Abstract
The brain is not routinely surveyed by lymphocytes and is defined as an immuno-privileged site. However, viral infection of the brain results in the infiltration and long-term persistence of pathogen-specific CD8+ T cells. These cells survive without replenishment from the circulation and are referred to as resident memory T cells (Trm). Brain Trm selectively express the integrin CD103, the expression of which is dependent on antigen recognition within the tissue. After clearance of virus, CD8+ T cells persist in tight clusters, presumably at prior infection hot spots. Antigen persistence is not a prerequisite for T-cell retention, as suggested by the failure to detect viral genomes in the T-cell clusters. Furthermore, we show that an intracranial dendritic cell immunization regimen, which allows the transient introduction of antigen, also results in the generation of memory T cells that persist long term in the brain. Brain Trm die rapidly on isolation from the tissue and fail to undergo recall expansion after adoptive transfer into the bloodstream of antigen-challenged recipients. These ex vivo defects imply a dependency on the local milieu for function and survival. Cumulatively, this work shows that Trm are a specialized population of memory T cells that can be deposited in tissues previously thought to be beyond routine immune surveillance.
Keywords: resident memory T cells, viral infection, CD8, vesicular stomatitis virus, CD103
After the clearance of an acute infection, the greatly expanded effector CD8+ T-cell population dramatically contracts, leaving behind a small but numerically significant population of cells that enter the memory T-cell pool (1–3). Antigen-specific CD8+ memory T cells remain at stable levels for prolonged periods of time in the complete absence of antigen (4–6) by cytokine-driven homeostatic proliferation (7, 8). Some of these memory cells can enter peripheral tissues (9–11) and from there, form the front line of defense against reinfection (12–14). Nonetheless, elegant parabiosis studies connecting the blood streams of immune and naive mice showed that circulating memory T cells do not freely access all tissues during surveillance, with the brain and lamina propria of the gut identified as tissues with minimal routine memory T-cell infiltration (15).
In addition to the limited memory T-cell infiltration, the brain has several features that classify it as immune-privileged. These include the lack of regular lymphatics, the presence of the blood–brain barrier, which limits the entry of antibody, additional barriers to the entry of white blood cells, and the general MHC low nature of the resident cells in the steady state (16). Nonetheless, infection or inflammation within the brain dramatically increases its permeability to cells and effector molecules of the immune system. Penetration of T cells into the brain parenchyma is thought to be, in some cases, antigen-specific (17, 18) and highly dependent on the integrin α4β1 very late antigen 4 (VLA-4) interaction with the adhesion molecule vascular cell adhesion molecule 1 (VCAM-1) expressed on inflamed endothelial cells (19).
In addition to memory T-cell populations that traverse through peripheral tissues, a distinct memory T-cell population that persists long term within nonlymphoid tissue, commonly at sites of prior infection, has recently been documented. These memory T cells are resident in nature, self-renewing, and highly protective against subsequent infections, and they were termed resident memory T cells (Trm). Initially identified in the skin and sensory ganglia of mice latently infected with HSV (13, 20, 21) and in the gut (22), these cells were shown to rapidly acquire effector function on secondary pathogenic encounter and in certain situations, undergo extensive proliferation in situ in response to antigen presented locally by incoming inflammatory dendritic cells. Here, we extend these studies and examine the nature of Trm in the brain after viral infection.
Using an intranasal (i.n.) vesicular stomatitis virus (VSV) infection model, we show the generation of CD103+ Trm in the brain parenchyma. Retroviral knockdown of CD103 expression shows that the integrin not only phenotypically denotes the majority of the resident memory T cells in the brain but functionally aids in Trm generation and accumulation. We provide evidence for local DC presentation within the brain as a means to drive CD103 expression on infiltrating T cells and show that memory T cells can persist in the brain in the absence of persisting antigen. Furthermore, we show that brain Trm exhibit functional defects when removed from their site of residence, further supporting the idea that Trm are a distinct memory CD8+ T-cell population tailored to survive and function within their local environment.
Results
Persistence of CD8+ T Cells in the Brain After VSV Infection.
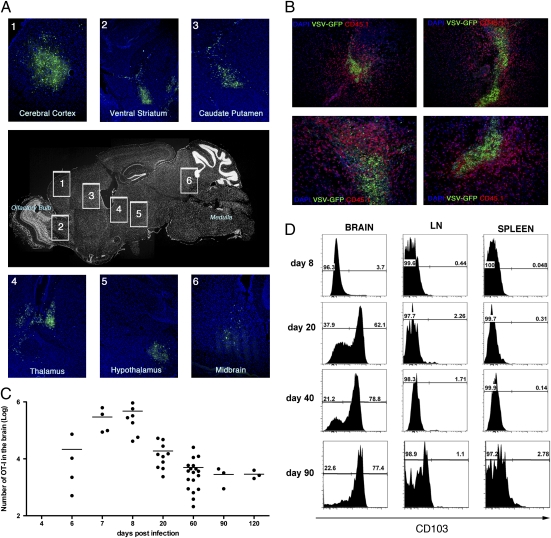
i.n. infection of mice with VSV results in an acute systemic infection with multiple organs, including the brain and lung, harboring replicating virus (Fig. S1A). B6 mice that had been seeded with low numbers of Ova-specific OT-I.CD45.1 T cells were infected with recombinant VSV that expresses GFP and a truncated form of Ovalbumin (Ova). By day 3 postinfection, distinct patches of GFP+ virus-infected regions could be detected throughout the brain (Fig. 1A), and by day 8 postinfection, large numbers of OT-I T cells had infiltrated the brain and could be found swarming around the last remnants of virus (GFP+)-infected regions (Fig. 1B). Although GFP+-infected cells could not be detected in the brain beyond day 8 postinfection, OT-I T cells persisted there for at least 120 d after infection (Fig. 1C).
Fig. 1.
Systemic infection after intranasal VSV infection. Mice were seeded with naïve OT-I.CD45.1 T cells before intranasal (i.n.) infection with VSV-GFP-Ovalbumin. (A) Brains were recovered on day 3 p.i., and virus infected regions were identified based on GFP expression. (B) Brains were recovered on day 8 p.i. and stained with anti-CD45.1 (red), DAPI (blue), and VSV-GFP (green). (C) The graph depicts the number of OT-I cells in the brain at the indicated times postinfection as determined by flow cytometry. Data points represent individual mice, and horizontal bars indicate the mean. (D) The expression of CD103 on the OT-I T cells in the brain, cervical lymph node, and spleen at the indicated days.
Interestingly, the vast majority of the OT-I T cells persisting in the brain beyond day 20 postinfection expressed CD103—an integrin found on resident memory CD8+ T cells in the skin (13) and gut (22). We observed a dramatic up-regulation of CD103 expression level on OT-I cells in the brain between days 8 and 20 postinfection, after which time the ratio of CD103+ to CD103− T cells remained stable. In contrast, the vast majority of OT-I T cells in the spleen and lymph node (LN) lacked expression of this marker at all time points tested (Fig. 1D). Both the CD103+ and CD103− memory OT-I cells that persisted within the brain expressed comparable levels of several other phenotypic markers tested such as CD122, CD127, PD-1, CD69, and KLRG1 (Fig. S1B). However, the two subsets differed in granzyme B staining, where a much higher fraction of CD103+ cells expressed this effector molecule. Brain Trm expressed lower levels of CD122 than spleen memory cells and did not express PD-1. Thus, memory CD8+ T cells with a unique phenotype persist long term within the brain after viral infection.
Persistence of Trm Is Independent of the Circulating Memory T-Cell Population.
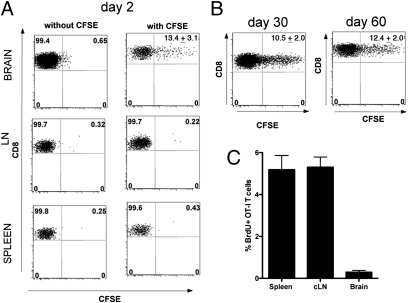
To question whether CD8+ T cells persisting within the brain after VSV infection were maintained by constant replenishment from the circulating memory T-cell pool or whether they represented a distinct self-sustaining population of memory T cells, we adapted a technique developed by McGill and Legge (23) to label lung resident T cells. Mice seeded with OT-I.CD45.1 CD8+ T cells were infected with VSV-Ovalbumin (VSV-OVA) (i.n.) and 20 d later, were injected intracranially with a solution of carboxyfluorescein succinimidyl ester (CFSE). Intracranial injection of CFSE labeled a proportion of the memory OT-I cells in the brain but did not label any cells in the spleen or draining deep cervical LN (Fig. 2A). The proportion of CFSE+ OT-I T cells in the brain remained stable for 60 d after injection (Fig. 2B), implying minimal replenishment from a circulating memory T-cell population. Memory OT-I CD8+ T cells introduced into the blood stream of mice infected with either VSV or VSV-OVA 20 d earlier failed to infiltrate the brain (Fig. S2), which further argues against routine memory T-cell surveillance of the brain (15).
Fig. 2.
Memory T cells persisting within the brain after VSV infection are resident. Mice were seeded with naïve OT-I T cells before infection with VSV-OVA (i.n.). On day 20 p.i., CFSE was injected intracranially, and the percentage of CFSE+ OT-I T cells was monitored in the brain, spleen, and deep cervical LN on days (A) 2, (B) 30, and 60 after CFSE injection. Representative flow cytometry profiles are depicted. Values represent the mean ± SEM. (C) Mice were seeded with naïve OT-I T cells before infection with VSV-OVA (i.n.). On day 20 p.i., mice were administered BrdU daily for 1 wk. Shown is the mean amount of BrdU incorporation in OT-I T cells in the spleen, cervical LN, and brain.
The minimal amount of CFSE dilution observed in Fig. 2B could also imply that the T cells in the brain are undergoing slow homeostatic division. To further investigate this, BrdU was administered to mice for 7 d, commencing on day 20 postinfection (p.i.). with VSV-OVA. The OT-I T cells in the brain undergo slower homeostatic division compared with the cells in the spleen and LN (Fig. 2C). A similar finding has been reported previously for resident memory T cells that persist in the skin (13).
Cumulatively, the data strongly imply that the memory T-cell population that resides within the brain persists with slow homeostatic division and without replenishment from the circulating memory T-cell pool.
Requirement for Antigen Recognition in the Brain for CD103 Expression.
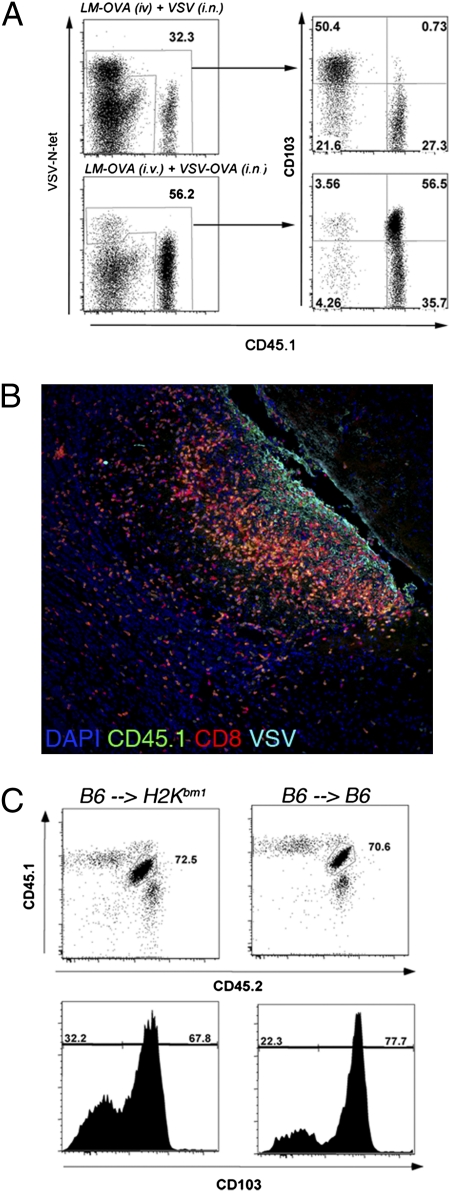
The majority of antigen-specific T cells persisting within the brain after i.n. VSV infection express CD103 (Fig. 1D). We next determined if the expression of CD103 was restricted to only T cells that had seen antigen in the brain or whether the inflammatory environment within the brain promoted CD103 expression. To do this, we systemically activated OT-I CD8+ T cells by infecting mice i.v. with recombinant Listeria monocytogenes (LM) that produces OVA (LM-OVA). Additionally, in the same mice, we generated nonspecific inflammation within the brain by the i.n. administration of wild-type VSV. The brains of these mice were recovered on day 30 postinfection, and the expression of CD103 on VSV-specific VSV-N tetramer+ cells and VSV-nonspecific OT-I T cells was examined. Consistent with our previous findings, the majority of VSV-N tetramer+ CD8+ T cells persisting within the brain expressed CD103. In striking contrast, the CD45.1+ OT-I T cells, which had not seen their antigen within the brain, did not express this marker (Fig. 3A Upper). Conversely, when mice were infected i.v. with LM-OVA and i.n. with VSV-OVA, a scenario where OVA antigen was present within the brain, the OT-I T cells that persisted in the brain did up-regulate CD103 (Fig. 3A Lower). Thus, the expression of CD103 is dependent on T cells encountering antigen within the brain. Interestingly, OT-I cells recruited to the brain after i.n. VSV infection could be detected around VSV antigen+ regions, suggesting that nonspecific T cells are subjected to the same environmental cues as their antigen-specific counterparts (Fig. 3B).
Fig. 3.
Requirement for antigen recognition within the brain for expression of CD103 on Trm. (A) Mice were seeded with naïve OT-I T cells and were infected with LM-OVA i.v. and either VSV or VSV-OVA i.n. On day 20 p.i., the level of expression of CD103 on OT-I cells (CD45.1) and polyclonal VSV N-specific CD8+ T (VSV-N-tet) cells isolated from the brain was determined. (B) Mice were seeded with naïve OT-I T cells (CD45.1) and were infected with LM-OVA i.v. and VSV i.n. On day 6 p.i., the brain were recovered and stained for VSV antigen (aqua), CD8 (red), CD45.1 (green), and DAPI (blue). (C) Naïve OT-I.CD45.1/2 cells were transferred into either [B6 →H2Kbm1] or [B6 →B6] radiation chimeras before infection with VSV-OVA (i.n.). On day 20 p.i., the level of CD103 expression on OT-I CD8+ T cells in the brain was determined.
CD8+ T cells may encounter antigen presented by professional antigen-presenting cells (APCs) and infected parenchymal cells. To identify the cell type driving CD103 expression within the brain, we generated C57BL/6 into H2Kbm1 bone marrow chimeras [B6 → H2Kbm1]. In these chimeric mice, the parenchymal cells cannot present antigen to OT-I T cells, whereas bone marrow-derived APCs are able to present antigen to OT-I T cells. Control chimeric mice [B6 → B6], where both the parenchyma and APCs can present antigen to OT-I T cells, were also generated. These mice were seeded with low numbers of OT-I.CD45.1/2 T cells before i.n. infection with VSV-OVA. Analysis of the brain on day 20 p.i. revealed a similar level of expression of CD103 on persisting OT-I CD8+ T cells, irrespective of parenchymal presentation of the appropriate MHC molecule (Fig. 3C). These data imply that antigen presentation by professional bone marrow-derived APCs alone can support CD103 expression by Trm.
Retrovirus Knockdown of CD103 Reduces the Retention of Memory T Cells in the Brain.
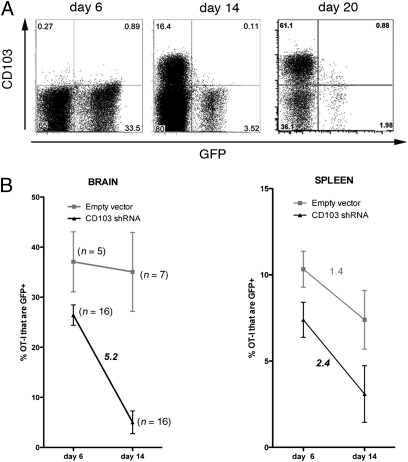
The integrin CD103 is expressed on the majority of pathogen-specific memory CD8+ T cells persisting within the brain after VSV infection. To determine if CD103 expression is involved in retaining memory T cells within the brain, we used a retroviral knockdown strategy to inhibit CD103 expression and monitored the effect that this had on T-cell retention. OT-I T cells infected with CD103 (αE) RNAi virus failed to up-regulate CD103 when cultured in vitro in the presence of TGFβ, validating a successful gene knockdown (Fig. S3).
A 1:1 mixture of either CD103 RNAi retrovirus- or empty vector-transduced GFP+ OT-I cells and nontransduced GFP− OT-I cells was adoptively transferred into mice, which were then infected i.n. with VSV-OVA. Although both the empty vector and CD103 RNAi-infected OT-I cells expanded and infiltrated the infected brain at early time points, the cells unable to express CD103 failed to persist as efficiently as control OT-I cells (Fig. 4 A and B). This implies that up-regulation of CD103 aids in T cell persistence within the brain by enhancing either T cell survival or retention. CD103+ OT-I T cells present in the brain on day 10 post–VSV-OVA infection displayed higher levels of expression of the prosurvival molecule Bcl-2 compared with their CD103− counterparts, suggesting a potential survival advantage at this acute time point (Fig. S4). Thus, CD103 expression not only phenotypically marks the majority of the resident memory T cells in the brain but has functional relevance in promoting T-cell accumulation within the brain.
Fig. 4.
Retrovirus knockdown of CD103 in OT-I cells reduces Trm accumulation in the brain. RNAi CD103 retrovirus- or control retrovirus-transduced OT-I.CD45.1 transgenic CD8+ T cells (CD45.1+GFP+) were adoptively transferred into naïve mice together with nontransduced OT-I cells (CD45.1+GFP−) followed by VSV-OVA infection. (A) Representative flow cytometry profiles depicting CD103 expression on RNAi CD103-transduced OT-I (GFP+) and nontransduced cells (GFP−) in the brains of mice at the indicated times. (B) The percentage of retrovirus-transduced (either control vector or RNAi CD103) OT-I persisting within the brain after VSV-OVA infection. Data represents the mean ± SEM, with the values indicating the fold reduction.
Brain Resident Memory CD8+ T Cells Persist in Clusters.
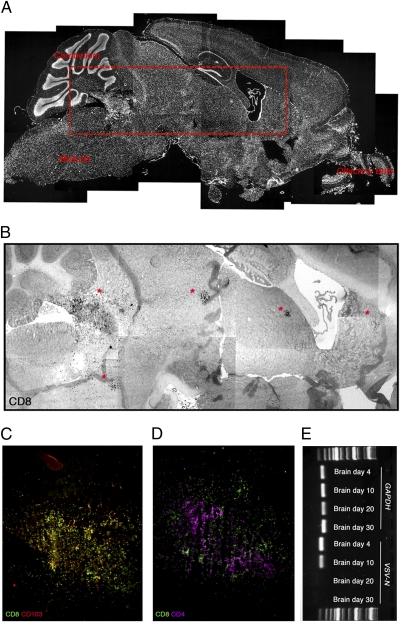
The CD8+ T cells persisting within the brain after VSV infection were located outside the vasculature (CD31+) and within the brain parenchyma (Fig. S5). Interestingly, some of the CD8+ T cells that persisted within the brain adopted a cluster configuration (Fig. 5 A and B). The majority of the CD8+ T cells in the cluster were CD103+ (Fig. 5C). Furthermore, some T-cell clusters also contained CD4+ T cells (Fig. 5D). We suspected that these CD8+ T cells may be congregating around prior infection hot spots (Fig. 1A) and the last remnants of persisting viral antigen. To assess if VSV genomic RNA (gRNA) was localized to the T-cell clusters, we used laser capture microdissection to excise T-cell clusters from the brains of mice at various times after infection and attempted to amplify viral gRNA. Although we could readily detect viral gRNA in tissue dissected from brains at days 4 and 10 postinfection, we were unable to amplify viral gRNA from day-20 and day-30 brain clusters (Fig. 5E). In addition, we could not detect antigen presentation to Ova-specific T cells in vitro after dendritic cell (DC) isolation from the brain at day 20 p.i. (Fig. S6). Although T cell cluster formation in the brain likely represents cells huddling around areas of prior viral infection, this result suggests that it is unnecessary for virus to persist within these clusters for T cells to sustain this configuration.
Fig. 5.
Brain resident memory CD8+ T cells persist in clusters. Brains were recovered from mice on day 21 p.i. with VSV-OVA (i.n.), cut, and stained with (A) DAPI (white), (B) anti-CD8 (black), (C) anti-CD8 (green) and anti-CD103 (red), or (D) anti-CD8 (green) and anti-CD4 (purple). (E) Detection of VSV gRNA in laser-capture microdissected areas of brain on day 4, 10, 20, and 30 p.i. with VSV-OVA.
Local DC Immunization Can Generate Trm.
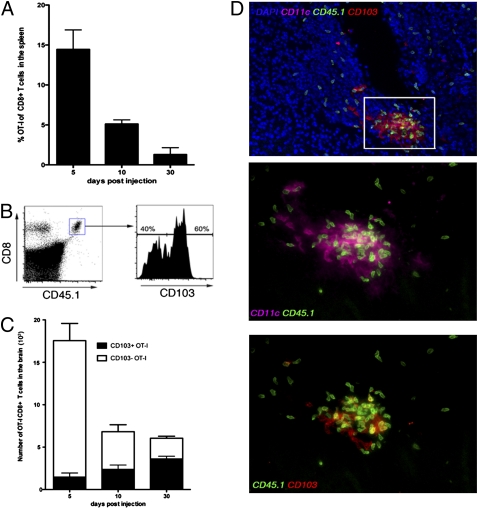
To formally address whether persisting antigen is required for the persistence of T cells within the brain, we injected peptide-loaded DCs into the brains of mice to question if this would result in the long-term deposition of CD8+ T cells at the local site of injection. Intracranial injection of DCs loaded with OVA peptide (DC-OVA) resulted in OT-I CD8+ T-cell priming, with the OT-I T cells representing 15% of the total CD8+ T cells in the spleen on day 5 postinjection (Fig. 6A). This efficient CD8+ T-cell response was probably caused by T-cell priming in the peripheral lymphoid organs because of antigen drainage. Analysis of the brains of these animals showed that OT-I T cells infiltrated and persisted within the brain for at least 30 d. Interestingly, by 30 d after DC immunization, 60% of the OT-I T cells remaining in the brain expressed CD103 (Fig. 6 B and C). However, when DC-OVAs were delivered i.v. into mice, low numbers of OT-I T cells persisted in the brain, and these cells did not express CD103 (Fig. S7). Furthermore, when DC-OVAs were administered i.v. and unloaded DCs were administered intracranially, we failed to observe CD103 up-regulation on cells in the brain, implying that tissue damage alone caused by the intracranial injection was insufficient to drive CD103 expression on persisting memory T cells (Fig. S7).
Fig. 6.
Local DC immunization can generate Trm. OVA peptide-pulsed DCs were injected intracranially into mice seeded with naïve OT-I.CD45.1 CD8+ T cells. (A) The percentage OT-I of the total CD8+ T cell population in the spleen at the indicated times after DC immunization. The bars represent the mean ± SEM. (B) Representative flow cytometry profile depicting the level of expression of CD103 on OT-I cells in the brain on day 30 after DC immunization. (C) The absolute number of CD103+ and CD103− cells in the brain on day 5, 10, and 30 post-DC immunization was determined. The bars represent the mean ± SEM. (D) Immunohistochemistry of the brain staining for CD11c (purple), CD45.1(green), CD103 (red), and DAPI (blue) on day 10 after DC peptide immunization.
We were able to observe OT-I T-cell interaction with DCs within the brain on day 10 after DC-OVA intracranial injection (Fig. 6D). Furthermore, the OT-I T cells that expressed CD103 at this early time point were those cells preferentially interacting with CD11c+ DCs within the brain, further indicating that local antigen presentation in the tissue may be necessary to acquire this phenotype.
Injecting peptide-pulsed DCs into the brain represents a means to transiently introduce antigen into the tissue. In this setting, CD8+ T cells infiltrate and persist, suggesting that the long-term residence of memory CD8+ T cells in the brain occurs irrespective of persisting antigen.
Brain Trm Do Not Function or Survive Well After Dissociation from the Tissue in Which They Reside.
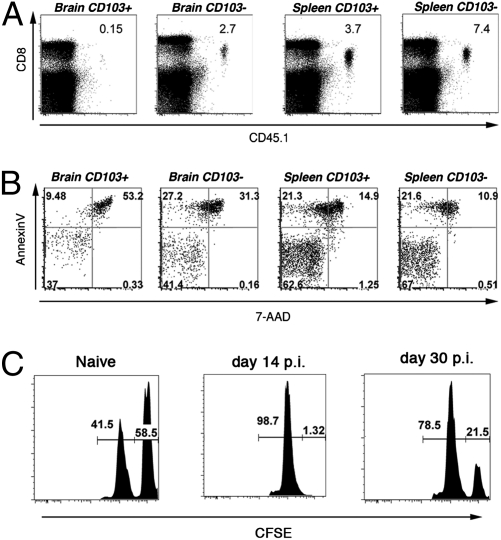
We sorted CD103+ and CD103− OT-I CD8+ T cells from the brains and spleens of mice on day 20 postinfection with VSV-OVA and adoptively transferred low numbers of these memory T cells i.v. into naïve recipients. These adoptive host mice were subsequently challenged by either i.n. infection with VSV-OVA (Fig. 7A) or i.v. infection with LM-OVA (Fig. S8 A and B). Remarkably, although splenic CD103+ and CD103− memory OT-I cells as well as brain-residing CD103− memory expanded in response to secondary challenge, brain resident CD103+ memory T cells did not mount a recall response.
Fig. 7.
Resident memory T cells do not function or survive well after dissociation from the tissue in which they reside. Mice were seeded with OT-I.CD45.1 T cells before i.n. infection with VSV-OVA. On day 20 p.i., OT-I cells were recovered from the brain and spleen of mice, sorted into CD103+ and CD103−, and adoptively transferred into naïve recipient mice that were challenged with VSV-OVA (i.n.). (A) Representative flow cytometry profiles of the spleen on day 8 p.i. Numbers represent the proportion of OT-I of the total CD8+ T cell population. (B) Memory OT-I.CD103+ and CD103− cells were sorted from the brain and spleen of mice and cultured in vitro for 12 h. Cells were stained with Annexin V and 7-AAD, and the percentage of dead cells (Annexin V+/− and 7-AAD+) was determined by flow cytometry. (C) A 1:1 ratio of CFSE-labeled peptide-pulsed targets (CFSEhi) and unpulsed (CFSElo) cells were injected intracranially into either naïve mice or mice infected i.n. 14 or 30 d earlier with VSV-OVA. The percentage killing was determined 14 h later.
We suspected that the lack of recall expansion exhibited by the CD103+ tissue resident population after isolation and adoptive transfer reflected a defect in survival after dissociation from the tissue. In support of this, annexin V and 7-amino-actinomycin D (7-AAD) staining indicated that CD103+ memory T cells isolated from the brain underwent the greatest level of death in culture (Fig. 7B). Memory T cells recovered from the spleen, irrespective of their CD103 expression, survived better ex vivo compared with tissue-bound memory cells. We excluded the possibility that the isolation procedure used to free the CD8+ T cells from the brain was driving this cell death, because when we injected splenic memory cells intracranially and recovered these cells from the brain, they showed no impairment in in vitro survival (Fig. S9A) and normal reexpansion after adoptive transfer into antigen-challenged recipients (Fig. S9B).
Although unable to undergo recall expansion after transfer, CD103+ and CD103− memory OT-I cells isolated from the brain made comparable levels of IFNγ after short-term peptide restimulation, indicating that these cells were not completely defective ex vivo (Fig. S10). Furthermore, intracranial injection of peptide-loaded targets into memory mice indicated that resident cells can kill targets when left in situ (Fig. 7C). Although we cannot distinguish if the CD103+ or CD103− population of cells is responsible for the observed in vivo killing, it is noteworthy that the majority of the granzyme B+ cells persisting within the brain after VSV infection express CD103 (Fig. S1B).
Thus, it seems that resident memory T cells represent a unique population of memory T cells highly dependent on their local milieu for survival and function.
Discussion
Infection results in the generation of memory T cells that are widely dispersed throughout the body (9, 24). Nonetheless, peripheral tissues that were directly involved in the infection usually contain elevated numbers of pathogen-specific memory T cells (11, 13, 25, 26). It is possible that these memory CD8+ T cells remain on site because of persisting antigen within the tissue. Indeed, antigen can persist after the clearance of an invading pathogen, reflected by the ability of LN DCs to activate T cells long after the clearance of the acute infection. Prolonged antigen presentation in LNs has been documented to occur after persistent infections (27, 28) as well as after infection with certain RNA viruses that are rapidly cleared, namely VSV and influenza (29–32). After intranasal VSV infection resulting in virus reaching the brain through the olfactory bulb, we observed the long-term persistence of memory CD8+ T cells in the brain. We suspected that the cluster configuration of these cells in the brain reflected T cell accumulation around depots of persisting antigen. Although we cannot perform longitudinal studies on one mouse, we suspect that regions of intense viral replication early on (Fig. 1) represent the sites of later CD8 cluster formation (Fig. 5). However, we could not detect viral gRNA in these T cell-enriched areas. Furthermore, we show that a DC immunization regimen, which allows the transient introduction of antigen, also results in the generation of memory T cells that persist long term in the brain and display a similar phenotype to resident memory T cells that persist after infection. Thus, it seems that the long-term residence of memory CD8+ T cells in the brain can occur irrespective of persisting antigen.
It is increasingly apparent that the role of DCs in the T-cell response goes far beyond initial antigen presentation for T-cell activation in the LN or spleen. Recently, there has been an increased focus on identifying the role of DCs at sites of infection or peripheral antigen depots. Depletion of nonmigratory pulmonary DC exacerbates influenza infection because of impairment in the developing CD8+ T-cell response (33). In addition, this group showed that local presentation of IL-15 by these pulmonary DCs increases T cell survival and accumulation in the lung (34). In the skin, dermal DCs have been shown to present antigen to both effector and regulatory CD4+ T cells to drive cytokine production (35). Furthermore, local antigen presentation by inflammatory DCs within sensory ganglia containing a reactivating HSV infection drives the proliferation of memory CD8+ T cells directly at the site of infection (21). In the work presented here, we show the antigen-driven up-regulation of CD103 on infiltrating effector CD8+ T cells in the brain, and we show that this can occur irrespective of antigen presentation by nonhematopoietic cells. Furthermore, we show that the up-regulation of CD103 facilitates T-cell accumulation within the brain. The expression of adhesion molecules, including VLA-1, CD103, and CD11a, have previously been shown to be important in the retention and survival of memory T cells within the gut, skin, and lung (36–40). Our data imply that the expression of CD103 in the brain is linked to local antigen presentation by DCs. Thus, beyond initial antigen presentation in the LN or spleen, professional APCs at the site of infection can help fine tune the immune response by influencing T-cell effector function, proliferation, survival, and residency.
We observe that memory CD8+ T cells persisting within the brain after VSV infection often localize in clusters. Interestingly, a recent intravital microscopy study of brain slices after Toxoplasma gondi infection identified a population of CD8+ T cells within the brain with a highly constrained pattern of migration (41). This work also showed the development of a local reticular fiber network within the brain after infection. Such scaffolding was absent from a naïve brain and was thought to develop only after infection or inflammation to facilitate lymphocyte migration. Thus, T-cell migration within the brain may be dictated by the nature and extent of the reticular fiber network induced by the inflammatory stimulus.
Resident memory T cells persisting within the brain function poorly on removal from the tissue. They fail to undergo recall expansion on adoptive transfer into the bloodstream of pathogen-challenged recipients and seem to die rapidly when placed in culture. Despite this inability to function outside the tissue, we show that brain Trm can kill targets efficiently in situ. It is still unclear what factors are important in the maintenance and long-term survival of Trm. It is interesting to speculate that, in addition to the known memory T cell homeostatic cytokines IL-7 and IL-15 (42), which we have not detected in elevated amounts within the recovering brain, resident memory T cells that express low levels of CD122 (Fig. S2) may become dependent on other local factors in their environment for survival. The inability of brain Trm to function on isolation further supports the notion that Trm are a unique memory T-cell population highly tailored to survive and function within their local environment (43).
Our findings show that infection can result in the long-term modification of the brain microenvironment. Specifically, cells of the immune system, which are largely excluded from the naïve brain, are deposited as long-term residents. It is important to consider that these brain-resident T cells could be problematic, because they persist in an environment not accustomed to routine immune surveillance or immune tolerance. Nevertheless, these cells may provide enhanced protection on secondary infection or during remitting–relapsing latent infections. Resident memory T cells are often found at mucosal and cutaneous surfaces, which are common portals of entry into the body for many pathogens and have been promoted as the first line of defense against infection. Although it is unlikely that an infection begins within the brain, generally, if a pathogen reaches the brain, prognosis is poor. Hence, the deposition of Trm within the brain may serve as the last line of defense against neuroinvasive pathogens that have successfully evaded the systemic immune response.
Materials and Methods
Mice.
C57BL/6, B6.C-H2-Kbm1/ByJ (H2Kbm1), and B6.SJL-PtprcaPep3b/BoyJ (CD45.1) mice were obtained from Jackson Laboratory and housed in specific pathogen-free conditions in the animal facilities at the University of Washington. OT-I T-cell receptor (TCR) transgenic mice congenic for Ly5.1 were bred and maintained in the same facilities. All experiments were done in accordance with the Institutional Animal Care and Use Committee guidelines of the University of Washington.
T-Cell Adoptive Transfer and Infections.
Mice received 104 naïve OT-I.CD45.1 CD8+ T cells through i.v. injection before infection. Memory cells were sorted from the brains and spleens of mice at the indicated times postinfection using a FACS Aria II, and 3 × 103 cells were transferred into mice i.v. before infection.
Mice were infected i.n. with 5 × 104 pfu of either wild-type or recombinant VSV that expresses GFP and a secreted form of OVA (44). Mice were infected i.v. with 2,000 cfu of recombinant LM that expresses a secreted form of OVA (LM-OVA). Growth and quantitation of LM-OVA was performed as described previously (45).
Viral Titer Determination.
The presence of infectious VSV in tissue samples was determined using standard pfu assays on confluent Vero cell monolayers.
Generation of Bone Marrow-Derived DCs.
Bone marrow flushed from tibias and femurs of C57BL/6 mice was resuspended at 1 × 106/mL in RPMI 1640 supplemented with 2.5 mM Hepes, 5.5 × 10−5 M mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin, 5 mM glutamine, 10% FBS, 10 ng/mL GM-CSF, and 10 ng/mL IL-4. Cells were incubated at 37 °C with 7% CO2 and cultured for 6 d with a media change on day 3. Maturation of the DCs was induced by adding LPS (Sigma-Aldrich) at 1 μg/mL during the last 20 h of culture. Dendritic cells were loaded with 10−6 M OVA257–264 peptide at 37 °C for 45 min. DCs were washed and resuspended in 30 μL of HBSS, and 2 × 105 cells were injected intracranially into mice.
Intracranial CFSE Administration and in Vivo Killing.
Mice were injected intracranially with 1 mM CFSE in a volume of 30 μL. Splenocytes were pulsed with 1 μM OVA peptide or were unpulsed and then labeled with CFSE at a final concentration of 10 μM for OVA peptide-pulsed cells (CFSE-high) and 1 μM for non–peptide-pulsed cells (CFSE-low). The cells were mixed at a ratio of 1:1, and a total of 1 × 106 cells were injected intracranially into recipient animals. Brains were recovered 14 h later.
Immunohistochemistry.
Tissues were fixed in 4% paraformaldehyde, frozen in Optimal Cutting Temperature (OCT) medium cut, and stained with the following antibodies: anti-CD103 (2E7), anti-CD4 (GK1.4), and anti-CD8 (53-6.7) purchased from eBioscience and anti-CD45.1 (A20) purchased from BioLegend. Slides were mounted with Vectashield containing DAPI (Vector Laboratories). Images were acquired using a fluorescence microscope and were analyzed using Adobe Photoshop.
RT-PCR and Laser Capture Microdissection.
Tissues were recovered and fixed on ice in 1% paraformaldehyde for 5 min before embedding in cryo-preservative solution. Sections were cut, placed onto polyethylene napthalate (PEN) membrane slides (Leica), and dehydrated in ethanol. Microdissection was performed using a Leica AS Laser Micro Dissection (LMD) microscope. T-cell clusters were identified on a consecutive serial section that had been stained with fluorescent antibodies as a guide. RNA was extracted using a PicoPure RNA Isolation kit (Arcturus) following the manufacturer's instructions. Synthesis of cDNA and real-time PCR was performed using SuperScript III Platinum Two-Step qPCR Kit with SYBR Green (Invitrogen) following the manufacturer's recommendations of using 10 mM of primers for either VSV-N (5′-CGGAGGATTGACGACTAATGC-3′ and 5′- ACCATCCGAGCCATTCGA-3′) or GAPDH (5′- TGTAGACCATGTAGTTGAGGTCA-3′ and 5′-AGGTCGGTGTGAACGGATTTG-3′). Amplification and detection were performed with an ABI Prism 7700. The amplification profile consisted of two holds, the first at 50 °C for 2 min and the second at 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s.
Flow Cytometry.
Single cell suspensions were prepared from spleens and LN by mechanical disruption. Brains were enzymatically digested for 1 h at 37 °C in 3 mL of collagenase type 3 (3 mg/mL in RPMI 1640 supplemented with 2% FBS), and lymphocytes were separated on a percoll gradient. Cells were stained for 25 min on ice with the appropriate mixture of monoclonal antibodies and washed with PBS with 1% BSA. The following conjugated monoclonal antibodies were obtained from BD Pharmingen or eBioscience: anti CD8α, CD45.1, CD122, CD127, PD-1, and CD103. The VSV-N tetramer was purchased from the Immune Monitoring Lab, Fred Hutchinson Cancer Research Center, Seattle, WA. For analysis of the homeostatic turnover of memory cells, 1 mg BrdU was injected i.p. on 7 consecutive d. Uptake was detected with a BrdU Flow kit according to the manufacturer's instructions (BD Pharmingen).
For the analysis of intracellular levels of IFNγ, single cell suspensions were incubated in the presence or absence of 1.0 μM peptide for 1 h at 37 °C, followed by a 4-h incubation in the presence of Brefeldin A. Cells were analyzed on a FACSCanto II using Flowjo software (Tree Star).
In Vitro Survival Assay.
Cells were cultured for 12 h in RPMI 1640 supplemented with 2% FCS, 2 mM glutamine, 5 × 10−5 M 2-mercaptoethanol, and antibiotics. Annexin V staining was performed using an Annexin V staining kit (BD Pharmingen) following the manufacturer's instructions.
Retroviral Transduction of CD8+ T Cells.
shRNA sequences were determined using RNAi-Codex software (http://cancan.cshl.edu/cgi-bin/Codex/Codex.cgi) and subcloned into MSCV-LTRmiR30-PIG (LMP) vector (Open Biosystems) between XhoI and EcoRI sites. Clones were verified by restriction site analysis and sequencing.
For retroviral transduction, OT-I cells were stimulated with peptide-pulsed splenocytes. The activated OT-I cells were spin-infected on day 2 postactivation in the presence of 4 μg/mL polybrene (Sigma-Aldrich) and 100 U/mL IL-2, and they were maintained for 2 d in IL-2–containing medium until sorting.
Supplementary Material
Acknowledgments
L.M.W is supported by an Overseas Biomedical Fellowship from the National Health and Medical Research Council of Australia. This work was supported by the Howard Hughes Medical Institute and National Institutes of Health Grants AI-19335 and A1083019 (to M.J.B.).
Footnotes
This article is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2008.
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010201107/-/DCSupplemental.
References
- 1.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 2.Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol. 2004;16:323–333. doi: 10.1016/j.smim.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 4.Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 5.Müllbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 7.Tan JT, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 9.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 11.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 12.Hogan RJ, et al. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 14.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- 15.Klonowski KD, et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 16.Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 17.Bartholomäus I, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 18.Galea I, et al. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204:2023–2030. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 20.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 22.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGill J, Legge KL. Cutting edge: Contribution of lung-resident T cell proliferation to the overall magnitude of the antigen-specific CD8 T cell response in the lungs following murine influenza virus infection. J Immunol. 2009;183:4177–4181. doi: 10.4049/jimmunol.0901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall DR, et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koelle DM, et al. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogan RJ, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 27.Stock AT, Jones CM, Heath WR, Carbone FR. Rapid recruitment and activation of CD8(+) T cells after herpes simplex virus type 1 skin infection. Immunol Cell Biol. 2010 doi: 10.1038/icb.2010.66. Epub May 11, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Cockburn IA, et al. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog. 2010;6:e1000877. doi: 10.1371/journal.ppat.1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zammit DJ, Turner DL, Klonowski KD, Lefrançois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner DL, Cauley LS, Khanna KM, Lefrançois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81:2039–2046. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jelley-Gibbs DM, et al. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178:7563–7570. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- 32.Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30:277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conrad C, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- 37.Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 38.Ray SJ, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 39.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 40.Masson F, et al. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol. 2007;179:845–853. doi: 10.4049/jimmunol.179.2.845. [DOI] [PubMed] [Google Scholar]
- 41.Wilson EH, et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Wakim LM, Bevan MJ. From the thymus to longevity in the periphery. Curr Opin Immunol. 2010;22:274–278. doi: 10.1016/j.coi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner MJ, Jellison ER, Lingenheld EG, Puddington L, Lefrançois L. Avidity maturation of memory CD8 T cells is limited by self-antigen expression. J Exp Med. 2008;205:1859–1868. doi: 10.1084/jem.20072390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacks JA, Bevan MJ. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J Immunol. 2008;180:4570–4576. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.