtained from the Arabidopsis Stock Center. Construction of the plasmids used for generating the transgenic plants is described in SI Methods.
Plant Growth Conditions and Mutant Analysis.
Plants were grown at 23 °C under a 16-h light/8-h dark cycle in plant growth rooms or chambers. For infection with H.a. Noco2, spores at a concentration of 5 × 104 spores/mL were sprayed onto the plants, which were then maintained in a growth chamber with high humidity (>80%) at 18 °C under a 12-h light/12-h dark cycle for 1 wk. For gene expression analysis, RNA was extracted using Takara RNAiso reagent. Reverse transcription was carried out using the Takara M-MLV RTase cDNA synthesis kit. Real-time PCR was performed using Takara SYBR Premix Ex. The primers used to amplify ICS1 were 5′-gaactcaaatctcaacctcc-3′ and 5′-actgcgacgagagaagaaac-3′. The primers used for amplification of Actin1, PR-1, and PR-2 were described previously (12). SA was extracted and measured by HPLC as described previously (23).
EMSA.
Expression and purification of the SARD1 and SARD2 proteins from E. coli is described in SI Methods. The 181-bp DNA fragment used in EMSA was amplified by PCR using primers 7F and 7R (Table S1). The probe was end-labeled by incubating 10 pmol of double-stranded DNA in a 40-μL reaction with 20 units of polynucleotide kinase (New England Biolabs) and 40 μCi of [γ-32P]ATP. After labeling, the DNA was diluted to 100 μL total volume. Approximately 20 ng of the purified protein was mixed with 100 ng of poly[dI-dC], 1 μL of labeled probe (0.1 pmol per reaction), and 4 μL of 5× binding buffer [50 mM Hepes (pH 7.5), 375 mM KCl, 6.25 mM MgCl2, 25% glycerol, 1 mM DTT) in a 20-μL reaction. The mixture was incubated on ice for 30 min and then run on a 4% (wt/vol) native polyacrylamide gel in 0.5× TGE buffer (12.5 mM Tris, 95 mM glycine, 0.5 mM EDTA; pH 8.8). The gel was dried and autoradiographed after electrophoresis.
Supplementary Material
Acknowledgments
We thank Dr. Guangming He for help with ChIP experiments, Patrick Gannon and Dr. Jacqueline Monaghan for a critical reading of the manuscript, the Arabidopsis Stock Center for the T-DNA insertion mutants, Dr. Jane Parker (Max-Planck Institute for Plant Breeding Research, Cologne, Germany) for eds1-2 (Col), Dr. Christiane Nawrath (University of Lausanne, Switzerland) for eds5-3 and sid2-1, and Dr. Jane Glazebrook (University of Minnesota, MN) for pad4-1 mutant seeds. Financial support was provided by the Chinese Ministry of Science and Technology (Yuelin Zhang).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005225107/-/DCSupplemental.
References
- 1.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 2.Mishina TE, Zeier J. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 2007;50:500–513. doi: 10.1111/j.1365-313X.2007.03067.x. [DOI] [PubMed] [Google Scholar]
- 3.Vlot AC, Dempsey DA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 4.Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- 5.Métraux JP, et al. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen JB, Hammerschmidt R, Zook MN. Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv. syringae. Plant Physiol. 1991;97:1342–1347. doi: 10.1104/pp.97.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White RF. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–412. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- 8.Ward ER, et al. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell. 1991;3:1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Görlach J, et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell. 1996;8:629–643. doi: 10.1105/tpc.8.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaffney T, et al. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- 11.Dong X. NPR1, all things considered. Curr Opin Plant Biol. 2004;7:547–552. doi: 10.1016/j.pbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Tessaro MJ, Lassner M, Li X. Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell. 2003;15:2647–2653. doi: 10.1105/tpc.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mou Z, Fan W, Dong X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;113:935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 14.Nawrath C, Métraux JP. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell. 1999;11:1393–1404. doi: 10.1105/tpc.11.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, et al. ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell. 2009;21:2527–2540. doi: 10.1105/tpc.108.065193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao M, et al. Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe. 2009;6:34–44. doi: 10.1016/j.chom.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Reddy VS, Ali GS, Reddy AS. Genes encoding calmodulin-binding proteins in the Arabidopsis genome. J Biol Chem. 2002;277:9840–9852. doi: 10.1074/jbc.M111626200. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, et al. Arabidopsis CaM-binding protein CBP60g contributes to MAMP-induced SA accumulation and is involved in disease resistance against Pseudomonas syringae. PLoS Pathog. 2009;5:e1000301. doi: 10.1371/journal.ppat.1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glazebrook J, Rogers EE, Ausubel FM. Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics. 1996;143:973–982. doi: 10.1093/genetics/143.2.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker JE, et al. Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell. 1996;8:2033–2046. doi: 10.1105/tpc.8.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Zhang Y, Clarke JD, Li Y, Dong X. Identification and cloning of a negative regulator of systemic acquired resistance, SNI1, through a screen for suppressors of npr1-1. Cell. 1999;98:329–339. doi: 10.1016/s0092-8674(00)81962-5. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, et al. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell. 2007;19:731–749. doi: 10.1105/tpc.106.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.