Abstract
The early events that determine the decision between lymphocyte tolerance and activation are not well-understood. Using a model of systemic self-antigen recognition by CD4+ T cells, we show, using single-cell biochemical analyses, that tolerance is characterized by transient signaling events downstream of T-cell receptor engagement in the mammalian target of rapamycin (mTOR) and NF-κB pathways. Parallel studies done by live cell imaging show that the key difference between tolerance and activation is the duration of the T cell–antigen presenting cell (APC) interaction, as revealed by stable T-cell immobilization on antigen encounter. Brief T cell–APC interactions result in tolerance, and prolonged interactions are associated with activation and the development of effector cells. These studies show that the duration of T cell–APC interactions and magnitude of associated TCR-mediated signaling are key determinants of lymphocyte tolerance vs. activation.
Keywords: autoimmunity, CD4 T cell, lymphopenia
A fundamental problem in understanding autoimmunity is to elucidate the mechanisms underlying the choice between lymphocyte tolerance and activation. This issue has been difficult to address, because methods for following the responses of lymphocytes to specific antigen encounter in vivo are limited. It is well established that there are multiple phosphorylation events downstream of T-cell receptor (TCR) signaling, which likely determine the fate of T cells upon recognition of their cognate antigen. Links between downstream signaling events and anergy induction have been shown for numerous TCR regulated pathways. For example, decreased levels of phosphorylated s6 protein (pS6), which are dependent on AKT (protein kinase B)/mammalian target of rapamycin (mTOR) activity, have been linked to anergy induction (1–3). Rapamycin blockade of mTOR signaling and thus, decreased pS6 levels can lead to anergy induction both in vitro and in vivo (1). The induction of anergy has also been shown to be dependent on the levels of nuclear factor of activated T cells-dependent genes in the absence of activator protein-1 signaling (4) and has been linked to impaired NF-κB activation in T cells that are rendered anergic in vitro (5). Many of the signaling studies, however, have been done in vitro using highly simplified models of T-cell tolerance.
Recent studies using live cell imaging have shown that the priming of naïve T cells takes place in distinct phases characterized first by short interactions with dendritic cells (DCs) (6, 7), leading to more stable conjugates, and ultimately, resulting in renewed motility and cytokine secretion from the T cells (6). Whether the interactions of T cells with DCs are similar or different during the induction of tolerance is not definitively established. The earliest studies’ imaging tolerance of CD4+ or CD8+ T cells used a DEC-205 antigen-targeting system in the absence (tolerance) or presence (priming) of external DC maturation stimuli, such as an anti-CD40 antibody (8–10). These studies showed that antigen-specific CD8+ T cells exhibited only brief contacts with DCs in situations of tolerance but prolonged interactions during activation (8, 9), where there is an intercellular adhesion molecule-1-dependent establishment of CD8+ T-cell memory upon stable interactions (10). However, in a similar targeting system using CD4+ cells, it was reported that there was an early arrest of T cells in both instances of tolerance and activation, with both regaining motility over time (8). Because of differing conclusions in these studies, there is still conflict in the field on whether there exists a rapid stop of T cells on DCs under conditions of tolerance. A major limitation of these studies is that they followed the fate of T cells in response to an exogenous antigen that was targeted to DCs and not an endogenously produced true self-antigen presented via intrinsic pathways.
To address the mechanisms underlying the induction and breakdown of T-cell tolerance in vivo, we have used a combination of single-cell biochemical analyses and live cell imaging. For these studies, we have exploited an experimental system in which we can determine the T-cell response to a transgene-encoded, endogenous, soluble self-antigen [ovalbumin (sOVA)]. Using this model, we have previously shown that, in a lymphocyte-sufficient host (sOVA-Tg), interaction of CD4+ T cells with the systemic antigen leads to profound unresponsiveness of T cells followed by their deletion. In marked contrast, interaction of the same T cells with the same antigen in a lymphopenic (sOVA-Tg/Rag−/−) host leads to persistent T-cell activation and an inflammatory disease that is representative of systemic, T cell-mediated autoimmunity (11, 12). The state of T-cell tolerance that develops in lymphocyte-replete sOVA-Tg recipients is characterized by a lack of cytokine production including IL-2, IFNγ, and IL-17. In lymphopenic sOVA-Tg/Rag−/− recipients, the disease that develops is characterized by the presence of IFNγ- and IL-17—producing effector cells. In addition, disease progresses even in the absence of IFNγ, but the loss of IL-17 reduces the skin inflammation associated with disease progression (11, 12). In previous work, we have established this experimental model and shown that it has great potential for analyzing how the choice between tolerance and activation is made. The mechanistic studies reported in the paper are our initial endeavors to address this problem.
Our results emphasize the importance of an intact immune system in determining the consequences of antigen recognition: tolerance in the presence of an intact lymphoid compartment and autoimmunity in the absence of endogenous lymphocytes. This model is relevant to understanding the links between immunodeficiency and autoimmunity, disorders such as graft vs. host disease, and more generally, the mechanisms that determine the choice between tolerance and autoimmunity. This experimental model has several important strengths: the antigen is an endogenously produced self-antigen, the distinction between tolerance and activation is absolute and invariable, and the responses of single cells can be followed quantitatively after specific antigen recognition.
To examine the early T-cell response, prior to the generation of effector cells, we used a combination of two-photon microscopy and analysis of intracellular signaling proteins within hours after T-cell transfer, allowing us to determine the differences in the initiation of the T-cell response under conditions of tolerance or activation. Our data show that activation of T-cell receptor signaling pathways downstream of mTOR and NF-κB is increased and prolonged in situations of productive activation. Additionally, our studies have identified two phases in the T cell–antigen presenting cell (APC) interaction: (i) under conditions of both tolerance and activation, the antigen-specific cells stop at an early time point on APCs, and (ii) this early stop signal is transient in situations of tolerance but the arrest phase persists in settings of autoimmunity. These data suggest that failure of self-tolerance is caused by prolonged T cell–APC interactions and concomitant T-cell signals, whereas transient interactions and signaling lead to abortive activation that may define the tolerant phenotype.
Results
Lymphopenia Results in Failure of Tolerance to an Endogenously Produced Self-Antigen.
To define the functional consequences of T-cell encounter with an endogenous antigen under conditions of activation and unresponsiveness, 1 × 106 DO11.10 Rag−/− CD4+ T cells (DO11.10) were transferred into either sOVA-Tg or sOVA-Tg/Rag−/− hosts. Lymph nodes (LNs) were harvested at various time points posttransfer, and cytokine production in the DO11.10 cells was then examined by flow cytometry. In sOVA-Tg recipient mice, no cytokine production was detected in the DO11.10 cells at any time point assayed, whereas in sOVA-Tg/Rag−/− mice, both IFNγ- and IL-17–producing populations emerged between 2 and 5 d posttransfer (Fig. 1 A and B). As previously shown, the IL-17 response waned after day 5, whereas the IFNγ response progressively increased in sOVA-Tg/Rag−/− mice (Fig. 1 A and B) (11). Additional analysis of tolerant DO11.10 cells shows that they are also unable to produce the cytokines IL-13, IL-2, IL-10, and IL-4 upon restimulation and are primarily Foxp3− (Fig. S1 A and B). We then followed the expression of activation markers and the proliferation of OVA-specific cells in both hosts. As early as 3 h posttransfer, DO11.10 cells began to up-regulate the activation markers CD25 and CD44 and did so to a similar extent over the initial 18 h assayed in both sOVA-Tg and sOVA-Tg/Rag−/− mice compared with cells transferred to control wild-type BALB/c mice (WT BALB/c). However, by day 5, a greater percentage of DO11.10 cells in sOVA-Tg mice had down-regulated CD25, whereas in sOVA-Tg/Rag−/− mice, the cells retained a greater amount of CD25 expression and showed a slight decrease in CD44 expression (Fig. 1C). In addition, expression of CD62L on these cells was increased in sOVA-Tg mice compared with sOVA-Tg/Rag−/− mice, suggesting a propensity for tolerant cells to be retained in the LNs compared with the effectors found in autoimmune settings (Fig. S1C). To analyze T-cell proliferation, we assayed Ki67 staining and found that DO11.10 cells enter into cell cycle by 48 h posttransfer in both sOVA-Tg and sOVA-Tg/Rag−/− mice, but this proliferation is maintained at high levels only in the antigen-specific cells in sOVA-Tg/Rag−/− mice (Fig. 1D). These data indicate that OVA reactive cells undergo similar early activation patterns in all assays, independent of the functional outcome of antigen recognition. However, full activation, as measured by activation marker expression and Ki67 staining, wanes under conditions of tolerance but is maintained under conditions of uncontrolled activation.
Fig. 1.
Early activation of antigen-specific cells under conditions of tolerance or autoimmunity. 1 × 106 CD4+ T cells were purified from DO11.10 Rag−/− mice and adoptively transferred i.v. into WT BALB/c, sOVA-Tg, and sOVA-Tg/Rag−/− recipients. At the indicated time points posttransfer, cells were harvested for analysis. (A) Representative FACS plots of CD4+ T cells that were isolated and restimulated for 18 h with OVA-pulsed dendritic cells for intracellular staining of IL-17A and IFNγ. (B) Cumulative data for the four experiments shown in A. (C) Lymph node cells were stained directly ex vivo for the activation markers CD44 and CD25. Data are representative of three individual experiments. (D) Spleen cells were assayed for Ki67 expression. Data are representative of three comparable experiments. All data in FACS plots are gated on donor CD4+KJ-126+ donor cells.
Prolonged Biochemical Signals Under Conditions of Activation.
To gain insight into the early events upon TCR engagement that influence the decision between tolerance and activation, we analyzed the activation kinetics of T-cell signals downstream of the TCR after antigen recognition. Intracellular pS6 and total IκBα were measured in self-reactive DO11.10 T cells after transfer to recipient mice. The S6 ribosomal protein is phosphorylated downstream of the AKT/mTOR pathway and has been shown to integrate multiple signals including the MAPK pathway, whereas IκBα levels correlate with the level of NF-κB activation, which is triggered by numerous pathways including MAPK (13, 14). We chose to study these biochemical signals as representative readouts of TCR signal transduction. 1 × 106 DO11.10 cells were transferred into WT BALB/c, sOVA-Tg, and sOVA-Tg/Rag−/− mice, and we analyzed a range of time points to better define the early response to self-antigen encounter under conditions of tolerance and activation. Background levels of pS6 were detected from DO11.10 cells transferred to WT BALB/c mice at all time points analyzed (Fig. 2 A–C). Comparable background levels were also detected on transfer to lymphopenic BALB/c Rag−/− mice not expressing the cognate antigen (Fig. S2A). In contrast, antigen-specific cells began to up-regulate pS6 as early as 3 h posttransfer into both sOVA-Tg and sOVA-Tg/Rag−/− hosts, and by 8 h, pS6 was present in a majority of the antigen-specific DO11.10 cells. Interestingly, as the percentage and mean fluorescence intensity of pS6+ cells continued to rise over time in lymphopenic sOVA-Tg/Rag−/− mice, pS6 levels in DO11.10 cells transferred into sOVA-Tg mice began to decline before 24 h (Fig. 2 A–C). The pooled results of pS6 assays in individual mice from multiple experiments show that, despite similar induction of pS6, these signals are both increased and prolonged in autoimmune sOVA-Tg/Rag−/− mice compared with tolerant sOVA-Tg mice (Fig. 2 B and C).
Fig. 2.
The tolerant phenotype is associated with transient pS6 induction. 1 × 106 DO11.10 Rag−/− CD4+ T cells were adoptively transferred i.v. into WT BALB/c, sOVA-Tg, and sOVA-Tg/Rag−/− recipients. Spleens were isolated at the indicated time points posttransfer for flow cytometry. (A) Representative histograms of pS6. (B) Summary of time course pS6 data for four individual experiments. (C) Mean fluorescence intensity (MFI) of pS6 levels for four individual experiments. All data in histograms and bar graphs are gated on CD4+KJ-126+ donor cells.
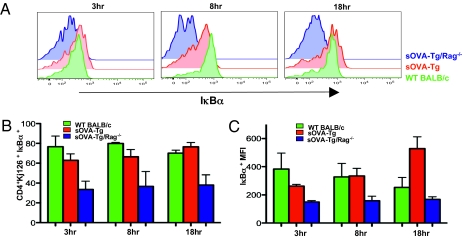
Assays for IκBα showed that, at 3 h posttransfer, levels of total IκBα were similar in DO11.10 cells transferred into sOVA-Tg and WT BALB/c mice, whereas levels in sOVA-Tg/Rag−/− mice were reduced, suggesting degradation of IκB and activation of NF-κB under conditions of T-cell activation and autoimmunity (Fig. 3 A–C). By 8 h posttransfer, there was a slight reduction of IκBα levels in cells isolated from sOVA-Tg mice compared with WT BALB/c, but the decrease seen in sOVA-Tg/Rag−/− mice was much more dramatic. In addition, the activation of NF-κB (as shown by IκBα degradation) seen in sOVA-Tg/Rag−/− mice was maintained throughout all of the early time points assayed but lost in sOVA-Tg mice (Fig. 3 A–C). Thus, the magnitude of NF-κB activation shows a clear correlation with the functional outcome of antigen encounter.
Fig. 3.
The tolerant phenotype is associated with reduced IκB degradation. 1 × 106 DO11.10 Rag−/− CD4+ T cells were adoptively transferred i.v. into WT BALB/c, sOVA-Tg, and sOVA-Tg/Rag−/− recipients. Spleens were isolated at the indicated time points posttransfer for flow cytometry. (A) Representative histograms of total IκBα. (B) Summary of time course data for three individual experiments of IκBα. (C) MFI of IκBα levels for three individual experiments. All data in histograms and bar graphs are gated on CD4+KJ-126+ donor cells.
Self-Reactive CD4+ T Cells Receive Early Stop Signals upon Antigen Encounter.
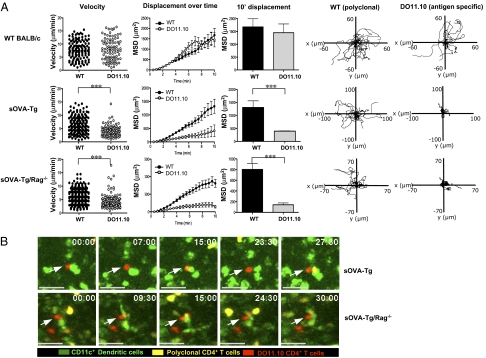
To determine if the early differences found in TCR signaling are a reflection of the T cell–APC interaction, we focused our studies on the nature of cell–cell contacts during the initiation of the immune response using two-photon imaging. We cotransferred 1 × 106 5-(and 6) carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD4+ T cells isolated from WT BALB/c hosts (polyclonal) as an internal control for cell movement with 1 × 106 antigen-specific, 5-(and 6)-{[(4 chloromethyl) benzoyl]amino}tetramethylrhodamine (CMTMR)-labeled DO11.10 T cells into WT BALB/c, sOVA-Tg, and sOVA-Tg/Rag−/− mice. Inguinal LNs were excised and imaged by two-photon laser scanning microscopy (TPLSM). At 4 h posttransfer, in WT BALB/c recipients (in the absence of cognate antigen), polyclonal and antigen-specific (DO11.10) cells moved freely through the LN, with no significant differences in their measured velocity, mean square displacement, and random migration paths (Fig. 4A and Movie S1). Comparable cell movement was also seen upon transfer to lymphopenic BALB/c Rag−/− mice (Fig. S2B Upper). At 4 h posttransfer in both sOVA-Tg and sOVA-Tg/Rag−/− recipient mice expressing self-antigen, polyclonal cells moved freely, whereas antigen-specific (DO11.10) cells showed a significant decrease in both their velocity and mean square displacement (Fig. 4A and Movies S2 and S3). These data suggest that early after antigen encounter, self-reactive T cells stop on APCs upon transfer to both tolerant and autoimmune hosts. Therefore, the induction of tolerance requires initial antigen recognition that is sufficient to induce a stop signal between T cells and APCs.
Fig. 4.
Self-reactive CD4+ T cells receive an early stop signal under conditions of both tolerance and autoimmunity. (A) A 1:1 ratio of 1–3 × 106 CD4+ T cells from DO11.10 Rag−/− (CMTMR-labeled) and WT BALB/c (CFSE-labeled) mice were adoptively transferred i.v. into WT BALB/c, sOVA-Tg, or sOVA-Tg/Rag−/− recipients. Inguinal lymph nodes were isolated 4 h posttransfer and imaged by TPLSM. The velocity (micrometers per minute), mean square displacement, and 10′ mean square displacement for polyclonal CD4+ T cells and DO11.10 CD4+ T cells are shown. In WT BALB/c mice, polyclonal cells had a mean velocity of 7.693 ± 0.3063 μm/min, whereas DO11.10 cells had a mean velocity of 7.933 ± 0.4218 μm/min. In sOVA-Tg recipient mice, polyclonal cells had a mean velocity of 6.539 ± 0.2385 μm/min, whereas DO11.10 cells had a mean velocity of 4.007 ± 0.1746 μm/min. In sOVA-Tg/Rag−/− recipient mice, polyclonal cells had a mean velocity of 6.290 ± 0.1694 μm/min, whereas DO11.10 cells had a mean velocity of 3.988 ± 0.1590 μm/min. Tracks of individual polyclonal CD4+ T cells and DO11.10 CD4+ T cells are also shown. Data are representative of three individual experiments containing two to three imaged time lapses each. (B) Time lapse images showing antigen-specific DO11.10 cells (red) stopped on CD11c+ DCs (green), whereas polyclonal cells (yellow) continue to traffic through the lymph node. Arrows point to arrested DO11.10 CD4+ T cells. Calibration stamp represents 20 micrometers. Data are representative of two individual experiments containing two to three imaged time lapses each. ***P ≤ 0.001 using a two-tailed, unpaired t test.
It is generally accepted that naïve T cells recognize their cognate antigen on DCs within the LN, although the APCs that normally present endogenous self-antigens are not clearly defined. To determine if the antigen-specific cells were receiving a stop signal through conventional DCs, we crossed sOVA-Tg recipient mice onto a CD11c-GFP reporter background, enabling us to follow T-cell interactions with the GFP-labeled DC population. Again, polyclonal and DO11.10 T cells were cotransferred into recipient mice and visualized by TPLSM at 4 h posttransfer. The antigen-specific DO11.10 cells (red) transferred to both sOVA-Tg and sOVA-Tg/Rag−/− mice were seen associated with GFP-labeled CD11c+ DCs (green) in interactions that remained stable for the entire 30 min time lapse imaged (Fig. 4B and Movies S4 and S5). In the same local environment, the control polyclonal cells (yellow) moved freely. These data confirm that antigen-specific cells are receiving an early stop signal from CD11c+ DCs under conditions of both tolerance and activation.
Release of T Cells from APCs Under Conditions of Tolerance.
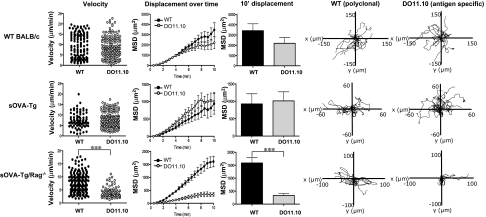
It has been shown that the duration of T-cell interactions with DCs can influence the resulting effector response (6, 15, 16). To determine if the interactions seen between DO11.10 cells and DCs are maintained after the early time point, we next examined T-cell motility in LNs by TPLSM at 18 h posttransfer. As seen at the earlier 4 h time point, both polyclonal and DO11.10 cells transferred to WT BALB/c showed no significant differences in velocity or mean square displacement, suggesting unrestricted T-cell motility (Fig. 5 Top and Movie S6). As seen before, comparable cell movement was also seen on transfer to lymphopenic BALB/c Rag−/− mice (Fig. S2B Lower). However, the behavior of the antigen-specific DO11.10 cells was strikingly different under conditions of activation compared with tolerance. At this late time point, the DO11.10 cells in sOVA-Tg/Rag−/− mice remained stopped on DCs and displayed significantly reduced velocity and mean square displacement (Fig. 5 Bottom and Movie S7). Similar results were seen in lymphopenic mice that lack only αβT cells (sOVA-Tg/TCRα−/−) (Fig. S3 and Movies S8 and S9). In contrast, DO11.10 cells in sOVA-Tg mice regained their motility, similar to the cotransferred, polyclonal control cells (Fig. 5 Middle and Movie S10). These data indicate that, under conditions of lymphopenia-induced autoimmunity, T cells form long-lived interactions with DCs, which culminate in strong activation, including the induction of strong intracellular biochemical signaling; however, in the presence of a full immune compartment, antigen-specific cells form early contacts with DCs, but these interactions and the subsequent biochemical signals are not maintained and the functional outcome is tolerance.
Fig. 5.
Self-reactive CD4+ T cells in tolerant hosts have regained their motility at 18 h posttransfer. A 1:1 ratio of 1–3 × 106 CD4+ T cells from DO11.10 Rag−/− (CMTMR-labeled) and WT BALB/c (CFSE-labeled) mice were adoptively transferred i.v. into WT BALB/c, sOVA-Tg, or sOVA-Tg/Rag−/− recipients. Inguinal lymph nodes were isolated 18 h after transfer and imaged by TPLSM. The velocity (micrometers per minute), mean square displacement, and 10′ mean square displacement for polyclonal CD4+ T cells and DO11.10 CD4+ T cells were determined. In WT BALB/c hosts, polyclonal cells had a mean velocity of 8.980 ± 0.4973 μm/min, whereas DO11.10 cells had a mean velocity of 8.693 ± 0.3998 μm/min. For sOVA-Tg mice, polyclonal cells had a mean velocity of 7.567 ± 0.4079 μm/min, whereas DO11.10 cells had mean velocity of 7.915 ± 0.2812 μm/min. In sOVA-Tg/Rag−/− mice, polyclonal cells had a mean velocity of 11.16 ± 0.4494 μm/min, whereas DO11.10 cells had a mean velocity of 3.133 ± 0.1969 μm/min. Tracks of individual polyclonal CD4+ T cells and DO11.10 CD4 T cells are shown. Data are representative of three individual experiments containing two to three imaged time lapses each. ***P ≤ 0.001 using a two-tailed, unpaired t test.
Discussion
The studies reported in this paper lead to several important conclusions that are relevant to defining the choice between T-cell tolerance and activation. First, there are many similarities in the early responses of T cells exhibiting either tolerance or activation, despite the fact that the functional outcomes after antigen recognition are vastly different. Even under conditions of tolerance, T cells form stable interactions with DCs and receive early signals through the TCR, which facilitate activation marker expression, initiate proliferation, and promote intracellular signaling, including the induction of pS6 and degradation of IκBα. Second, the choice between tolerance and activation is determined mainly by the duration and magnitude of the T cell–APC interaction. In sOVA-Tg recipients with an intact lymphoid compartment in which the antigen-specific cells become tolerant, the interactions with APCs are transient. The stop signals are evident at 4 h, but the T cells are released from APCs by 18 h. In striking contrast, in lymphopenic recipients where the transferred T cells undergo massive activation, the interactions are stable for at least 18 h. The biochemical consequences of these prolonged T cell–APC interactions in lymphopenic hosts include the activation of intracellular signaling pathways, shown in our studies by the induction of pS6 and degradation of IκB, which correlates with activation of NF-κB. Functionally, these changes are sufficient to induce stable and prolonged expression of activation markers, massive proliferation, and generation of effector T cells.
It has been shown that T cells need prolonged and perhaps, multiple encounters with APCs to initiate their full responses and that these signals are integrated for optimal effector potential (16). Our results suggest that the increased signaling seen in sOVA-Tg/Rag−/− recipient mice is the consequence of the duration of interactions between T cells and APCs presenting cognate antigen. It is also possible that there exists a feedback loop such that signaling leads to enhanced adhesion, which further augments intracellular signaling. It is not known which biochemical and functional responses require sustained antigen recognition. Our studies begin to provide a glimpse of the consequences of different durations of antigen encounter by correlating the T-cell response with the duration of the initial stop signals and the concomitant biochemical signals.
A fundamental question that arises from these studies is why lymphopenia promotes prolonged T cell–APC interactions and the subsequent biochemical and functional responses of the T cells. In the absence of endogenous T cells (sOVA-Tg/Rag−/− and sOVA-Tg/TCRα−/− recipients), we observe the formation of long-lived conjugates between T cells and APCs, whereas only initial arrest is seen in mice with an intact T-cell compartment (sOVA-Tg) (Fig. 5 and Fig. S3). One possibility is that regulatory T cells (Tregs) are absent from the lymphopenic recipients and that these cells can inhibit the interactions between T cells and dendritic cells, preventing T-cell arrest and/or reducing the duration of contacts (17, 18). We have depleted endogenous Tregs from sOVA-Tg mice by treating the mice with an anti-CD25 antibody (PC61) before transfer of DO11.10 T cells. Imaging studies under these conditions show no differences in the motility of antigen-specific T cells after depletion (Fig. S4A), with the caveat that Treg depletion is not complete, leaving ∼4% of CD4+ Foxp3+ T cells after antibody treatment compared with ∼9% in untreated mice (Fig. S4B).
An additional possibility that could account for transient T-cell contacts in sOVA-Tg mice is the lack of costimulation or a less mature DC population. However, we have not observed consistent phenotypic differences in DCs isolated from sOVA-Tg and sOVA-Tg/Rag−/− mice at these early time points, suggesting that the DCs are not spontaneously activated in the latter. To determine if DC activation was able to promote sustained interactions, we treated sOVA-Tg mice with agonistic anti-CD40 antibodies at the time of T-cell transfer. Upon DC activation under these conditions, no changes were observed in the motility of antigen-specific T cells at 18 h compared with mice that did not receive the activating antibody (Fig. S5). These data suggest that DC activation status may not be the primary difference accounting for prolonged interactions in sOVA-Tg/Rag−/− mice compared with sOVA-Tg mice.
It seems likely that the transient signals seen in intact sOVA-Tg recipients may be because endogenous lymphocytes compete with DO11.10 cells for growth factors and costimulation. The competing lymphocytes may be nonspecific or may be specific for ligands that cross-react with the antigen recognized by the DO11.10 cells. Lymphopenia is known to be a powerful trigger for autoimmunity, consistent with the importance of competition with an intact immune system for maintaining self-tolerance. Our studies show that tolerance can be broken by reducing competition with endogenous T cells, and these data show that the duration of TCR signals received is the key factor in determining whether self-reactive cells become tolerant or undergo full activation.
Materials and Methods
Mice.
All experimental mice were used at 5–8 wk of age. WT BALB/c mice were purchased from Charles River Laboratory. Transgenic mice expressing the DO11.10 TCR were obtained from K. Murphy (Washington University, St. Louis, MO) and were crossed onto a Rag2−/− background for use as donor cells. Soluble OVA transgenic mice (sOVA-Tg) express a soluble form of OVA as described (19). sOVA-Tg mice were also used on a Rag2−/− and a TCRα−/− background as described (20). CD11c-GFP mice were obtained from Jackson Laboratories and crossed onto the sOVA-Tg Rag2−/− mice. All mice were bred and maintained in a specific pathogen-free facility in accordance with the guidelines of the Laboratory Animal Resource Center of the University of California.
Preparation of Splenic Single-Cell Suspensions.
After stimulation in vivo, the spleens of recipient mice were harvested as previously described (21). At the indicated time points, spleens were dissociated in PBS containing 1.6% paraformaldehyde (Electron Microscopy Sciences) for 15 min at room temperature. Cell suspensions were then passed through a 70-μm filter, and ice-cold methanol was added to a final concentration of 80% methanol.
Staining and Flow Cytometry.
Fixed and permeabilized cells were prepared for flow cytometric analysis by intracellular cytokine staining as previously described (11). Splenic single-cell suspensions were used for intracellular signaling analysis. The following antibodies were used to identify and analyze donor and recipient cell populations: Ki67 (B56; BD Biosciences), B220 (RA3-6B2; BD Biosciences), CD90.2 (30-H12; Biolegend), CD4 (RM4-5; BD Biosciences), CD11b (M1/70; BD Biosciences), antikeyhole limpet hemocyanin control Ab (X40; BD Biosciences), CD90.1 (HIS51; BD Biosciences), and anti-DO11.10 TCR (KJ1-26; BD Biosciences). pS6 ribosomal protein (Ser235/236, 2F9) and IκBα (L35A5) were purchased from Cell Signaling Technologies.
Cell Preparations, Purifications, and Adoptive Transfer.
CD4+ T cells for adoptive transfer were purified from spleens and LNs of WT BALB/c or DO11.10 Rag2−/− mice using the EasySep Mouse CD4+ T-cell enrichment kit (StemCell Technologies) with the addition of anti-CD25 biotin for removal of endogenous Tregs. After antibody addition, the cells were run over a magnetic column (Miltenyi). For microscopy experiments, cells were either labeled with 20 μM CMTMR or 5 μM CFSE (Invitrogen) for 30 min at 37 °C; 1 × 106 unlabeled DO11.10 Rag2−/− (for flow cytometry samples) or a 1:1 mix of labeled (1–3 × 106 each) WT BALB/c and DO11.10 Rag2−/− (for TPLSM) were transferred i.v. into recipient mice.
TPLSM.
For imaging of polyclonal and antigen-specific T cells, inguinal LNs were immobilized on coverslips with the efferent lymphatics adhered to the coverslip. During imaging, LNs were kept at a temperature between 35.5 °C and 37 °C in a flow chamber perfused with RPMI medium without phenol red (Gibco) saturated with 95% O2/5% CO2. For two-photon imaging, we used a custom resonant scanning instrument based on published designs (22). A custom 4D acquisition module in the VideoSavant digital video recording software (IO Industries) was used for image acquisition (22). Samples were excited with a MaiTai TiSaphire laser (SpectraPhysics) tuned to a wavelength of 810 nm (or 910 nm when CD11c-GFP recipient mice were used). Emission wavelengths of 500–540 nm (for CFSE and GFP) and 567–640 nm (for CMTMR) were collected. After assessing the entire LN for the presence of transferred cells, images of up to 30 xy planes with 3 μm Z spacing were acquired every 30 s for 30 min.
TPLSM Data Analysis.
The cell tracks generated were individually verified for accuracy. To calculate velocity, mean square displacement and 10′ mean square displacement tracks that were as least 5 min in duration were used. All graphs depicting velocity and displacement are a combination of two representative time lapses for each condition. Data were visualized and analyzed using Imaris (Bitplane), MATLAB (Mathworks), and Metamorph (MDS) software.
Statistical Analysis.
All statistical analysis was done using Prism software (GraphPad). Error bars for all figures and any number that follows ± is SE.
Supplementary Material
Acknowledgments
We thank Carlos Benetiz (University of California, San Francisco, CA) for assistance in animal husbandry, Shu-wei Jiang (University of California, San Francisco, CA) for cell sorting, and Peter Krutzik (Stanford University, Stanford, CA) for the directly conjugated IκBα antibodies. In addition, we thank Jordan Jacobelli.(University of California, San Francisco, CA), Peter Beemiller (University of California, San Francisco, CA), and the University of California San Francisco biological imaging development center (San Francisco, CA) for assistance with TPLSM experiments and analysis software. This work was supported by National Institutes of Health Grants F32 AI077199 (to S.D.K.), AI52116 (to M.F.K.), U19 AI057229 (to G.P.N.), HHSN272200700038C (to G.P.N.), P01 AI35297 (to A.K.A.), and R01 AI64677 (to A.K.A.). This work was also supported by the Larry L. Hillblom Foundation (to R.S.F.) and the Leukemia and Lymphoma Foundation (to M.F.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010560107/-/DCSupplemental.
References
- 1.Zheng Y, et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmond RJ, Emery J, Okkenhaug K, Zamoyska R. MAPK, phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. J Immunol. 2009;183:7388–7397. doi: 10.4049/jimmunol.0902294. [DOI] [PubMed] [Google Scholar]
- 4.Macián F, et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 5.Chiodetti L, Choi S, Barber DL, Schwartz RH. Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol. 2006;176:2279–2291. doi: 10.4049/jimmunol.176.4.2279. [DOI] [PubMed] [Google Scholar]
- 6.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 7.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shakhar G, et al. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hugues S, et al. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 10.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: From protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto S, Kimball SR, Safer B. Signal transduction pathways that contribute to increased protein synthesis during T-cell activation. Biochim Biophys Acta. 2000;1494:28–42. doi: 10.1016/s0167-4781(00)00208-6. [DOI] [PubMed] [Google Scholar]
- 15.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 16.Celli S, Garcia Z, Bousso P. CD4 T cells integrate signals delivered during successive DC encounters in vivo. J Exp Med. 2005;202:1271–1278. doi: 10.1084/jem.20051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tadokoro CE, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohr J, Knoechel B, Kahn EC, Abbas AK. Role of B7 in T cell tolerance. J Immunol. 2004;173:5028–5035. doi: 10.4049/jimmunol.173.8.5028. [DOI] [PubMed] [Google Scholar]
- 20.Knoechel B, Lohr J, Kahn E, Abbas AK. The link between lymphocyte deficiency and autoimmunity: Roles of endogenous T and B lymphocytes in tolerance. J Immunol. 2005;175:21–26. doi: 10.4049/jimmunol.175.1.21. [DOI] [PubMed] [Google Scholar]
- 21.O'Gorman WE, et al. The initial phase of an immune response functions to activate regulatory T cells. J Immunol. 2009;183:332–339. doi: 10.4049/jimmunol.0900691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bullen A, Friedman RS, Krummel MF. Two-photon imaging of the immune system: A custom technology platform for high-speed, multicolor tissue imaging of immune responses. Curr Top Microbiol Immunol. 2009;334:1–29. doi: 10.1007/978-3-540-93864-4_1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.