Investigation of tocochromanols, lipophilic molecules that protect against fatty acid peroxidation, is still providing surprises. They are uniquely synthesized by photosynthetic organisms, widely studied in relation to their photoprotective role in photosynthesis, and are of importance in human nutrition as vitamin E. An article in PNAS from the DellaPenna laboratory (1) makes a significant step forward by showing that tocochromanols are essential for maintaining the viability of Arabidopsis thaliana seeds, suggesting that tocochromanols are a key component in the evolution of desiccation-resistant seeds.
Tocochromanols Protect Against Lipid Peroxidation
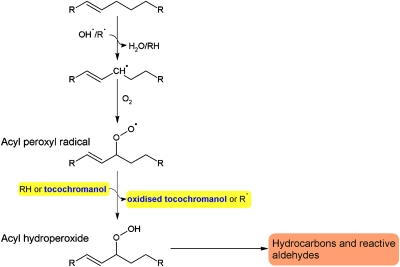
Lipid peroxidation is a chain reaction in which unsaturated fatty acids in lipids of various types are oxidized, producing highly reactive products that can cause further cellular damage. Polyunsaturated fatty acids (PUFAs) are particularly susceptible to peroxidation, a chain reaction initiated by hydroxyl radicals (Fig. 1). The first reasonably stable products, fatty acyl hydroperoxides, are still sufficiently reactive to produce aldehydes (reactive electrophiles) that cause cellular damage and induce the expression of defense-related genes (2, 3). This process is seen in the rancidification of fats and oils stored in the air. Chain reactions are minimized by preventing initiation or propagation. Initiation is minimized by antioxidant systems that decrease hydroxyl radical production (4). The tocochromanols are effective in preventing the propagation of peroxidation. Of these, α-tocopherol and γ-tocopherol (1) are the most common in plants (5, 6). Tocochromanols consist of a hydrophobic isoprenoid tail that is anchored into membrane bilayers, whereas a more hydrophilic hydroxychroman ring sits nearer the surface. To prevent propagation of fatty acid peroxidation, the hydroxyl group on the hydroxychroman ring readily donates H to acyl peroxyl radicals to form an acyl hydroperoxide and a tocopheroxyl radical (Fig. 1). Tocochromanols also react with singlet oxygen, a reactive form of oxygen produced during photosynthesis. This suggests an important role in protection of the photosynthetic thylakoid membranes, which are rich in PUFAs and therefore susceptible to peroxidation initiated by singlet oxygen and other reactive oxygen species formed during photosynthesis (7).
Fig. 1.
Peroxidation of an unsaturated fatty acyl chain is initiated by a hydroxyl radical (OH·) or an acyl peroxyl radical (R·). Tocochromanols scavenge acyl peroxyl radicals and therefore minimize their reaction with further acyl chains. Details of the tocochromanol oxidation products are not shown, but most of these can be converted back to tocochromanol.
Rancid Lipids and Seed Longevity
The article by Mène-Saffrané et al. (1) focuses attention on plastochromanol-8 (PC-8), a less well-studied tocochromanol. Seeds, particularly oil seeds that store triacylglyerols (TAGs) as a food reserve in oil bodies, are rich in γ-tocopherol (8). Previously, the DellaPenna group showed that Arabidopsis thaliana mutants lacking homogentisate phytyltransferase (VTE2) are unable to synthesize tocopherols. The mutant seeds had reduced longevity during storage and suffered fatty acid peroxidation during germination (8), suggesting an important role for tocopherols in protecting seeds that use TAGs as a food reserve. The present article extends these observations and shows that PC-8 plays a key role in preventing fatty acid peroxidation in Arabidopsis seeds. The authors demonstrated this function by making a double mutant affected in VTE2 and VTE1 (tocopherol cyclase). Tocopherol cyclase forms the hydroxychroman ring of tocochromanols. The role of VTE1 in PC-8 synthesis was recently demonstrated in Arabidopsis (9). In these studies, as in the present article, PC-8 was not detected in the vte1 mutant, leading to the suggestion that plastoquinol-9 is the precursor of PC-8. The double vte1 vte2 mutant lacks both tocopherols and PC-8 (1). Its seeds rapidly lose viability after harvesting owing to catastrophic fatty acid peroxidation, clearly indicating a role for tocochromanols, including PC-8, in protecting unsaturated fatty acids from peroxidation. The results also show that PC-8 and tocopherols are essential for the long-term viability and maintenance of vigor of Arabidopsis seeds. Because PC-8 concentration is low compared with the tocopherols, the results suggest that its longer prenyl tail improves its antioxidant efficiency or helps to target it to the oil bodies. The rate constants of the reaction of tocopherols and PC-8 with singlet oxygen are similar (10), as might be expected from the identical structure of their hydroxychroman rings; thus, prenyl chain length must be important in the effectiveness of PC-8.
Significantly, Mène-Saffrané et al. assessed the viability of seeds stored in dry conditions, the usual situation for crop plants. However, seeds that lie dormant in soil are fully hydrated or at least will go through cycles of wetting and drying. It would be interesting to know whether the protective effect of tocochromanols is specific to desiccated cells or also required to maintain the viability of hydrated dormant seeds. Under hydrated conditions, other antioxidant and repair systems may operate. Also, it would be interesting to know whether tocochromanols, particularly PC-8, are also involved in the desiccation resistance of photosynthetic cells in algae, bryophytes, and the angiosperm resurrection plants.
The present results agree with previous observations that seed longevity is oxygen dependent and that oxidative damage is a determinant of desiccation resistance (11, 12). Glutathione redox state has been suggested as another determinant of viability in the desiccated state: the glutathione pool oxidizes over time until viability is lost at a specific redox state (13). The relationship between glutathione and tocochromanols in desiccated cells is not known—are they protecting the same cellular components, and do they interact? Because metabolism is slow in anhydrobiotic conditions, another question to be resolved is whether oxidized tocochromanols can be regenerated in dry seeds or whether tocochromanol content falls substantially during storage. The intriguing possibility that selection for longevity and vigorous seedling growth in crop plants has inadvertently increased their seed tocochromanol levels (14) is mentioned by Mène-Saffrané et al. Increased seed tocopherol has been engineered in Arabidopsis seeds by expression of homogentisate phytyltransferase, driven bya seed-specific promoter (15). The seedlings were not more resistant to oxidative stress during germination, but it would be interesting to know whether they show
Tocochromanols are essential for maintaining the viability of Arabidopsis thaliana seeds.
increased longevity after harvest. A class of protective hydrophilic proteins that accumulate during seed maturation is the dehydrins (hydrophilins). Various roles in protecting desiccated proteins and membranes have been proposed for these proteins (16). A histidine-rich dehydrin binds metals with high affinity (17) and could therefore act as an antioxidant by scavenging redox active metals, minimizing initiation of fatty acid peroxidation by preventing hydroxyl radical formation through the Fenton reaction.
Tocochromanols and Photosynthesis
Tocopherols and the precursor 2,3-dimethyl-6-phytyl-1,4-benzoquinol (1) are important in protecting photosynthesis from photooxidative stress. However, a strong phenotype is only revealed under under extreme conditions or in combination with mutations in other photoprotective components (6, 7). The emergence of PC-8 as a key player in protecting seed lipids and its effectiveness as a singlet oxygen scavenger suggest that the role of tocochromanols in photoprotection of photosynthesis needs further investigation using the vte1 vte2 double mutant.
Conclusion
Mène-Saffrané et al. (1) show very clearly the key role of tocochromanols in Arabidopsis seed longevity and vigor, indicating their importance in the formation of desiccation-resistant seeds. Further investigation could determine if this role is equally important in seeds that store more starch than oil. Nonantioxidant roles have been proposed for tocochromanols (18), including possible effects on membrane function in the cold or under desiccation (19). Not commented upon by the authors is the ability of a few vte1 vte2 double mutants to survive the seedling stage and apparently grow normally. Therefore, unlike their hydrophilic antioxidant “colleague” ascorbic acid (20), tocochromanols may not be absolutely essential for plant growth, at least under laboratory conditions. Clearly, more remains to be learned about the biological functions of this intriguing group of molecules.
Footnotes
The author declares no conflict of interest.
See companion article on page 17815 in issue 41 of volume 107.
References
- 1.Mène-Saffrané L, Jones AD, DellaPenna D. Plastochromanol-8 and tocopherols are essential lipid soluble antioxidants during seed desiccation and quiescence in Arabidopsis. Proc Natl Acad Sci USA. 2010;107:17815–17820. doi: 10.1073/pnas.1006971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber H, Chételat A, Reymond P, Farmer EE. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J. 2004;37:877–888. doi: 10.1111/j.1365-313x.2003.02013.x. [DOI] [PubMed] [Google Scholar]
- 3.Sattler SE, et al. Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell. 2006;18:3706–3720. doi: 10.1105/tpc.106.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford Univ Press; 1999. [Google Scholar]
- 5.Falk J, Munné-Bosch S. Tocochromanol functions in plants: Antioxidation and beyond. J Exp Bot. 2010;61:1549–1566. doi: 10.1093/jxb/erq030. [DOI] [PubMed] [Google Scholar]
- 6.Mène-Saffrané L, DellaPenna D. Biosynthesis, regulation and functions of tocochromanols in plants. Plant Physiol Biochem. 2010;48:301–309. doi: 10.1016/j.plaphy.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell. 2005;17:3451–3469. doi: 10.1105/tpc.105.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell. 2004;16:1419–1432. doi: 10.1105/tpc.021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szymańska R, Kruk J. Plastoquinol is the main prenyllipid synthesized during acclimation to high light conditions in Arabidopsis and is converted to plastochromanol by tocopherol cyclase. Plant Cell Physiol. 2010;51:537–545. doi: 10.1093/pcp/pcq017. [DOI] [PubMed] [Google Scholar]
- 10.Gruszka J, Pawlak A, Kruk J. Tocochromanols, plastoquinol, and other biological prenyllipids as singlet oxygen quenchers-determination of singlet oxygen quenching rate constants and oxidation products. Free Radic Biol Med. 2008;45:920–928. doi: 10.1016/j.freeradbiomed.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Leprince O, Vertucci CW, Hendry GAF, Atherton NM. The expression of desiccation-induced damage in orthodox seeds is a function of oxygen and temperature. Physiol Plant. 1995;94:233–240. [Google Scholar]
- 12.Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 13.Kranner I, Birtić S, Anderson KM, Pritchard HW. Glutathione half-cell reduction potential: A universal stress marker and modulator of programmed cell death? Free Radic Biol Med. 2006;40:2155–2165. doi: 10.1016/j.freeradbiomed.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Velasco L, Goffman FD. Tocopherol, plastochromanol and fatty acid patterns in the genus Linum. Plant Syst Evol. 2000;221:77–88. [Google Scholar]
- 15.Xi DM, Liu WS, Yang GD, Wu CA, Zheng CC. Seed-specific overexpression of antioxidant genes in Arabidopsis enhances oxidative stress tolerance during germination and early seedling growth. Plant Biotechnol J. 2010;8:796–806. doi: 10.1111/j.1467-7652.2010.00509.x. [DOI] [PubMed] [Google Scholar]
- 16.Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008;148:6–24. doi: 10.1104/pp.108.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara M, Fujinaga M, Kuboi T. Metal binding by citrus dehydrin with histidine-rich domains. J Exp Bot. 2005;56:2695–2703. doi: 10.1093/jxb/eri262. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: A critical review. Free Radic Biol Med. 2008;44:739–764. doi: 10.1016/j.freeradbiomed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Maeda H, Sage TL, Isaac G, Welti R, Dellapenna D. Tocopherols modulate extraplastidic polyunsaturated fatty acid metabolism in Arabidopsis at low temperature. Plant Cell. 2008;20:452–470. doi: 10.1105/tpc.107.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]