Abstract
Sleep and wakefulness are regulated primarily by inhibitory interactions between the hypothalamus and brainstem. The expression of the states of rapid eye movement (REM) sleep and non-REM (NREM) sleep also are correlated with the activity of groups of REM-off and REM-on neurons in the dorsal brainstem. However, the contribution of ventral brainstem nuclei to sleep regulation has been little characterized to date. Here we examined sleep and wakefulness in mice deficient in a homeobox transcription factor, Goosecoid-like (Gscl), which is one of the genes deleted in DiGeorge syndrome or 22q11 deletion syndrome. The expression of Gscl is restricted to the interpeduncular nucleus (IP) in the ventral region of the midbrain–hindbrain transition. The IP has reciprocal connections with several cell groups implicated in sleep/wakefulness regulation. Although Gscl−/− mice have apparently normal anatomy and connections of the IP, they exhibited a reduced total time spent in REM sleep and fewer REM sleep episodes. In addition, Gscl−/− mice showed reduced theta power during REM sleep and increased arousability during REM sleep. Gscl−/− mice also lacked the expression of DiGeorge syndrome critical region 14 (Dgcr14) in the IP. These results indicate that the absence of Gscl and Dgcr14 in the IP results in altered regulation of REM sleep.
Keywords: homeobox transcription factor, mouse behavior, ventral brainstem
In vertebrates and invertebrates, sleep is defined behaviorally as a reversible quiescence which is regulated in a circadian and homeostatic manner, accompanied by an increased threshold to respond to external stimuli (1). In mammals and birds, sleep is classified further into rapid eye movement (REM) sleep and non-REM (NREM) sleep based on specific brain-activity patterns and muscle tonus detected by electroencephalography/electromyography (EEG/EMG). In rodents, NREM sleep is defined by high-amplitude, low-frequency waves on the EEG, typified by the presence of the 1- to 4-Hz (i.e., delta) frequencies. In contrast, REM sleep is characterized by power in the 6- to 12-Hz (i.e., theta) frequency band, which is derived primarily from hippocampal activity, combined with a loss of skeletal muscle tone. Switching between the sleeping and wakeful states is regulated primarily by inhibitory interactions between the hypothalamus and brainstem (2, 3). Switching between NREM and REM states is regulated further by inhibitory interactions between populations of neurons in the brainstem (3, 4). Although dopaminergic neurons in the ventral midbrainstem have been implicated in regulating sleep and wakefulness (5), the role of the ventral brainstem in sleep regulation has not been as well studied as the role of the dorsal brainstem.
It has been reported that lesions of the bilateral fasciculus retroflexus, a major input to the interpeduncular nucleus (IP), result in reduced REM sleep time (6, 7). The IP is located on the midline in the ventral region of the midbrain–hindbrain transition and is evolutionarily conserved from fish to mammals. It has reciprocal connections with the median raphe nucleus (MnR), dorsal raphe nucleus (DRN), laterodorsal tegmental nucleus (LDTg), and nucleus incertus (NI) (8–13), which are implicated in the regulation of sleep and wakefulness and the generation of hippocampal theta waves (2, 3, 14, 15). In addition, the IP receives input from the basal forebrain via the fasciculus retroflexus directly or relayed at the medial habenular nucleus. In turn, the IP innervates the basal forebrain (16). Because the basal forebrain is known to regulate the vigilance state, this reciprocal pattern of innervation also supports a potential role of the IP in sleep mechanisms.
However, no studies to date have examined whether the IP is involved in sleep, in part because the size and position of the IP make it difficult to lesion the IP or inject it locally without damaging bilateral dorsal brainstem nuclei and fibers of passage. A recent comprehensive approach to gene expression in the mouse brain revealed that a homeobox transcription factor Goosecoid-like (Gscl), also known as “Gsc2,” has an expression pattern restricted to the IP (17). Gscl is one of the genes deleted in patients who have DiGeorge syndrome or 22q11 deletion syndrome, who have a variety of psychiatric symptoms (18). We thus examined sleep/wakefulness parameters in Gscl−/− mice (19) under baseline conditions and also studied REM sleep rebound after REM sleep deprivation and the sensory threshold to arousal during sleep in these mice.
Results
Gscl Expression Is Restricted to the IP.
We examined the expression pattern of Gscl mRNA at different developmental stages. In the adult brain, Gscl mRNA is expressed exclusively in the caudal (IPc) and lateral (IPl) subnuclei of IP (Fig. 1 A and B). During embryonic development, the expression of Gscl mRNA is restricted to the developing ventral midbrain/pons transitional region, a future IP region (Fig. 1 C and D), as reported previously (17, 20). Loss of Gscl does not alter subnuclear structures in the Nissl-stained IP, and there is no difference in position and proportion between Gscl-positive and Gscl-negative subnuclei.
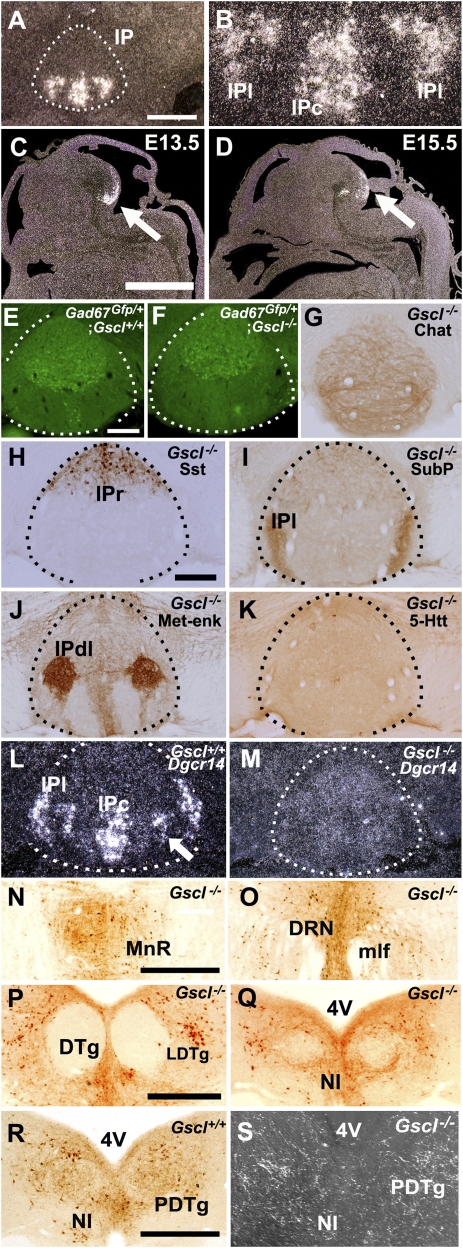
Fig. 1.
Normal anatomical structure of the IP of a Gscl-deficient mouse. (A) Gscl mRNA expression is restricted to the IP (delineated by broken lines). (B) High-magnification view of A shows that Gscl mRNA is expressed in the IPc and IPl. (C and D) During the embryonic stage, Gscl mRNA expression is restricted to the developing ventral midbrain/pons transition (arrow). (E and F) Both Gscl+/+; Gad67Gfp/+ and Gscl−/−; Gad67Gfp/+ mice have diffuse and moderate GFP expression in the entire IP (delineated by broken lines) with strong expression in the rostral subnucleus. (G–K) The IP of Gscl−/− mice exhibits immunoreactivities for ChAT (G), somatostatin (Sst) (H), substance P (SubP) (I), Met-enkephalin (Met-enk) (J), and 5-HT transporter (5-HTT) (K). (L) Gscl+/+ mice have marked expression of Dgcr14 in the IPc, IPl, and part of the intermediate subnucleus (arrow). (M) Gscl−/− mice did not show increased expression of Dgcr14 mRNA in the IP subnucleus. (N–R) Retrograde tracing from the IP after injection of a retrograde tracer, cholera toxin B, in the IPl. Labeled fibers and cells were recognized in the MnR (N), DRN (O), LDTg (P), and NI (Q) of Gscl−/− mice and in the NI of Gscl+/+ mice (R). (S) Injection of an anterograde tracer, AAV-GFP, in the IPl revealed GFP-positive fibers in the PDTg and NI of Gscl−/− mice. mlf, medial longitudinal fasciculus. (Scale bars: 300 μm in A, C, N, P, and R; 150 μm in E and H.)
IP neurons contain several inhibitory neurotransmitters, including GABA, somatostatin, and substance P, and the IP receives projections of cholinergic, serotonergic, and substance P-containing fibers (9, 16, 21). We examined whether the loss of Gscl alters the neurochemical characteristics of the IP neurons and the input fibers. When Gscl+/− mice were crossed with the Gad67-Gfp knock-in line (22), Gscl−/−; Gad67Gfp/+ mice showed diffuse and moderate GFP expression in the entire IP with strong expression in the rostral subnucleus, similar to Gscl+/+; Gad67Gfp/+ mice (Fig. 1 E and F). Consistent with the previous reports on wild-type mice (9, 16, 21), both Gscl−/− and Gscl+/+ mice showed (i) diffuse choline acetyltransferase (ChAT) immunoreactivity in the IP (Fig. 1G); (ii) strong somatostatin immunoreactivity in the rostral and apical subnuclei (Fig. 1H); (iii) moderate substance P immunoreactivity in the IP with prominent immunoreactivity in the lateral subnucleus (Fig. 1I); (iv) Met-enkephalin immunoreactivity strongly in the dorsolateral subnucleus and moderately in the rostral and caudal subnuclei (Fig. 1J); and (v) diffuse serotonin (5-HT) transporter immunoreactivity in the entire IP with scattered strong immunoreactive cells (Fig. 1K). Loss of Gscl had no appreciable effects on Gad67-Gfp expression, or immunoreactivity for ChAT, somatostatin, substance P, and 5-HT transporter outside the IP. We also examined the expression of DiGeorge syndrome critical region 14 (Dgcr14, also known as “Es2”) mRNA, a gene adjacent to Gscl on both the human and mouse chromosomes. In the Gscl+/+ mouse brain, Dgcr14 mRNA was strongly expressed in the IPc, IPl, and a part of the intermediate subnuclei (Fig. 1I), similar to the expression pattern of Gscl mRNA in the IP (Fig. 1B). Gscl−/− mice, however, lacked the expression of Dgcr14 mRNA in the IP (Fig. 1J). In contrast to Gscl mRNA, Dgcr14 mRNA showed a diffuse and weak expression pattern in the entire brain of wild-type mice; this diffuse expression was conserved in Gscl−/− mice.
To examine whether loss of Gscl affected fiber connections to the IP, we injected a retrograde tracer, cholera toxin B, into the lateral subnucleus of the IP. Labeled cells were recognized in the MnR (Fig. 1K), DRN (Fig. 1L), LDTg (Fig. 1M), NI (Fig. 1N), median septal nucleus, nucleus of the diagonal band, lateral hypothalamus, supramammillary nucleus, and medial habenular nucleus of Gscl−/− mice. These nuclei were the same as those previously described in wild-type mice (Fig. 1O) (8–10, 12, 13, 23). Injection of an anterograde tracer, an adeno-associated viral vector containing the gene for GFP (AAV-GFP), in the IPl of Gscl−/− and Gscl+/+ mice showed dense efferent fibers throughout pontine midline structures, including the MnR, DRN, LDTg, NI, and posterodorsal tegmental nucleus (PDTg) (Fig. 1P), as previously described (8, 9, 11). Thus, we found no apparent differences between the two genotypes in the afferent and efferent fiber connections to and from the IP, although there were small differences in the number of labeled cells and fibers among all tracer-injected brains because of inevitable differences in the exact locations and amounts of tracer injected.
Gscl-Deficient Mice Show Reduced REM Sleep Time.
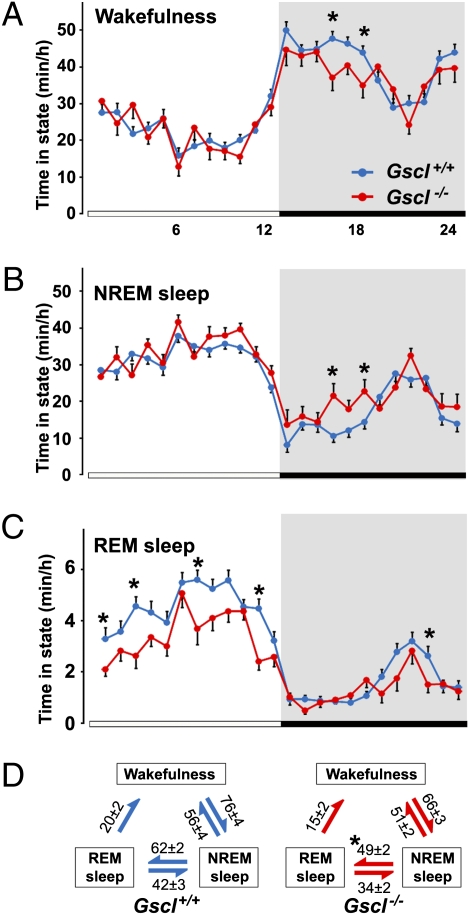
Gscl−/− mice exhibited a decrease in both total time and episode frequency of REM sleep during the light period and over 24 h when compared with Gscl+/+ mice (Fig. 2 and Table 1). However, no significant difference was noted in the duration of REM sleep episodes in Gscl−/− and Gscl+/+ mice (Table 1). REM sleep latency was increased during the light period and over 24 h in Gscl−/− mice. In addition to a slight but significant increase in total NREM sleep time, Gscl−/− mice exhibited a longer mean duration and reduced frequency of NREM sleep episodes when compared with wild-type mice during the light period and over 24 h (Table 1). This observation indicates that the NREM sleep phase is more consolidated in the light period in Gscl−/− mice than in Gscl+/+ mice. Wakefulness time and mean episode duration were similar in Gscl−/− and wild-type mice, although we noted a tendency toward shorter total wakefulness time during the dark period in Gscl−/− mice (Fig. 2 and Table 1). Importantly, the number of transitions from NREM sleep to REM sleep was reduced selectively in Gscl−/− mice (Fig. 2D). This finding is consistent with a reduced number of REM sleep episodes, a shorter total REM sleep time, and longer duration of NREM sleep episodes. In other words, Gscl−/− mice tend to “skip” REM sleep episodes during NREM sleep.
Fig. 2.
Sleep and wakefulness in Gscl-deficient mice. (A–C) Circadian variation in wakefulness, NREM sleep, and REM sleep in Gscl+/+ (n = 12) and Gscl−/− mice (n = 6). Data (mean ± SEM) are expressed as minutes per hour spent in each stage, averaged from EEG/EMG recordings during three consecutive 24-h periods. (D) Values indicate the number (mean ± SEM) of transitions between wakefulness, NREM sleep, and REM sleep per 24 h. Gscl−/− mice (Right) showed reduced transitions from NREM sleep to REM sleep compared with Gscl+/+ mice (Left). Data (mean + SEM) were subjected to ANOVA with repeated measurements followed by the Tukey post hoc test. *P < 0.05.
Table 1.
Sleep/wakefulness parameters
| Wakefulness |
NREM sleep |
REM sleep |
|||||||
| Period | Gscl+/+ | Gscl−/− | P | Gscl+/+ | Gscl−/− | P | Gscl+/+ | Gscl−/− | P |
| 24 h | |||||||||
| Time (min) | 764 ± 11 | 730 ± 20 | 0.071 | 596 ± 10 | 649 ± 19 | 0.011 | 79.6 ± 2.6 | 61.0 ± 2.3 | <0.0001 |
| Duration (s) | 686 ± 46 | 689 ± 42 | 0.965 | 318 ± 11 | 400 ± 17 | 0.0002 | 77.6 ± 1.8 | 74.5 ± 2.1 | 0.303 |
| Frequency (episode/h) | 2.36 ± 0.14 | 1.84 ± 0.08 | 0.003 | 4.01 ± 0.15 | 3.17 ± 0.07 | <0.0001 | 2.17 ± 0.08 | 1.63 ± 0.06 | <0.0001 |
| REM sleep latency (min) | 8.13 ± 0.25 | 10.8 ± 0.43 | <0.0001 | ||||||
| 12-h light period | |||||||||
| Time (min) | 275 ± 8.0 | 273 ± 5.9 | 0.88 | 385 ± 6.4 | 402 ± 6.0 | 0.066 | 60.0 ± 2.6 | 44.7 ± 2.0 | <0.0001 |
| Duration (s) | 519 ± 39 | 579 ± 41 | 0.35 | 325 ± 12 | 430 ± 22 | 0.0001 | 81.6 ± 2.3 | 79.6 ± 2.6 | 0.602 |
| Frequency (episode/h) | 2.36 ± 0.13 | 1.79 ± 0.10 | 0.001 | 4.99 ± 0.16 | 3.74 ± 0.12 | <0.0001 | 3.16 ± 0.15 | 2.26 ± 0.09 | <0.0001 |
| REM sleep latency (min) | 8.17 ± 0.28 | 10.8 ± 0.46 | <0.0001 | ||||||
| 12-h dark period | |||||||||
| Time (min) | 490 ± 9.0 | 457 ± 16 | 0.054 | 210 ± 8.8 | 247 ± 15 | 0.032 | 19.5 ± 0.99 | 16.4 ± 1.1 | 0.059 |
| Duration (s) | 981 ± 91 | 850 ± 67 | 0.26 | 311 ± 14 | 366 ± 13 | 0.009 | 70.8 ± 2.1 | 65.5 ± 2.0 | 0.085 |
| Frequency (episode/h) | 2.34 ± 0.18 | 1.93 ± 0.12 | 0.075 | 3.02 ± 0.19 | 2.59 ± 0.15 | 0.087 | 1.17 ± 0.06 | 0.98 ± 0.07 | 0.065 |
| REM sleep latency (min) | 8.11 ± 0.29 | 11.0 ± 0.61 | 0.0003 | ||||||
Data are expressed as mean ± SEM for Gscl+/+ mice (n = 12) and Gscl−/− mice (n = 6). All parameters were derived from EEG/EMG recordings for three consecutive 24- h periods. Statistical comparisons are by Student's t test. Significant changes (P < 0.05) are shown in bold type.
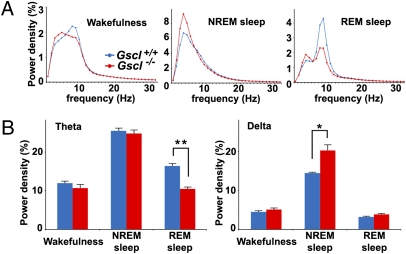
Reduced Theta Power in Gscl-Deficient Mice.
EEG spectral analysis of Gscl+/+ and Gscl−/− mice during wakefulness, NREM sleep, and REM sleep revealed that EEG power density in the theta frequency range (6–12 Hz) during REM sleep was reduced significantly in Gscl−/− mice compared with Gscl+/+ mice (P = 0.002) (Fig. 3). In addition, we noted that EEG power density in the delta frequency range (1–4 Hz) during NREM sleep was greater in Gscl−/− mice than in Gscl+/+ mice (P = 0.03) (Fig. 3).
Fig. 3.
EEG spectral analysis of Gscl−/− mice. (A) EEG spectral profiles of Gscl+/+ mice (blue line, n = 12) and Gscl−/− mice (red line, n = 6) during wakefulness (Left), NREM sleep (Center), and REM sleep (Right). The average EEG spectra were normalized to total EEG power from 1–32 Hz in 1-Hz bins. (B) Gscl−/− mice (red bar) exhibited a reduced power density in the theta frequency band (Left) during REM sleep and a greater power density in the delta frequency band (Right) during NREM sleep, when compared with Gscl+/+ mice (blue bar). Data (mean + SEM) were analyzed with ANOVA followed by the Tukey post hoc test. *P < 0.05; **P < 0.005.
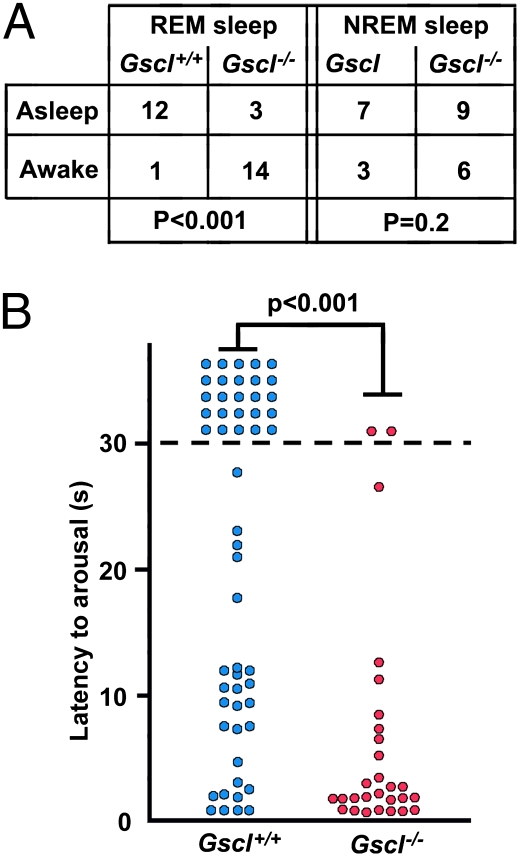
Increased Arousability During REM Sleep in Gscl-Deficient Mice.
While making vigilance-state recordings in Gscl−/− mice, we observed that Gscl−/− mice seemed excessively sensitive to external stimuli during sleep. To examine the arousability of Gscl−/− mice, we tested their arousal threshold during REM and NREM sleep using acoustic stimuli. In 14 of 17 trials during REM sleep, Gscl−/− mice (n = 4) were awakened in response to a standardized acoustic stimulus, but Gscl+/+ mice (n = 5) remained asleep in 12 of 13 trials (P < 0.001; Fig. 4A). In contrast, there was no significant difference in the arousal response to acoustic stimuli during NREM sleep (P = 0.2). To confirm this finding with a different modality of stimuli, we measured the time to awaken in response to combined acoustic, olfactory, and visual stimuli caused by moving a Latex glove close to a mouse. Gscl−/− mice had significantly shorter latencies to awake than Gscl+/+ mice during REM sleep (Fig. 4B).
Fig. 4.
Arousal response to stimuli during sleep. (A) (Left) During REM sleep, Gscl−/− mice (n = 4) tended to be awakened in response to acoustic stimuli, but Gscl+/+ mice (n = 5) remained asleep (χ2 test; P < 0.001). (Right) There was no significant difference between Gscl−/− mice and Gscl+/+ mice in the arousal response to an approaching object during NREM sleep (P = 0.2). Numbers in the table denote the number of stimulus trials. (B) The latency of Gscl−/− mice (n = 4) in response to an approaching object during REM sleep was shorter than that of Gscl+/+ mice (n = 5) (Mann–Whitney's u test, P < 0.001). Circles represent individual trials.
Reduced REM Sleep Rebound in Gscl-Deficient Mice.
To examine the homeostatic regulation of REM sleep, Gscl−/− mice were deprived of REM sleep from Zeitgeber time (ZT)6 to ZT12, and their REM sleep time then was examined from ZT12 to ZT24, when there was no significant difference between Gscl+/+ and Gscl−/− mice in baseline REM sleep time (Fig. 2C). After REM sleep deprivation, both Gscl+/+ and Gscl−/− mice spent longer in REM sleep than under baseline conditions (Fig. 5A), but both the extent and duration of the REM sleep rebound were less in Gscl−/− mice than Gscl+/+ mice. REM sleep deprivation did not affect NREM sleep time in either genotype (Fig. 5B).
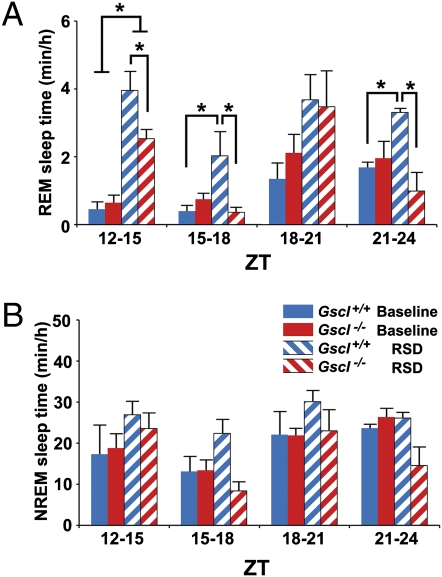
Fig. 5.
REM sleep rebound after REM sleep deprivation. (A) After 6 h of REM sleep deprivation (RSD) from ZT6–12, the time spent in REM sleep is displayed for each 3-h period during the recovery phase from ZT12–24. Both Gscl+/+ mice (n = 5) and Gscl−/− mice (n = 4) spent increased time in REM sleep from ZT12–15 compared with baseline. Gscl−/− mice exhibited a shorter REM sleep time than Gscl+/+ mice during ZT12–15, ZT15–18, and ZT21–24. (B) The time spent in NREM sleep after 6 h of RSD for each 3-h period from ZT 12–24. RSD did not alter NREM time in either Gscl+/+ or Gscl−/− mice. Data (mean ± SEM) were analyzed with ANOVA followed by the Tukey post hoc test. *P < 0.05.
Discussion
The present study has shown that Gscl−/− mice spend less time in REM sleep, express fewer REM sleep episodes, and have fewer transitions from NREM sleep to REM sleep. Furthermore, these mice have reduced theta power and increased arousability during REM sleep. In view of the restricted expression of Gscl to the IP combined with a specific loss of expression of Dgcr14 in the IP of Gscl−/− mice, these results indicate that the normal function of the IP is required for REM sleep regulation.
Although Gscl−/− mice showed reduced theta power, the EEG pattern of REM sleep still was clearly different from that of NREM sleep and of wakefulness. Moreover, we staged REM sleep based on both the appearance of theta wave and loss of muscle tone. Thus, it is unlikely that shorter total time of REM sleep or reduced REM sleep rebound of Gscl−/− mice resulted from a misscoring of REM sleep.
The IP is located at the ventral region of the midbrain–hindbrain transition and has afferent and efferent connections with the basal forebrain and brainstem. These connections suggest that the IP may function as an interface between the basal forebrain and brainstem in the modulation of brain function and behavior. Although the functional role of the IP remains unknown (16), several findings have suggested that the IP may be associated with sleep and wakefulness. Unlike most brain regions, glucose utilization in the IP is increased during REM sleep as well as under anesthesia (24–26). Moreover, bilateral lesions of the fasciculus retroflexus, the major afferent path from the IP, decrease the time spent in REM sleep (6, 7). To date, however, no report has directly examined the role of the IP in sleep mechanisms, primarily because research has tended to focus on the dorsal region of the brainstem (2–4) and because surgical procedures targeting the IP inevitably damage bilateral dorsal brainstem nuclei as well as fibers connecting the hypothalamus with the brainstem nuclei.
Among IP subnuclei, Gscl and Dgcr14 are expressed mainly in the IPc and IPl structures. These subnuclei send efferent fibers containing serotonergic REM-off neurons to the MnR and DRN and efferent fibers containing cholinergic REM-on neurons to the LDTg (3, 9, 11, 27). In addition, the IPc and IPl subnuclei send efferent fibers to the NI (8, 9, 11), which relays ascending projections from the nucleus pontis oralis to the medial septal nucleus, a pathway implicated in hippocampal theta generation (14). Moreover, the IP sends a small number of efferent fibers to the hippocampus and medial septal nucleus (9, 11). These connections provide an anatomical basis for the IP as a regulator of hippocampal theta and REM sleep.
Another interesting phenotype of Gscl−/− mice is increased arousability, specifically during REM sleep, in response to external stimuli. The elevated arousability is unlikely to be caused by disturbed peripheral sensory processing or increased anxiety, because Gscl−/− mice respond normally to acoustic or visual stimuli during NREM sleep and wakefulness and show normal anxiety behavior (28). External stimuli may activate wake-promoting neurons in the brainstem to switch from sleep to wakefulness (2, 29). Although the increased arousability of Gscl−/− mice suggests an altered regulation of wake-promoting neurons in response to sensory stimuli, further studies are needed to elucidate the detailed mechanisms.
Gscl−/− mice exhibited an REM sleep rebound after deprivation, but the magnitude and duration of the rebound was smaller than in wild-type mice. Because Gscl−/− mice spend less time in REM sleep than wild-type mice under baseline conditions, the reduced REM sleep rebound may result from a smaller need for REM sleep in Gscl−/− mice after 6 h of REM sleep deprivation. However, it also is possible that the mechanisms of REM sleep rebound per se are affected in the knock-out mice.
The accentuated expression in the IP of Dgcr14 was absent in Gscl−/− mice, in which the entire Gscl gene was replaced with the puromycin resistance and hygromycin resistance genes (19). Another strain of Gscl−/− mice, in which the entire Gscl gene was replaced with the neomycin resistance gene, also showed a loss of Dgcr14 expression in the IP (30). Dgcr14 is located only 2 kb downstream of Gscl, with the same transcription direction, suggesting that Gscl contains a cis regulatory element required for the high expression of Dgcr14 in IP subnuclei. Downstream of Dgcr14 but in the opposite transcription direction are two genes, Testis-specific serine kinase 1 (Tssk1) and Tssk2, that are expressed in the brain only in the piriform cortex [ref. 18 and Allen Brain Atlas (http://mouse.brain-map.org/)]. Hence, loss of Gscl and Dgcr14 expression in the IP may be sufficient to cause the altered regulation of sleep/wakefulness behavior in Gscl−/− mice. Gscl is a paralogue of goosecoid and a homeobox transcription factor that recognizes a specific DNA sequence (31) and interacts with ring finger protein 4 (32). In addition, Dgcr14 is a nuclear protein with a coiled-coil domain (33). These findings suggest that loss of Gscl and Dgcr14 may alter gene- or protein-expression profiles in the IPc and IPl subnuclei, resulting in a functional abnormality.
Gscl and Dgcr14 are among the genes deleted in most individuals with DiGeorge syndrome or 22q11 deletion syndrome (20, 33). These patients have multiple neuropsychiatric symptoms and are susceptible to schizophrenia (18, 34, 35). Moreover, it has been reported that polymorphisms of DGCR14 are significantly associated with schizophrenia (36). Interestingly, Df(16)A+/− mice with a microdeletion including the Gscl and Dgcr14 genes showed reduced synchrony of hippocampal theta with the neuronal activity of the prefrontal cortex (37). Together with these findings, the present results suggest that loss of Gscl and Dgcr14 affects the regulation of hippocampal theta and REM sleep, possibly contributing to the psychiatric symptoms frequently seen in patients who have 22q11 syndrome.
Materials and Methods
Animals.
Gscl−/− mice and littermate Gscl+/+ mice were derived from Gscl+/− parents that were backcrossed for more than six generations to the C57BL/6J strain (19). Gad67Gfp/+ mice were previously described (22) and crossed to the Gscl+/− line. Mice were provided food and water ad libitum, maintained on a 12-h light/dark cycle at all times, and were under controlled temperature and humidity conditions. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center at Dallas and were carried out in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Genotypes were determined by the amplification of genomic DNA by PCR.
EEG/EMG Electrode Implantation.
For chronic EEG/EMG monitoring, 12- to 14-wk-old Gscl−/− and wild-type male mice were anesthetized with 40 mg/kg ketamine and 4 mg/kg xylazine, and the cranium was exposed. Four electrode pins were lowered to the dura under stereotaxic control, and two flexible wires for EMG recording were inserted in the neck muscle and then attached to the skull with dental cement. The electrodes for EEG signals were positioned over the frontal and occipital cortices [anteroposterior (AP): 0.5 mm, mediolateral (ML): 1.3 mm, dorsoventral (DV): −1.3 mm; and AP: −4.5 mm, ML: 1.3 mm, DV: −1.3 mm]. After recovery from anesthesia, the mice were housed individually and tethered to a counterbalanced arm (Instech Laboratories) that allowed the free movement and exerted minimal weight. All mice were allowed 14 d of recovery from surgery and habituation to the recording conditions.
EEG/EMG Analysis.
EEG/EMG signal was recorded continuously for three consecutive 24-h periods. EEG/EMG signals were amplified using a Grass Model 78 (Grass Instruments), filtered (EEG: 0.3–300 Hz; EMG: 30–300 Hz), digitized at a sampling rate of 250 Hz, and displayed using custom polygraph software. The vigilance state in each 20-s epoch was classified as NREM sleep, REM sleep, or wakefulness by visual inspection of the EEG and EMG signals by two independent observers blinded as to genotype. Total time spent in wakefulness, NREM, and REM sleep was derived by summing the total number of 20-s epochs in each state. Mean episode durations were determined by dividing the total time spent in each state by the number of episodes of that state. Mean REM sleep latency was determined by averaging the time elapsed from the beginning of a continuous NREM sleep episode to the beginning of the subsequent REM sleep episode. Epochs containing movement artifacts were included in the state totals but excluded from subsequent spectral analysis. EEG signals were subjected to a fast Fourier transform analysis from 1 to 32 Hz with a 1-Hz bin using MatLab (MathWorks). EEG power density in each frequency bin was expressed as a percentage of the mean total EEG power over all frequency bins and vigilance states.
Arousability Test During REM and NREM Sleep.
EEG/EMG-implanted 14- to 15-wk-old male mice were tested during the light period (ZT 6–10) in a cage equipped with a speaker. An experimenter monitored EEG/EMG signals in a room adjacent to the recording room. An 8-kHz, 70-dB, 500-ms pulse of a sinusoidal tone was delivered during NREM and REM sleep episodes. The number of trials in which mice reacted to the sound, as seen in robust EMG signals, were counted over the total number of trials. A similar study used a Latex glove attached to the end of a long metal rod as the external stimulus during videotape recording. Ten seconds after the onset of REM sleep under continuous EEG/EMG monitoring, an experimenter gently moved a glove from a distance of 3 m from the mouse to a distance of 5 cm. The latency to awaken was scored in real time from the onset of stimulus to apparent wakefulness as indicated by the EEG/EMG signals. All experiments were conducted by an experimenter who was blinded as to mouse genotype.
REM Sleep Deprivation.
REM sleep deprivation was conducted for 6 h in the second half of the light period (ZT 6–12) by gentle handling under EEG/EMG monitoring. A REM transition was defined by the reduction of slow-wave amplitude and the appearance of theta wave intermixed with slow waves on the EEG, combined with diminishing EMG tonus. After REM sleep deprivation, the mice were kept in the same experimental cages with continuous recording of the EEG/EMG for a further 24 h. The vigilance state data during the recovery period were compared with baseline data recorded during the period before the deprivation procedure.
In Situ Hybridization and Histological Examinations.
Animals were deeply anesthetized with ketamine and xylazine and then perfused with PBS, followed by 4% paraformaldehyde. Brains or embryos were removed, postfixed overnight in 4% paraformaldehyde, and then equilibrated in 20% sucrose for 2 d. The brains and embryos then were sectioned on a freezing microtome at 35 μm and mounted on Matsunami Adhesive Silane-coated slide glass (Matsunami Glass). The sections were hybridized in situ to 35S-labeled Gscl or Dgcr14 sense and antisense probes synthesized from pGEM-T Easy (Promega) containing the sequence of Gscl or Dgcr14 mRNA, using a Maxiscript kit (Ambion) in the presence of 35S-CTP (Amersham). The slides were developed in Kodak D-19 and counterstained using Nissl stain. After fixation and sectioning of Gscl+/+; Gad67Gfp/+ and Gscl−/−; Gad67Gfp/+ brains as above, GFP fluorescence was observed under the fluorescence microscope. Immunohistochemistry was performed using a free-floating method. The brain sections were incubated with antibodies for ChAT (goat polyclonal, AB144; Millipore), somatostatin (rabbit polyclonal, AB5494; Millipore), substance P (rabbit polyclonal, AB1566; Millipore), Met-enkephalin (rabbit polyclonal, AB5026; Millipore), and 5-HT transporter (rabbit polyclonal, ab44520; Abcam) followed by incubation with biotinylated anti-rabbit or goat IgG, and then incubated in avidin–biotin–HRP conjugate (Vector). Positive immunoreactivity was visualized using 3,3′-diaminobenzidine (DAB).
Tracer Injection.
Under anesthesia 12- to 14-wk-old Gscl−/− and wild-type male mice were placed in a stereotaxic apparatus, and a fine glass pipette was positioned in the lateral subnucleus at coordinates (AP: −3.5 mm, ML: 0.3 mm, DV: 4.8 mm) according to a mouse brain atlas (38). After the injection of tracer, the pipette was withdrawn slowly, and the incision was closed with sutures. The mouse survived for 7 d before being perfused with 4% paraformaldehyde. The brain then was processed for immunostaining.
As a retrograde tracer, 200 nL of 1% cholera toxin B (List Biotechnological Labs) was injected. Immunostaining was performed using anti-goat cholera toxin B antibody (List Biotechnological Labs) and DAB. As an anterograde tracer, 50–100 nL of AAV-GFP (Harvard Gene Therapy Initiative Research Vector Core Facility) was used. Brain sections were incubated with rabbit anti-GFP antibody (Molecular Probes). GFP-positive fibers were visualized with DAB and observed under dark-field microscopy.
Acknowledgments
We thank Y. Yanagawa (Gunma University, Maebashi, Japan) for providing Gad67 knock-in mice; C. Lee, S. Dixon, R. Floyd, and M. Thornton for technical support; T. Motoike, Y. Ikeda, H. Kumagai, I. Chang, A. Chang, S. Ogawa, J. Long, and A. Tsai for critical discussions and manuscript review. M.Y. is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by the Perot Family Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Hendricks JC, Sehgal A, Pack AI. The need for a simple animal model to understand sleep. Prog Neurobiol. 2000;61:339–351. doi: 10.1016/s0301-0082(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 2.Saper CB, Chou TC, Scammell TE. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 3.Pace-Schott EF, Hobson JA. The neurobiology of sleep: Genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 4.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 5.Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11:113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Haun F, Eckenrode TC, Murray M. Habenula and thalamus cell transplants restore normal sleep behaviors disrupted by denervation of the interpeduncular nucleus. J Neurosci. 1992;12:3282–3290. doi: 10.1523/JNEUROSCI.12-08-03282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valjakka A, et al. The fasciculus retroflexus controls the integrity of REM sleep by supporting the generation of hippocampal theta rhythm and rapid eye movements in rats. Brain Res Bull. 1998;47:171–184. doi: 10.1016/s0361-9230(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 8.Goto M, Swanson LW, Canteras NS. Connections of the nucleus incertus. J Comp Neurol. 2001;438:86–122. doi: 10.1002/cne.1303. [DOI] [PubMed] [Google Scholar]
- 9.Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J Comp Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- 10.Olucha-Bordonau FE, et al. Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J Comp Neurol. 2003;464:62–97. doi: 10.1002/cne.10774. [DOI] [PubMed] [Google Scholar]
- 11.Shibata H, Suzuki T. Efferent projections of the interpeduncular complex in the rat, with special reference to its subnuclei: A retrograde horseradish peroxidase study. Brain Res. 1984;296:345–349. doi: 10.1016/0006-8993(84)90071-4. [DOI] [PubMed] [Google Scholar]
- 12.Shibata H, Suzuki T, Matsushita M. Afferent projections to the interpeduncular nucleus in the rat, as studied by retrograde and anterograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. J Comp Neurol. 1986;248:272–284. doi: 10.1002/cne.902480210. [DOI] [PubMed] [Google Scholar]
- 13.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- 14.Nuñez A, Cervera-Ferri A, Olucha-Bordonau F, Ruiz-Torner A, Teruel V. Nucleus incertus contribution to hippocampal theta rhythm generation. Eur J Neurosci. 2006;23:2731–2738. doi: 10.1111/j.1460-9568.2006.04797.x. [DOI] [PubMed] [Google Scholar]
- 15.Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- 16.Klemm WR. Habenular and interpeduncularis nuclei: Shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–RA273. [PubMed] [Google Scholar]
- 17.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 18.Paylor R, Lindsay E. Mouse models of 22q11 deletion syndrome. Biol Psychiatry. 2006;59:1172–1179. doi: 10.1016/j.biopsych.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Saint-Jore B, et al. Goosecoid-like (Gscl), a candidate gene for velocardiofacial syndrome, is not essential for normal mouse development. Hum Mol Genet. 1998;7:1841–1849. doi: 10.1093/hmg/7.12.1841. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb S, et al. The DiGeorge syndrome minimal critical region contains a goosecoid-like (GSCL) homeobox gene that is expressed early in human development. Am J Hum Genet. 1997;60:1194–1201. [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmendinger LM, Moore RY. Interpeduncular nucleus organization in the rat: Cytoarchitecture and histochemical analysis. Brain Res Bull. 1984;13:163–179. doi: 10.1016/0361-9230(84)90018-2. [DOI] [PubMed] [Google Scholar]
- 22.Tamamaki N, et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 23.Groenewegen HJ, Wouterlood FG. Basal forebrain inputs to the interpeduncular nucleus in the rat studied with the Phaseolus vulgaris-leucoagglutinin tracing method. Brain Res Bull. 1988;21:643–649. doi: 10.1016/0361-9230(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 24.Herkenham M. Anesthetics and the habenulo-interpeduncular system: Selective sparing of metabolic activity. Brain Res. 1981;210:461–466. doi: 10.1016/0006-8993(81)90927-6. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy C, et al. Local cerebral glucose utilization in non-rapid eye movement sleep. Nature. 1982;297:325–327. doi: 10.1038/297325a0. [DOI] [PubMed] [Google Scholar]
- 26.McQueen JK, Martin MJ, Harmar AJ. Local changes in cerebral 2-deoxyglucose uptake during alphaxalone anaesthesia with special reference to the habenulo-interpeduncular system. Brain Res. 1984;300:19–26. doi: 10.1016/0006-8993(84)91336-2. [DOI] [PubMed] [Google Scholar]
- 27.Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- 28.Long JM, et al. Behavior of mice with mutations in the conserved region deleted in velocardiofacial/DiGeorge syndrome. Neurogenetics. 2006;7:247–257. doi: 10.1007/s10048-006-0054-0. [DOI] [PubMed] [Google Scholar]
- 29.Fulcher BD, Phillips AJ, Robinson PA. Modeling the impact of impulsive stimuli on sleep-wake dynamics. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78:051920. doi: 10.1103/PhysRevE.78.051920. [DOI] [PubMed] [Google Scholar]
- 30.Wakamiya M, Lindsay EA, Rivera-Pérez JA, Baldini A, Behringer RR. Functional analysis of Gscl in the pathogenesis of the DiGeorge and velocardiofacial syndromes. Hum Mol Genet. 1998;7:1835–1840. doi: 10.1093/hmg/7.12.1835. [DOI] [PubMed] [Google Scholar]
- 31.Gottlieb S, Hanes SD, Golden JA, Oakey RJ, Budarf ML. Goosecoid-like, a gene deleted in DiGeorge and velocardiofacial syndromes, recognizes DNA with a bicoid-like specificity and is expressed in the developing mouse brain. Hum Mol Genet. 1998;7:1497–1505. doi: 10.1093/hmg/7.9.1497. [DOI] [PubMed] [Google Scholar]
- 32.Galili N, Nayak S, Epstein JA, Buck CA. Rnf4, a RING protein expressed in the developing nervous and reproductive systems, interacts with Gscl, a gene within the DiGeorge critical region. Dev Dyn. 2000;218:102–111. doi: 10.1002/(SICI)1097-0177(200005)218:1<102::AID-DVDY9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Lindsay EA, Harvey EL, Scambler PJ, Baldini A. ES2, a gene deleted in DiGeorge syndrome, encodes a nuclear protein and is expressed during early mouse development, where it shares an expression domain with a Goosecoid-like gene. Hum Mol Genet. 1998;7:629–635. doi: 10.1093/hmg/7.4.629. [DOI] [PubMed] [Google Scholar]
- 34.Karayiorgou M, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Schizophrenia Consortium Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, et al. Transmission disequilibrium test provides evidence of association between promoter polymorphisms in 22q11 gene DGCR14 and schizophrenia. J Neural Transm. 2006;113:1551–1561. doi: 10.1007/s00702-005-0420-3. [DOI] [PubMed] [Google Scholar]
- 37.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Elsevier; 2004. [Google Scholar]