Conventional vaccines, going back to the work of Jenner and Pasteur, are based on a rather direct mimicry of the offending pathogen using an attenuated or killed version of the microbe or purified or recombinant proteins from the microbe surface. These vaccines have been enormously successful against a range of pathogens. However, the conventional approaches have faltered for other pathogens, such as HIV, that have evolved an arsenal of molecular tricks to avoid immune responses. In such cases, alternate strategies are being investigated. The report by Ofek et al. (1) in PNAS describes a promising approach for presenting a vaccine target in the context of a protein or “scaffold” that lacks some of the defensive features of a pathogen such as HIV. The report does not deliver an HIV vaccine, but it takes an important step forward.
Nonconventional strategies for bacterial vaccine development are already on firm ground. “Reverse vaccinology,” in which the complete repertoire of bacterial surface antigens is determined, the ability of individual antigens to elicit immunity in animal models is investigated, and a combination of vaccine antigens is then chosen, has led to the successful development of a vaccine to serogroup B Neisseria meningitidis (2). This microbe is the most common cause of meningococcal disease in the developed world and has defied conventional vaccine approaches for decades. In the viral vaccine arena, the greatest problems are posed by the highly variable viruses, such as HIV and hepatitis C virus, and to a lesser extent, influenza virus. Typically, the immunodominant antibody responses to these viruses are directed to the most variable parts of the virus, but a vaccine should ideally elicit functional antibodies to conserved regions [broadly neutralizing antibodies (bNAbs)] that can protect against a wide spectrum of global circulating isolates. How can we design vaccines that elicit bNAbs? Fortunately, a subset of individuals infected with these viruses generally make bNAbs, and it is proposed that monoclonal versions of the bNAbs can provide valuable information to allow us to design vaccines that can “reelicit” the bNAbs in a “reverse engineering” strategy (3).
For HIV, a number of bNAbs have been described (4), one of which named 2F5 (5) has been shown to neutralize more than 50% of a large panel of global isolates and to protect against mucosal challenge in a macaque model (6). 2F5 recognizes a continuous epitope in a region of the HIV gp41 envelope surface protein close to the virus membrane, designated the membrane proximal external region (MPER) (7) that is conformationally flexible and assumes mostly helical conformations. However, crystallographic studies have been carried out with a range of peptides to suggest that the core 2F5 epitope adopts an extended kinked structure in complex with the antibody (8–10). A number of reports show that the 22-aa antibody H3 loop, which typically forms the heart of the antibody-combining site, does not contact the peptide epitope but is essential for virus recognition and neutralization (11–13). The H3 loop, which has considerable hydrophobic character at its apex, may contact the virus membrane, a region of the envelope glycoproteins distinct from the core epitope, or both; controversy surrounds the extent of any contact of 2F5 with the membrane and its designation as polyreactive (12–18).
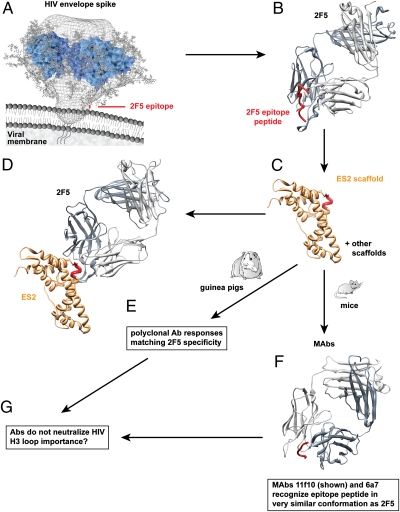
Ofek et al. (1) began their quest to reelicit 2F5-like antibodies by designing a series of epitope scaffolds using computational methods (Fig. 1). They searched the Protein Data Bank for structures that had exposed stretches of peptide sequence in a conformation similar to that of the 2F5 epitope and might therefore accept a 2F5 epitope transplant. Following some refinements, including the introduction of stabilizing substitutions, they came up with five scaffolds, designated ES1 to ES5, that would express the 2F5 epitope in the context of a graft. The scaffolds were then investigated in terms of affinity for 2F5, the results of which were typically and encouragingly in the nanomolar range, and in terms of the rigidity of the peptide epitope. The structure of a scaffold showing the highest affinity and rigidity, ES2, was determined to a resolution of 2.8 Å, and the 2F5 epitope graft was shown to be in a conformation relatively similar (Cα rmsd = 0.7 Å) to that of the peptide bound to 2F5. Even better structural correspondence (Cα rmsd ∼0.2 Å) was seen when the structure of a complex of ES2 and 2F5 was solved and compared with that of the epitope peptide bound to 2F5. Thus, the graft seemed to have “taken” in structural terms in the protein scaffold.
Fig. 1.
The epitope scaffolding strategy. (A) 2F5 is a broadly neutralizing anti-HIV antibody that recognizes a conserved continuous epitope close to the viral membrane on the glycoprotein gp41 of the surface envelope spike. (B) An epitope peptide adopts an extended kinked structure when bound to 2F5, as shown by crystallography. Grafting of the peptide into different scaffolding proteins selected from computational analyses identifies a number that present the epitope in the extended kinked conformation (C) and bind 2F5 tightly (D). (E) Immunization of small animals with scaffolds yields polyclonal antibody responses that match the specificity of 2F5 closely. (F) mAbs from immunized mice recognize the epitope peptide in the extended kinked conformation. (G) However, scaffold-elicited antibodies do not neutralize HIV, indicating that the scaffold design may need modification to induce antibody features (possibly the long H3 loop with hydrophobic character) to allow close approach of antibodies to the viral membrane.
The next step was to investigate the behavior of the scaffolds as immunogens. Guinea pigs were immunized with scaffolds, either singly or in combination. The strongest antibody responses to the graft were seen for those grafts showing the least rigidity, notably ES5. These responses were also among those that mapped most similarly to 2F5 when examined in terms of their reactivity with modified epitope peptides. In contrast, animals immunized with free or cyclized epitope peptides showed serum antibody reactivity profiles with modified peptides quite distinct from 2F5, indicating that the antibodies elicited were unlike 2F5 and that the free and cyclized peptides are inferior to the scaffolds as potential vaccine candidates.
Mice were then immunized either with scaffold ES5 or with ES5 followed by ES1, and mAbs were isolated. Two mAbs from the second immunization procedure showed liganded structures in which the epitope peptide was in a conformation remarkably similar to that in 2F5–peptide complexes. Further, the surfaces of the antibody combining sites in the two mAbs were chemically very similar to those of 2F5, although there were differences in some of the details. Perhaps the most significant difference between the two mAbs and 2F5 was the absence of a long H3 loop in the former.
The string of successes achieved by Ofek et al. (1) faltered at the last stage in that antibodies from scaffold immunization did not significantly neutralize HIV, indicating that the antibodies do not bind to the 2F5 epitope in the context of the virus. The most likely explanation is a failure to elicit antibodies with a long hydrophobic H3 loop. An alternative explanation is that the mode of binding of the antibodies to the core epitope differs somehow from that of 2F5, for example, in terms of the angle of epitope approach.
In a parallel study to that of Ofek et al. (1), Correia et al. (19) applied the epitope scaffolding approach to another well-characterized broadly neutralizing anti-MPER antibody designated 4E10 (7, 20). This antibody binds a continuous epitope even closer to the viral membrane than 2F5; like 2F5, it requires a relatively long H3 loop (18 aa), with hydrophobic residues at its apex, which a number of studies suggest interacts with the virus membrane and contributes to neutralization. The conformation of the core peptide epitope bound to 4E10 is largely helical (21). Epitope scaffolds were designed, some of which had extremely high affinities (picomolar) for 4E10, ≈1,000-fold higher than the peptide alone for 4E10. Crystallographic studies of both unliganded and 4E10-complexed scaffolds showed a high degree of structural mimicry of the 4E10–peptide complex. Immunization of rabbits with one of the scaffolds generated strong serum antibody responses to the graft, which has shown very low immunogenicity in other environments. The scaffold serum responses mapped much like 4E10 itself. However, as for Ofek et al. (1), the serum antibodies did not neutralize HIV, and, again, the difficulty may be associated with a requirement for a long H3 loop.
In conclusion, the studies described establish the principle that epitopes can be grafted into protein scaffolds and used as immunogens to elicit antibodies that closely resemble the mAbs that inspired scaffold design. The scaffolds are superior immunogens in many respects to other presentations containing the epitope sequences, including peptide conjugates. This is an important development for rational vaccine design. In the case of HIV for the MPER antibodies studied, there appears to be a major complication in that the epitopes recognized consist not only of the core peptide but additional viral surface contacts, which, for 4E10 at least, include the virus membrane. The challenge now for the MPER epitopes is to develop design strategies that induce long H3 loops with appropriate hydrophobic character as well as mimicking 2F5 or 4E10 recognition of the core peptide.
Footnotes
The author declares no conflict of interest.
See companion article on page 17880.
References
- 1.Ofek G, et al. Elicitation of structure-specific antibodies by epitope scaffolds. Proc Natl Acad Sci USA. 2010;107:17880–17887. doi: 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinaudo CD, Telford JL, Rappuoli R, Seib KL. Vaccinology in the genome era. J Clin Invest. 2009;119:2515–2525. doi: 10.1172/JCI38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 4.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 5.Muster T, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessell AJ, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zwick MB, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ofek G, et al. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its epitope. J Virol. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pai EF, Klein MH, Chong P, Pedyczak A. Fab′–epitope complex from the HIV-1 cross-neutralizing monoclonal antibody 2F5. World Intellectual Property Organization. Patent WO-00/61618. 2000 [Google Scholar]
- 10.Julien JP, Bryson S, Nieva JL, Pai EF. Structural details of HIV-1 recognition by the broadly neutralizing monoclonal antibody 2F5: Epitope conformation, antigen-recognition loop mobility, and anion-binding site. J Mol Biol. 2008;384:377–392. doi: 10.1016/j.jmb.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Zwick MB, et al. The long third complementarity-determining region of the heavy chain is important in the activity of the broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2F5. J Virol. 2004;78:3155–3161. doi: 10.1128/JVI.78.6.3155-3161.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julien JP, et al. Ablation of the complementarity-determining region H3 apex of the anti-HIV-1 broadly neutralizing antibody 2F5 abrogates neutralizing capacity without affecting core epitope binding. J Virol. 2010;84:4136–4147. doi: 10.1128/JVI.02357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ofek G, et al. Relationship between antibody 2F5 neutralization of HIV-1 and hydrophobicity of its heavy chain third complementarity-determining region. J Virol. 2010;84:2955–2962. doi: 10.1128/JVI.02257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes BF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 15.Scherer EM, Zwick MB, Teyton L, Burton DR. Difficulties in eliciting broadly neutralizing anti-HIV antibodies are not explained by cardiolipin autoreactivity. AIDS. 2007;21:2131–2139. doi: 10.1097/QAD.0b013e3282a4a632. [DOI] [PubMed] [Google Scholar]
- 16.Apellaniz B, et al. Confocal microscopy of giant vesicles supports the absence of HIV-1 neutralizing 2F5 antibody reactivity to plasma membrane phospholipids. FEBS Lett. 2010;584:1591–1596. doi: 10.1016/j.febslet.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Matyas GR, Beck Z, Karasavvas N, Alving CR. Lipid binding properties of 4E10, 2F5, and WR304 monoclonal antibodies that neutralize HIV-1. Biochim Biophys Acta. 2009;1788:660–665. doi: 10.1016/j.bbamem.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Song L, et al. Broadly neutralizing anti-HIV-1 antibodies disrupt a hinge-related function of gp41 at the membrane interface. Proc Natl Acad Sci USA. 2009;106:9057–9062. doi: 10.1073/pnas.0901474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correia BE, et al. Computational design of epitope-scaffolds allows induction of antibodies specific for a poorly immunogenic HIV vaccine epitope. Structure. 2010;18:1116–1126. doi: 10.1016/j.str.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Stiegler G, et al. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2001;17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso RM, et al. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]