Abstract
NF-κB is a key transcription factor involved in the regulation of T-cell activation and proliferation upon engagement of the T-cell receptor (TCR). T cells that lack the IκB kinase (IKKβ) are unable to activate NF-κB, and rapidly undergo apoptosis upon activation. NF-κB activation following T-cell receptor engagement induces the expression of Mdm2 through interaction with NF-κB sites in its P1 promoter, and enforced expression of Mdm2 protected T cells deficient for NF-κB activation from activation-induced cell death. In T cells with intact NF-κB signaling, ablation or pharmacologic inhibition of Mdm2 resulted in activation-induced apoptosis. Mdm2 coprecipitates with p73 in activated T cells, and apoptosis induced by inhibition of Mdm2 was p73-dependent. Further, Bim was identified as a p73 target gene required for cell death induced by Mdm2 inhibition, and a p73-responsive element in intron 1 of Bim was characterized. Our results demonstrate a pathway for survival of activated T cells through NF-κB–induced Mdm2, which blocks Bim-dependent apoptosis through binding and inhibition of p73.
Keywords: apoptosis, T lymphocyte, Bim
During the course of an immune response, activated T lymphocytes undergo cell death by a variety of mechanisms (1). Such activation-induced cell death (AICD) can be mediated by expression of death ligands of the TNF family, including CD95 ligand (1) and TNF-related apoptosis-inducing ligand (2), and the engagement of their respective receptors. An independent mechanism of AICD involves the expression and stabilization of the proapoptotic BCL-2 protein, BIM (3). Another, less well characterized mode of AICD in T cells involves engagement of the p53 family member p73 (4). Intriguingly, this mechanism of T-cell death appears to be inhibited by the function of the transcription factor NF-κB, as T cells expressing a nondegradable mutant of its inhibitor, IκB, undergo AICD in a p73-dependent manner (5). Upon ligation of the T-cell receptor (TCR) and coreceptors, T cells activate NF-κB via the IκB kinase (IKK) complex, composed of IKKα, IKKβ, and IKKγ/NEMO (6). T cells deficient in NF-κB components (7) or lacking IKKβ (8, 9) develop normally provided TNFR1 is disrupted (10), but display defective proliferation (11) and accumulation of memory T cells (12, 13).
The p53 family of proteins includes p53, p63, and p73, and forms of these proteins produced by alternative splicing and/or alternative translation-start sites (14). A critical regulator of p53 is the E3-ubiquitin ligase, murine double minute-2 (MDM2), which binds directly to the NH2 terminus of p53 in the nucleus, inhibiting its transcriptional activity (15). The MDM2–p53 complex shuttles from the nucleus to the cytoplasm, where MDM2 targets p53 for proteasome-dependent degradation. MDM2 also binds to p73 and inhibits its transcriptional activity, but does not target p73 for degradation (16).
Expression of murine and human MDM2 is under the control of two promoters, P1 and P2 (17, 18). Whereas P2 is driven by p53 (17), transcription factors responsible for P1-mediated expression have not been identified to date. Interestingly, NF-κB has been shown to induce MDM2 expression in mouse embryonic fibroblasts (19), as an NF-κB–responsive element has been identified in intron 1 of MDM2.
Here, we explore the role of NF-κB in the expression of MDM2 in activated T cells and its role in preventing p73-dependent, activation-induced apoptosis. We further identify BIM as a p73 target in T lymphocytes, required for this form of AICD.
Results
To generate T cells defective in NF-κB signaling, we bred Ikkβ−/−Tnfr1−/− animals to rescue the embryonic lethality seen in Ikkβ−/− mice. Splenic T cells from 7-d-old Ikkβ+/+Tnfr1−/− or Ikkβ−/−Tnfr1−/− mice (hereafter referred to as Ikkβ+/+ or Ikkβ−/−) were stimulated with anti-CD3 plus anti-CD28, and as expected (5), apoptosis was dramatically elevated in activated Ikkβ−/− T cells (Fig. S1 A and B). Similarly, we found that Jurkat T cells, stably expressing an IκBαM superrepressor (20), underwent apoptosis upon stimulation with anti-CD3, whereas cells stably transduced with a control vector did not (Fig. S1C).
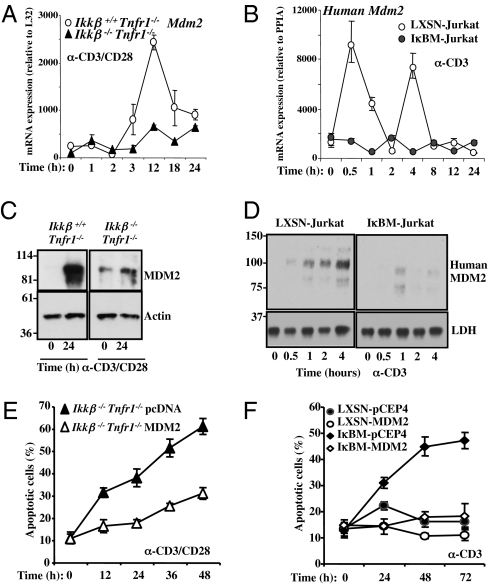
In mouse embryonic fibroblasts, NF-κB has been observed to drive the expression of MDM2, which regulates p53 function (19). Similarly, in both primary T cells (Fig. 1 A and C) and Jurkat cells (Fig. 1 B and D), activation led to increased mRNA and protein expression of MDM2. Ikkβ−/− T cells and Jurkat cells expressing IκBαM did not display increased MDM2 following activation.
Fig. 1.
Impaired Mdm2 up-regulation in primary Ikkβ−/−Tnfr1−/− and IκBM-Jurkat T cells upon TCR activation. (A) Total mRNA was isolated from anti-mCD3/anti-mCD28 activated Ikkβ+/+Tnfr1−/− and Ikkβ−/−Tnfr1−/− lymphocytes and assessed by real-time RT-PCR for Mdm2 mRNA expression. Absolute mRNA values were determined, normalized to L32, and reported as arbitrary units. (B) Total mRNA was isolated from anti-hCD3 (OKT3) activated LXSN- and IκBM-Jurkat T cells and assessed by real-time RT-PCR for human Mdm2 mRNA expression. Absolute mRNA values were determined, normalized to Cyclophylin (PPIA), and reported as arbitrary units. (C) Ikkβ+/+Tnfr1−/− and Ikkβ−/−Tnfr1−/− lymphocytes were activated for 24 h with anti-mCD3/anti-mCD28. MDM2 and Actin protein expression was detected by immunoblotting. (D) LXSN- and IκBM-Jurkat T cells were activated with anti-hCD3 (OKT3) for the indicated times. Human MDM2 and lactate dehydrogenase protein expression were assessed by Western blotting. (E) Primary Ikkβ−/−Tnfr1−/− lymphocytes were transiently transfected with pcDNA or pcDNA-MDM2 for 13 h and then activated for the indicated times with anti-mCD3/anti-mCD28. Apoptotic T cells were determined visually by nuclear chromatin staining with Hoechst 33342 dye. (F) The indicated cell lines were activated with soluble anti-hCD3 (OKT3) for the indicated time and apoptotic cells were counted as in E.
We next asked if the expression of MDM2 participates in NF-κB–dependent cell survival following T-cell activation. To ectopically express MDM2 in primary T cells, we optimized transduction to achieve expression of GFP in almost 50% of primary cells (Fig. S2C). We introduced a construct for transient expression of MDM2 in Ikkβ−/− T cells before activation with anti-CD3 plus anti-CD28 (Fig. 1E and Fig. S2A). Although activation of Ikkβ-null T cells resulted in extensive cell death, enforced expression of MDM2 inhibited apoptosis.
Similarly, we stably enforced MDM2 expression in Jurkat cells with or without IκBαM superrepressor (Fig. S2B). Although activation of Jurkat cells with anti-CD3 resulted in apoptosis in cells expressing IκBαM superrepressor, this cell death was inhibited by MDM2, but not by stable transduction with the control vector (Fig. 1F).
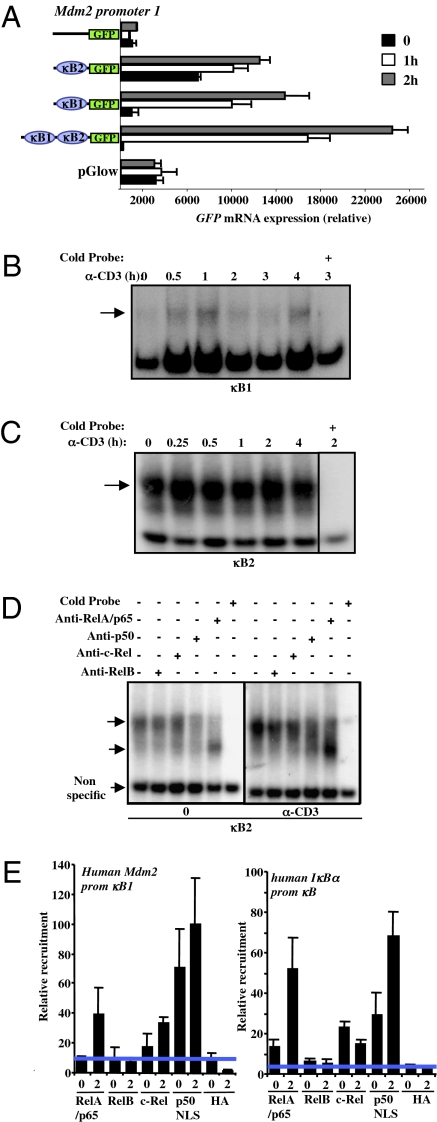
We identified six potential NF-κB sites in the human Mdm2 promoter (Fig. S3A) and found that two were active in reporter assays and electrophoretic mobility shift assays (EMSAs; Fig. 2 A–C). These sites in the P1 promoter, denoted κB1 and κB2 (Fig. S3A), drove reporter expression in activated Jurkat cells (Fig. 2A). EMSA analysis showed binding to κB1 following T cell activation (Fig. 2B). Consistent with earlier reports for other κB sites (21), NF-κB binding activity to the κB1 site appeared in two waves. Similarly, an observed background binding for κB2 was increased upon treatment with anti-CD3 (Fig. 2C). To explore the latter binding in more detail, we performed neutralization studies with antibodies to NF-κB components (Fig. 2D). We observed no role for c-Rel or RelB in the binding, but inhibition with anti-p50 antibody blocked both background and enhanced binding following activation. Addition of anti-RelA antibody caused the appearance of a different band, likely to be a p50 homodimer (22, 23). Thus, it is likely that, upon activation, p50/RelA binds to the κB2 site in the Mdm2 P1 promoter.
Fig. 2.
NF-κB regulates Mdm2 promoter 1. (A) LXSN-Jurkat T cells were cotransfected with the indicated GFP reporter plasmids with or without κB1 and κB2 sites and stimulated for 1 and 2 h with anti-hCD3 (OKT3). GFP mRNA was analyzed by real-time RT-PCR and normalized to LacZ mRNA expression as a transfection efficiency control. (B) NF-κB binding to a DNA probe containing the κB1-binding site in the human Mdm2 P1 promoter. Nuclear extracts were prepared from LXSN-Jurkat T cells treated with anti-hCD3 (OKT3) for the indicated time and incubated with a γ32P-labeled double-stranded oligonucleotide probe corresponding to the putative NF-κB site in the P1 promoter region. Specificity of binding to the κB sites was shown by competition with double-stranded oligonucleotides (cold probe). The arrow indicates specific binding. (C) As in B, use of a DNA probe containing the κB2-binding site in the human Mdm2 P1 promoter region. The arrows indicate specific binding. (D) As in C, use of the DNA probe containing the κB2-binding site with the addition of antibodies against p65/RelA, p50, c-Rel, and RelB to identify the subunits present in the bound complexes (arrows indicate p50/p65, p50/p50, and nonspecific complexes). (E) ChIP of NF-κB proteins detects NF-κB site in the human Mdm2 P1 promoter. Jurkat T cells were activated with anti-hCD3 (OKT3) for 2 h and ChIP was performed with the indicated antibodies. DNA was probed by quantitative PCR for the indicated sequences. Activation resulted in binding of p65 and p50 to both the established NF-κB site in the human IκB promoter and the κB1 site in the human Mdm2 promoter.
To further analyze the binding of NF-κB to the κB1 site, chromatin immunoprecipitation (ChIP) analysis was performed (Fig. 2E). The κB site in the IκB promoter (24) was used as a control. ChIP with anti-p50 precipitated both elements in resting and activated Jurkat T cells, whereas anti-RelA precipitated the elements only in activated T cells. Precipitation with anti–c-Rel was also observed. Together, these results implicate p50/RelA (and possibly p50/c-Rel) heterodimers in driving the Mdm2 P1 promoter following T-cell activation. Consistent with a role for p50/RelA in Mdm2 promoter activation, we found that both κB1 and κB2 promoter reporter expression was induced by coexpression of p50 and RelA in Jurkat cells (Fig. S3B) and primary T cells (Fig. S3C).
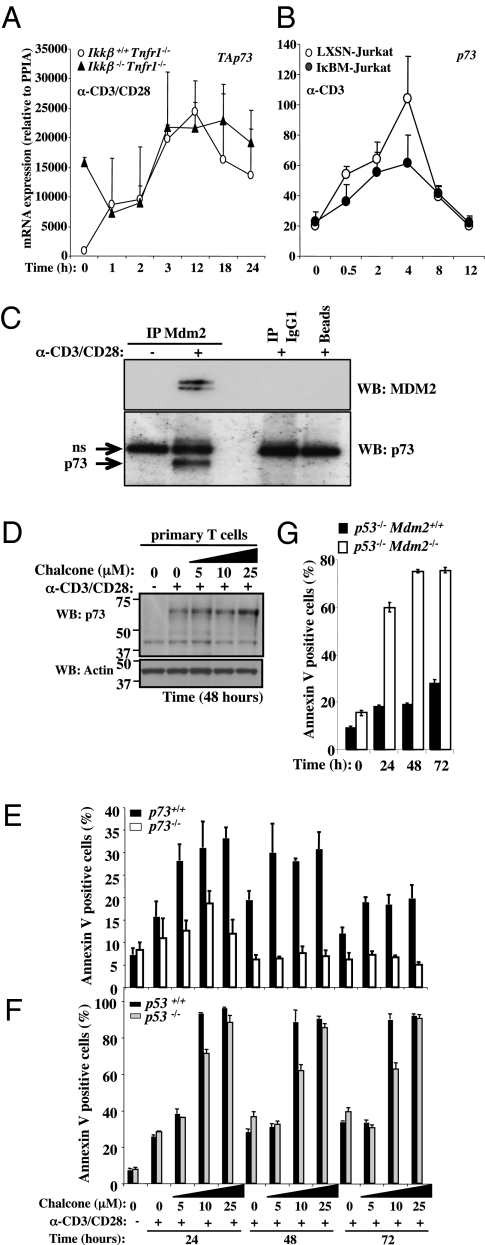
A role for p53 in this AICD, however, was unlikely, as Jurkat T cells lack p53 (25), and we were unable to detect significant p53 stabilization following activation of primary Ikkβ-deficient T cells. Previous studies (5, 26) had shown that cell death in activated T cells expressing an IκB superrepressor is dependent on the p53 family member p73. As described earlier (4), we detected induction of p73 mRNA expression by T-cell activation in both primary T cells and Jurkat cells, and this was unaffected by NF-κB status (Fig. 3 A and B). We therefore immunoprecipitated MDM2 from activated, primary WT T cells (Fig. 3C and Fig. S4 A and B). We found that p73 coprecipitated with MDM2 from activated but not resting T cells. The identification of p73 was validated using lysates from p73-deficient T cells (Fig. S4A). The effect of an MDM2 inhibitor on the binding of MDM2 to p73 was then examined. Chalcone is an MDM2 inhibitor that binds to MDM2 and competes for p53 binding (27). We found that chalcone effectively blocked the coprecipitation of p73 with MDM2 (Fig. S4B). The presence of chalcone had only a minor effect on levels of p73 following activation (Fig. 3D). This is consistent with the observations that MDM2 binds to and blocks the function of p73, but does not induce its degradation (16, 28). Further, we found that whereas the presence of chalcone promoted activation-induced apoptosis in WT T cells, T cells lacking p73 were strikingly resistant to this effect (Fig. 3E). In contrast, T cells lacking p53 were fully sensitive to activation-induced apoptosis in the presence of chalcone (Fig. 3F).
Fig. 3.
Ikkβ−/−Tnfr1−/− and IκBM-Jurkat T cells express TAp73 upon TCR activation. (A) Primary Ikkβ+/+Tnfr1−/− and Ikkβ−/−Tnfr1−/− lymphocytes were stimulated with anti-mCD3/anti-mCD28 for the indicated times. Total mRNA was isolated and assessed by real-time RT-PCR for TAp73 mRNA expression. Absolute mRNA values were determined, normalized to Cyclophylin (PPIA), and reported as arbitrary units. (B) LXSN- and IκBM-Jurkat T cells were stimulated with anti-hCD3 for the indicated times. Total mRNA was isolated and assessed by real-time RT-PCR for p73 mRNA expression. Absolute mRNA values were determined, normalized to Cyclophylin (PPIA), and reported as arbitrary units. (C) Primary T cells from C57BL/6 mice were untreated or stimulated for 24 h with plate-bound anti-mCD3/anti-mCD28. Immunoprecipitations (IP) with an anti-MDM2 Ab or isotype control were followed by immunoblot detection of MDM2 and p73 with HRP-coupled antibodies. Controls include the Ab isotype alone (IP IgG1) and beads incubated in the absence of Ab (beads). (Top) IP, anti-Mdm2; WB, anti-Mdm2. (Bottom) IP, anti-Mdm2; WB, anti-p73. (D) WT primary T cells were activated for 48 h with plate-bound anti-mCD3/anti-mCD28 in the presence or absence of increasing concentrations of MDM2 inhibitor (chalcone). Total cellular proteins were isolated. p73 and Actin protein expression was assessed by immunoblotting. (E) Primary T cells isolated from p73−/− and p73+/+ mice were activated with plate-bound anti-mCD3/anti-mCD28 for the indicated times in the presence of the indicated concentration of MDM2 inhibitor (chalcone) and were analyzed by flow cytometry for AlloPhycoCyanin (APC)-conjugated annexin V staining. Percentages of annexin V-positive cells are indicated. (F) Primary T cells isolated from p53−/− and p53+/+ mice were activated with plate-bound anti-mCD3/anti-mCD28 for the indicated times in the presence of the indicated concentration of MDM2 inhibitor (chalcone) and were analyzed by flow cytometry for APC-conjugated annexin V staining. Percentages of Annexin V-positive cells are indicated. (G) Primary T cells isolated from p53−/− and Mdm2−/− × p53−/− double KO mice were activated with plate-bound anti-mCD3/anti-mCD28 for the indicated times and were analyzed by Flow cytometry for APC-conjugated annexin V staining. Percentages of annexin V-positive cells are indicated.
Genetic ablation of MDM2 is embryonically lethal, but can be rescued by deletion of p53 (29, 30). We therefore examined the role of MDM2 in activated T-cell survival by comparing T cells from p53−/−Mdm2+/+ and p53−/−Mdm2−/− mice (Fig. 3G). Strikingly, the absence of MDM2 resulted in dramatic AICD in these T cells. Together, our results show that expression and function of MDM2 upon T-cell activation is required for survival of the cells.
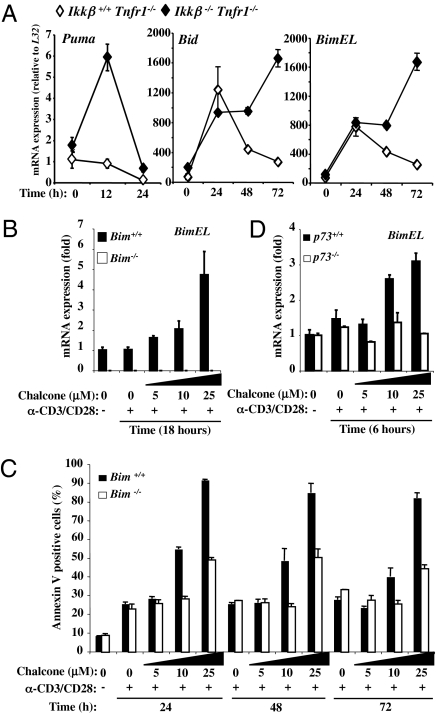
The transcriptional targets of p53 and p73 are distinct, but overlapping (31). The proapoptotic BH3-only proteins PUMA (32) and BID (33) are p53 targets required for p53-mediated apoptosis in different tissues. PUMA is also a transcriptional target of p73 (34–36). We therefore examined the expression of Puma and Bid following activation of Ikkβ+/+ and Ikkβ−/− T cells (Fig. 4A). In addition, we also examined the expression of another BH3-only protein, BIM, which is not a known p53 target, but has been implicated in activation-induced apoptosis in thymocytes (37) and mature T cells (38). Puma mRNA was selectively induced in activated T cells lacking IKKβ. Although both Bid and Bim were transiently induced following activation of Ikkβ+/+ T cells, levels of expression of both were dramatically enhanced in T cells lacking IKKβ (Fig. 4A).
Fig. 4.
p73 regulates Bim gene expression after TCR costimulation. (A) Primary lymphocytes from Ikkβ+/+Tnfr1−/− and Ikkβ−/−Tnfr1−/− mice were activated with plate-bound anti-mCD3/anti-mCD28 for the indicated times. Total mRNA was isolated and assessed by real-time RT-PCR for Puma, Bid, and BimEL mRNA expression. Absolute mRNA values were determined, normalized to L32, and reported as arbitrary units. (B) Primary Bim−/− and Bim+/+ T cells were activated with plate-bound anti-mCD3/anti-mCD28 for 18 h in the presence or absence of MDM2 inhibitor (chalcone). Total mRNA was isolated and assessed by real-time RT-PCR for BimEL mRNA expression. (C) Primary T cells isolated from Bim−/− and Bim+/+ mice were activated with plate-bound anti-mCD3/anti-mCD28 for the indicated times in the presence of the indicated concentration of MDM2 inhibitor (chalcone) and were analyzed by flow cytometry for APC-conjugated annexin V staining. Percentages of annexin V-positive cells are indicated. (D) Primary p73−/− and p73+/+ T cells were activated with plate-bound anti-mCD3/anti-mCD28 for 6 h in the presence of the indicated concentration of chalcone. Total mRNA was isolated and assessed by real-time RT-PCR for BimEL mRNA expression.
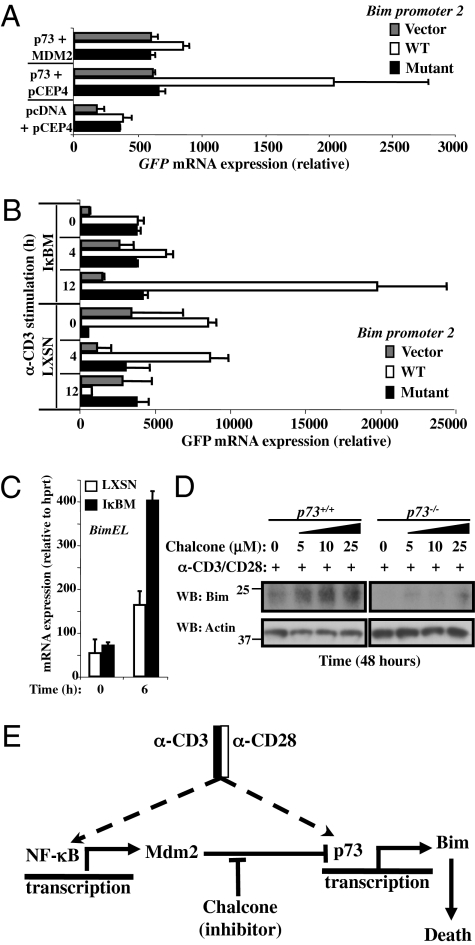
To determine if any or all of these BH3-only proteins play roles in p73-dependent, chalcone-promoted death of activated T cells, we used primary T cells from mice deficient in PUMA, BID, or BIM. Treatment with chalcone promoted activation-induced T-cell apoptosis, regardless of the status of PUMA (Fig. S5A) or BID (Fig. S5B). In contrast, T cells lacking BIM were resistant to the effects of chalcone (Fig. 4C). Consistent with these findings, Bim mRNA was elevated in primary T cells, activated in the presence of chalcone (Fig. 4B). T cells lacking p73 did not show this effect (Fig. 4D).
This suggested that Bim is expressed in response to p73 in activated T cells, provided the function of NF-κB or MDM2 is disrupted. We therefore examined the Bim promoter and identified a potential p73 target element (Fig. S6A). A reporter of 70 bp of the human Bim promoter containing the potential p73 binding site was cotransfected with p73 into Jurkat cells (Fig. 5A). Although p73 drove expression of this reporter, a mutation in the p73 site rendered the promoter unresponsive to p73. Coexpression of MDM2 prevented this activity of p73 on the Bim promoter. In keeping with these observations, activation of primary T cells in the presence of chalcone effectively induced expression from the Bim promoter reporter (Fig. S6B).
Fig. 5.
p73 binds and activates the bim promoter. (A) LXSN-Jurkat T cells were cotransfected with different constructs expressing human p73-α in the presence or not of Mdm2 with the indicated GFP reporter plasmids with WT p73 site (white bars), mutated p73 site (black bars), or empty vector (gray bars). GFP mRNA was analyzed by real-time RT-PCR and normalized to LacZ mRNA expression as a transfection efficiency control. (B) LXSN- and IκBM-Jurkat T cells were cotransfected with the indicated GFP reporter plasmids without (gray bars) or with WT p73 site (white bars) or mutated p73 site (black bars) from Bim P2 promoter region. GFP mRNA was analyzed by real-time RT-PCR and normalized to LacZ mRNA expression as a transfection efficiency control. Anti-hCD3 (OKT3) stimulation. (C) LXSN- and IκBM-Jurkat T cells were activated with anti-hCD3 (OKT3) for 6 h. Total mRNA was isolated and assessed by real-time RT-PCR for BimEL mRNA expression. (D) Primary T cells from p73+/+ and p73−/− were activated with plate-bound anti-mCD3/anti-mCD28 for 48 h in presence or not of the indicated concentration of chalcone. Total cellular proteins were isolated and Western blots were probed with antibodies against Bim and Actin. (E) Model of the NF-κB/Mdm2/p73/Bim pathway for survival and death of activated T cells.
We then examined the effect of NF-κB status on activation-induced expression of the Bim promoter reporter. Although only marginal reporter expression was observed upon activation of Jurkat cells with anti-CD3, this was dramatically enhanced in Jurkat expressing the IκBαM superrepressor (Fig. 5B). Mutation of the p73 site in the Bim promoter negated this effect. Consistent with these observations, we found that Jurkat cells expressing the IκBαM superrepressor induced expression of Bim mRNA (Fig. 5C). In primary WT T cells, a low level accumulation of BIM was observed following activation, and this was dramatically enhanced by chalcone (Fig. 5D). In contrast, activation of p73−/− T cells did not result in BIM expression with or without chalcone (Fig. 5D).
Discussion
We have defined a pathway in activated T cells in which NF-κB induces the expression of MDM2, which in turn binds to and inhibits p73-induced BIM expression, thereby preventing BIM-mediated apoptosis (Fig. 5E). NF-κB, induced upon T-cell activation, directly engages the Mdm2 P1 promoter, and in the absence of NF-κB function, T-cell activation leads to cell death that can be inhibited by enforced expression of MDM2.
NF-κB can drive MDM2 expression, without a direct interaction with the P2 promoter of Mdm2. Recently, an NF-κB component, BCL3, was found to bind in the first intron of Mdm2. We have found (Fig. S7A) that Bcl3 is expressed in activated WT but not Ikkβ-deficient T cells, but our results directly implicate p50/RelA (and perhaps p50/c-Rel) in binding to and promoting expression from the P1 promoter of Mdm2. This does not rule out a role for BCL3 in MDM2 expression as well. T cells from mice lacking p50 and c-Rel develop normally but die upon activation (39). This was attributed to a requirement for NF-κB for expression of the antiapoptotic protein BCL-2 and IL-2, either of which prevented AICD in the deficient T cells, as did ectopic expression of active AKT. As BCL-2 blocks BIM-dependent apoptosis (40) (implicated in the pathway we have elucidated), the effect of ectopic BCL-2 is not unexpected. Similarly, the ability of IL2 or AKT signaling to prevent apoptosis in this setting may involve the role of cytokines and AKT in inhibiting BIM expression via phosphorylation of FOXO3a (41). Recently, however, the absence of FOXO3 in T cells was observed to have no effect on the expansion or contraction of T cells in response to viral infection (42). At present, we do not know if FOXO3a (or another FOXO protein) participates in optimal BIM expression induced by p73.
Recently, p73-induced expression of BIM was implicated in cell death induced by prolonged mitotic arrest in fibroblasts (43). Three p73-binding elements distinct from that we found in the promoter region were identified in the first intron of Bim by ChIP analysis. Thus, although Bim does not appear to be a target of p53, it is a target of p73. Direct induction of Bim by p73 accounts for the role of BIM in p73-dependent apoptosis we observe in activated T cells with defective NF-κB activation, or in which MDM2 is inhibited.
We have found that T cells with normal NF-κB function but deficient in MDM2 (either by genetic ablation or pharmacologic inhibition) undergo cell death upon activation. Mice carrying one hypomorphic allele of MDM2 have been noted to display a marked lymphopenia that was attributed to p53 hyperactivation (44). This p53-dependent destruction of lymphoid cells was also observed in Mdm2−/− mice carrying a tamoxifen-responsive p53ERtam knock-in allele upon treatment with tamoxifen (45). In our studies, however, treatment with the MDM2 inhibitor chalcone resulted in activation-induced apoptosis in T cells lacking p53, but not in T cells lacking p73. Thus, T-cell activation appears to engage p73 but not p53, and the former is inhibited by NF-κB–induced MDM2. Intriguingly, we have found that T cell activation also engages expression of another p53 inhibitor, MDMX [also called MDM4 (46); Fig S7B]. At present, we do not know if MDMX can inhibit p73, or if chalcone interferes with MDMX function in this context. However, such expression of MDMX to inhibit p53 might explain why inhibition of MDM2 by chalcone does not appear to induce p53-dependent apoptosis in p73-deficient activated T cells. Indeed, irradiation of activated T cells results in p53-independent apoptosis. Interestingly, irradiation of activated T cells recruits p73 to the Bax promoter, and this effect is antagonized by p50/RelA (47).
When might a failure to engage NF-κB during T-cell activation occur during normal immune responses? Although engagement of the CD3-TCR complex activates IKK, coreceptor (e.g., CD28) signaling enhances NF-κB activation. Thus, at the initiation of immune responses, a failure to costimulate T cells may result in inefficient NF-κB activation, thereby engaging the death pathway we have elucidated. If so, the lack of p73 may allow such cells to persist. Intriguingly, p73-deficient mice often show early postnatal lethality, in part because of systemic inflammatory responses. The possible contribution of T cells to this effect has not been rigorously explored.
Materials and Methods
Cell Lines and Animals.
All mice (described in detail in SI Materials and Methods) were bred and housed under specific pathogen-free conditions in our animal facilities at La Jolla Institute for Allergy and Immunology (La Jolla, CA) and at St. Jude Children’ Research Hospital (Memphis, TN).
T Cells and T-Cell Lines.
Briefly, T cells were enriched from spleens by negative selection using a MACS system with microbeads conjugated to monoclonal anti-mouse CD45R (B220; isotype: rat IgG2a; clone:RA3-6B2; Miltenyi Biotec) following the manufacturer's instructions. LXSN-Jurkat and IκBM-Jurkat cells have been previously described (20). Primary T cells were stimulated in 96-well flat-bottom plates with plate-bound anti-mCD3 (clone 145–2C11) and anti-mCD28 (clone 37.51; antibodies coated in PBS solution at 10 μg/mL overnight at 4 °C; BD Pharmingen) and 0.5–1 × 106 T cells were added per well or Jurkat T cells were stimulated with soluble anti-hCD3 (OKT3; eBioscience), in the presence or absence of an MDM2 inhibitor [Chalcone (trans-4-Iodo, 4′-boranyl-chalcone); Calbiochem] was added as indicated. Following culture, cells were harvested at indicated times and apoptosis assessed as described (48).
Plasmid Constructs and Transfections.
Murine mdm-2 cDNA was amplified by RT-PCR from total RNA (SI Materials and Methods provides details). Human Mdm2 promoter constructs (κB1–κB2, κB1, κB2, and no κB sites) and human Bim promoter constructs were generated by PCR (SI Materials and Methods). Transient expression experiments were performed as described previously (2) (SI Materials and Methods).
Real-Time RT-PCR.
Total RNA isolation, reverse transcription, and real-time PCR were assessed as described previously (2). Primer sequences are available upon request.
EMSA.
Extracts and probes were prepared as previously described (49). SI Materials and Methods provides more details on EMSA.
Supplementary Material
Acknowledgments
We thank Emmanuel Dejardin, Scott Brown, Jean-François Peyron, Ulrich Maurer, Martina Malatesta, and A. Emre Sayan for their valuable scientific support. This work was supported by grants from the National Institutes of Health and Medical Research Council (United Kingdom), Ministero Sanità “Alleanza Contro il Cancro” Grant ACC12, Associazione Italiana per la Ricerca sul Cancro Grant 2008-2010_33-08, and Telethon Grant GGPO4110.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006163107/-/DCSupplemental.
References
- 1.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 2.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 3.Sandalova E, Wei CH, Masucci MG, Levitsky V. Regulation of expression of Bcl-2 protein family member Bim by T cell receptor triggering. Proc Natl Acad Sci USA. 2004;101:3011–3016. doi: 10.1073/pnas.0400005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lissy NA, Davis PK, Irwin M, Kaelin WG, Dowdy SF. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature. 2000;407:642–645. doi: 10.1038/35036608. [DOI] [PubMed] [Google Scholar]
- 5.Wan YY, DeGregori J. The survival of antigen-stimulated T cells requires NFkappaB-mediated inhibition of p73 expression. Immunity. 2003;18:331–342. doi: 10.1016/s1074-7613(03)00053-0. [DOI] [PubMed] [Google Scholar]
- 6.Weil R, Israël A. Deciphering the pathway from the TCR to NF-kappaB. Cell Death Differ. 2006;13:826–833. doi: 10.1038/sj.cdd.4401856. [DOI] [PubMed] [Google Scholar]
- 7.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 9.Li ZW, et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senftleben U, Li ZW, Baud V, Karin M. IKKbeta is essential for protecting T cells from TNFalpha-induced apoptosis. Immunity. 2001;14:217–230. doi: 10.1016/s1074-7613(01)00104-2. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Supprian M, et al. I kappa B kinase 2 deficiency in T cells leads to defects in priming, B cell help, germinal center reactions, and homeostatic expansion. J Immunol. 2004;173:1612–1619. doi: 10.4049/jimmunol.173.3.1612. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Supprian M, et al. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Vig M, Lyons J, Van Parijs L, Beg AA. Combined deficiency of p50 and cRel in CD4+ T cells reveals an essential requirement for nuclear factor kappaB in regulating mature T cell survival and in vivo function. J Exp Med. 2003;197:861–874. doi: 10.1084/jem.20021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levrero M, et al. The p53/p63/p73 family of transcription factors: Overlapping and distinct functions. J Cell Sci. 2000;113:1661–1670. doi: 10.1242/jcs.113.10.1661. [DOI] [PubMed] [Google Scholar]
- 15.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 16.Zeng X, et al. MDM2 suppresses p73 function without promoting p73 degradation. Mol Cell Biol. 1999;19:3257–3266. doi: 10.1128/mcb.19.5.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barak Y, Gottlieb E, Juven-Gershon T, Oren M. Regulation of mdm2 expression by p53: Alternative promoters produce transcripts with nonidentical translation potential. Genes Dev. 1994;8:1739–1749. doi: 10.1101/gad.8.15.1739. [DOI] [PubMed] [Google Scholar]
- 18.Jones SN, et al. Genomic organization of the mouse double minute 2 gene. Gene. 1996;175:209–213. doi: 10.1016/0378-1119(96)00151-5. [DOI] [PubMed] [Google Scholar]
- 19.Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFkappaB activation: A role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 20.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann A, Leung TH, Baltimore D. Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. EMBO J. 2003;22:5530–5539. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 23.Weih F, et al. p50-NF-kappaB complexes partially compensate for the absence of RelB: severely increased pathology in p50(-/-)relB(-/-) double-knockout mice. J Exp Med. 1997;185:1359–1370. doi: 10.1084/jem.185.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edelstein LC, Lagos L, Simmons M, Tirumalai H, Gélinas C. NF-kappa B-dependent assembly of an enhanceosome-like complex on the promoter region of apoptosis inhibitor Bfl-1/A1. Mol Cell Biol. 2003;23:2749–2761. doi: 10.1128/MCB.23.8.2749-2761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Y, Haines DS. The pathway regulating MDM2 protein degradation can be altered in human leukemic cells. Cancer Res. 1999;59:2064–2067. [PubMed] [Google Scholar]
- 26.Green DR. Death and NF-kappaB in T cell activation: Life at the edge. Mol Cell. 2003;11:551–552. doi: 10.1016/s1097-2765(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Knutson E, Wang S, Martinez LA, Albrecht T. Stabilization of p53 in human cytomegalovirus-initiated cells is associated with sequestration of HDM2 and decreased p53 ubiquitination. J Biol Chem. 2007;282:29284–29295. doi: 10.1074/jbc.M705349200. [DOI] [PubMed] [Google Scholar]
- 28.Bálint E, Bates S, Vousden KH. Mdm2 binds p73 alpha without targeting degradation. Oncogene. 1999;18:3923–3929. doi: 10.1038/sj.onc.1202781. [DOI] [PubMed] [Google Scholar]
- 29.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 30.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 31.Fontemaggi G, et al. Identification of direct p73 target genes combining DNA microarray and chromatin immunoprecipitation analyses. J Biol Chem. 2002;277:43359–43368. doi: 10.1074/jbc.M205573200. [DOI] [PubMed] [Google Scholar]
- 32.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 33.Sax JK, et al. BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol. 2002;4:842–849. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 34.Melino G, et al. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem. 2004;279:8076–8083. doi: 10.1074/jbc.M307469200. [DOI] [PubMed] [Google Scholar]
- 35.Ming L, et al. Sp1 and p73 activate PUMA following serum starvation. Carcinogenesis. 2008;29:1878–1884. doi: 10.1093/carcin/bgn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramadan S, et al. p73 induces apoptosis by different mechanisms. Biochem Biophys Res Commun. 2005;331:713–717. doi: 10.1016/j.bbrc.2005.03.156. [DOI] [PubMed] [Google Scholar]
- 37.Bouillet P, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 38.Hildeman DA, et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Y, et al. NF-kappa B RelA (p65) is essential for TNF-alpha-induced fas expression but dispensable for both TCR-induced expression and activation-induced cell death. J Immunol. 2001;166:4949–4957. doi: 10.4049/jimmunol.166.8.4949. [DOI] [PubMed] [Google Scholar]
- 40.O'Connor L, et al. Bim: A novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Möller C, et al. Stem cell factor promotes mast cell survival via inactivation of FOXO3a-mediated transcriptional induction and MEK-regulated phosphorylation of the proapoptotic protein Bim. Blood. 2005;106:1330–1336. doi: 10.1182/blood-2004-12-4792. [DOI] [PubMed] [Google Scholar]
- 42.Dejean AS, et al. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10:504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toh WH, Nam SY, Sabapathy K. An essential role for p73 in regulating mitotic cell death. Cell Death Differ. 2009;17:787–800. doi: 10.1038/cdd.2009.181. [DOI] [PubMed] [Google Scholar]
- 44.Mendrysa SM, et al. mdm2 Is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol Cell Biol. 2003;23:462–472. doi: 10.1128/MCB.23.2.462-473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vater CA, Bartle LM, Dionne CA, Littlewood TD, Goldmacher VS. Induction of apoptosis by tamoxifen-activation of a p53-estrogen receptor fusion protein expressed in E1A and T24 H-ras transformed p53-/- mouse embryo fibroblasts. Oncogene. 1996;13:739–748. [PubMed] [Google Scholar]
- 46.Parant J, et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet. 2001;29:92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- 47.Cianfrocca R, et al. RelA/NF-kappaB recruitment on the bax gene promoter antagonizes p73-dependent apoptosis in costimulated T cells. Cell Death Differ. 2008;15:354–363. doi: 10.1038/sj.cdd.4402264. [DOI] [PubMed] [Google Scholar]
- 48.Bouchier-Hayes L, Muñoz-Pinedo C, Connell S, Green DR. Measuring apoptosis at the single cell level. Methods. 2008;44:222–228. doi: 10.1016/j.ymeth.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busuttil V, et al. Blocking NF-kappaB activation in Jurkat leukemic T cells converts the survival agent and tumor promoter PMA into an apoptotic effector. Oncogene. 2002;21:3213–3224. doi: 10.1038/sj.onc.1205433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.