Abstract
Cardiac failure occurs when the heart fails to adapt to chronic stresses. Reactive oxygen species (ROS)-dependent signaling is implicated in cardiac stress responses, but the role of different ROS sources remains unclear. Here we report that NADPH oxidase-4 (Nox4) facilitates cardiac adaptation to chronic stress. Unlike other Nox proteins, Nox4 activity is regulated mainly by its expression level, which increases in cardiomyocytes under stresses such as pressure overload or hypoxia. To investigate the functional role of Nox4 during the cardiac response to stress, we generated mice with a genetic deletion of Nox4 or a cardiomyocyte-targeted overexpression of Nox4. Basal cardiac function was normal in both models, but Nox4-null animals developed exaggerated contractile dysfunction, hypertrophy, and cardiac dilatation during exposure to chronic overload whereas Nox4-transgenic mice were protected. Investigation of mechanisms underlying this protective effect revealed a significant Nox4-dependent preservation of myocardial capillary density after pressure overload. Nox4 enhanced stress-induced activation of cardiomyocyte hypoxia inducible factor 1 and the release of vascular endothelial growth factor, resulting in increased paracrine angiogenic activity. These data indicate that cardiomyocyte Nox4 is a unique inducible regulator of myocardial angiogenesis, a key determinant of cardiac adaptation to overload stress. Our results also have wider relevance to the use of nonspecific antioxidant approaches in cardiac disease and may provide an explanation for the failure of such strategies in many settings.
Keywords: cardiac remodeling, hypoxia inducible factor, reactive oxygen species
Heart failure results from disease stresses that chronically increase cardiac workload (1). The cardiac response to such insults involves remodeling of the cardiomyocytes, vasculature, and extracellular matrix that may initially be adaptive. Persistent stress, however, results in contractile dysfunction, fibrosis, ventricular dilatation, and capillary rarefaction. Specific pathways are thought to drive adaptive vs. maladaptive features of remodeling (1–3).
Increased reactive oxygen species (ROS) production is implicated in cardiac remodeling through several mechanisms, including the activation of signaling pathways that promote cardiomyocyte hypertrophy, abnormal excitation-contraction coupling, mitochondrial dysfunction, cell death, and extracellular matrix remodeling (4, 5). Clinical trials of antioxidants have yielded disappointing results, however, and effective therapies based on targeting ROS remain elusive. ROS in a stressed heart may emanate from several sources, including mitochondria, NADPH oxidases of the Nox family, uncoupled nitric oxide (NO) synthases, and xanthine oxidases, but the functional roles of individual sources remain unclear (5). It is recognized, however, that different sources may modulate distinct signaling pathways through regulated, spatially restricted ROS production (6). Indeed, some ROS-mediated effects are beneficial rather than detrimental, e.g., in cardioprotective signaling elicited by preconditioning (7).
Nox family enzymes generate ROS by catalyzing electron transfer from NADPH to molecular O2. Seven family members exist (Nox1–5 and Duox1–2), each based on a distinct catalytic subunit and with tissue-specific expression (8, 9). The prototypic Nox enzyme, Nox2, mediates microbicidal activity in phagocytes through generation of large amounts of superoxide (O2−). In nonphagocytic cells, however, Nox2 and other Nox proteins generate low levels of ROS that are involved in intracellular signaling (9). Previous studies using Nox2-null mice and other models showed that Nox2 in the heart is involved in the development of cardiac hypertrophy and contractile dysfunction induced by angiotensin II, pressure overload, or myocardial infarction (10–15). Nox4 differs from Nox2 and other Nox enzymes in that it is regulated mainly by its expression level and does not require agonist stimulation or association with regulatory subunits for activation (16–18). Recent studies also suggest that it generates predominantly H2O2 rather than O2− (17–20). Previous work showed that Nox4 is expressed at a low level in the adult mammalian heart and that its abundance increases during pressure overload (11), but its pathophysiological functions in vivo are unknown.
In this study, we generated a Nox4-null mouse model and a cardiomyocyte-targeted Nox4-transgenic model to elucidate the effects of Nox4 during cardiac stress. In marked contrast to the effects of Nox2 and other ROS sources, increases in cardiomyocyte Nox4 resulted in protection against pressure overload-induced adverse cardiac remodeling. Nox4 facilitated preservation of myocardial capillary density during pressure overload by regulating stress-induced cardiomyocyte hypoxia inducible factor 1 (Hif1) activation and release of vascular endothelial growth factor (VEGF), resulting in increased paracrine angiogenic activity. These data indicate that Nox4 is a unique stress-inducible regulator of myocardial angiogenesis that facilitates adaptation to cardiac overload stress.
Results
Myocardial Nox4 Expression Increases During Stress.
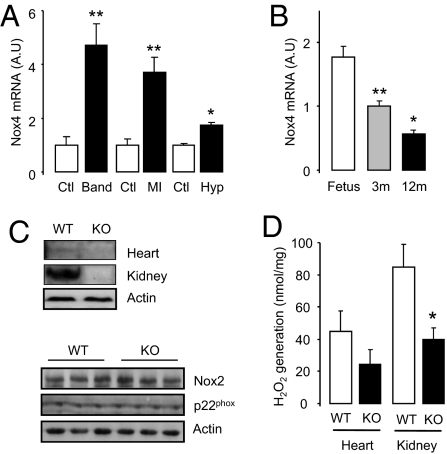
We first analyzed changes in myocardial Nox4 expression during development and in response to various stresses. Nox4 expression increased after in vivo pressure overload, myocardial infarction, or in vitro hypoxia (Fig. 1A). Nox4 expression was significantly lower in the myocardium of healthy young or old animals compared with fetal hearts (Fig. 1B). The increase in Nox4 protein expression after pressure overload was largely in cardiomyocytes (Fig. S1A). This induction of Nox4 is similar to that of so-called fetal genes that are reactivated in the adult heart during stress (3).
Fig. 1.
Cardiac Nox4 induction during stress and generation of Nox4-null mice. (A) Changes in Nox4 expression after pressure overload (Band), myocardial infarction (MI), or in vitro hypoxia (Hyp; 24 h) compared with respective controls (Ctl). **P < 0.01; n = 4–6/group. (B) Nox4 expression in 3-mo (3m)- and 12-mo (12m)-old mice compared with fetal heart. **P < 0.01; n = 5/group. (C) Western blots showing loss of Nox4 protein in heart and kidney of homozygous Nox4-KO mice (Top) and lack of change in cardiac Nox2 and p22phox levels (Bottom). (D) H2O2 production in heart and kidney of Nox4-KO compared with WT. *P < 0.05; n = 6/group.
Nox4-Null Mice Have Exaggerated Load-Induced Cardiac Dysfunction.
To evaluate the role of increases in Nox4 during cardiac stress, we generated mice deficient in Nox4 (Fig. S1B). Deletion of endogenous Nox4 resulted in a total loss of Nox4 protein and a small reduction in H2O2 production as assessed by a homovanillic acid assay (Fig. 1 C and D). Myocardial levels of Nox2 and p22phox were unaffected by Nox4 deletion (Fig. 1C). Nox4-null mice were born in the expected Mendelian ratio, bred normally, and showed no obvious abnormal baseline phenotype (Table S1).
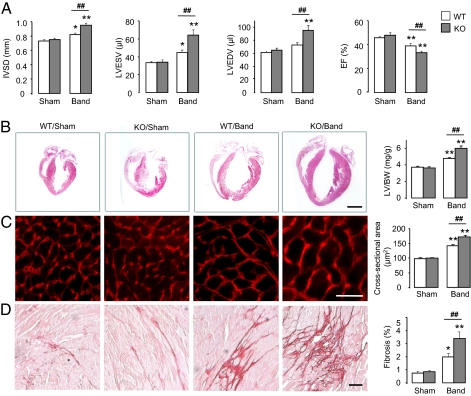
Basal cardiac size and function assessed by echocardiography were unchanged in Nox4-null mice (Fig. 2A). To assess the effects of the absence of Nox4 during cardiac stress, we performed suprarenal aortic constriction to generate a chronic pressure overload. The degree of pressure overload was similar in Nox4-null mice and wild-type (WT) littermates, and there was no difference in mortality. As expected, 6 wk of pressure overload caused contractile impairment and ventricular dilatation in wild-type mice. Nox4-null animals, however, developed significantly greater cardiac dilatation and contractile impairment than wild type (Fig. 2A). Nox4-null mice also developed exaggerated cardiac hypertrophy after chronic pressure overload at both the whole-heart and the cardiomyocyte level, as well as increased interstitial fibrosis (Fig. 2 B–D). These data therefore suggest that an increase in myocardial Nox4 expression is protective against the detrimental consequences of chronic pressure overload.
Fig. 2.
Nox4-null mice have exaggerated load-induced dysfunction. (A) Echocardiography of WT and KO mice subjected to 6 wk of chronic pressure overload. IVSD: interventricular septal diameter; LVESV: LV end-systolic volumes; LVEDV: LV end-diastolic volumes; EF: ejection fraction. (B) Representative H&E-stained longitudinal sections of WT and KO hearts. Scale bar: 2 mm. (C and D) Representative sections for cardiomyocyte area (WGA-stained) and interstitial fibrosis (Picrosirius red-stained), respectively. Scale bars: 20 μm. Mean data are shown at the right. **P < 0.01; *P < 0.05 for band vs. respective sham; ##P < 0.01 for KO band vs. WT band; n = 12–15/group. LV/BW: LV/body weight.
Cardiomyocyte-Targeted Nox4-Transgenic Mice Show No Basal Dysfunction.
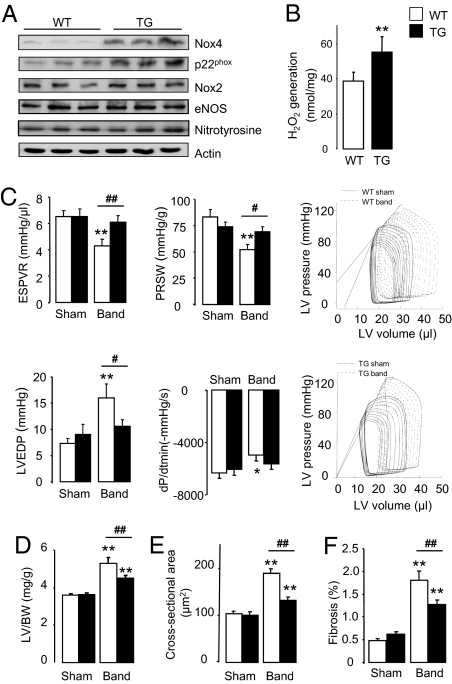
To further validate a protective role of Nox4 during cardiac overload, we generated transgenic mice with a cardiomyocyte-targeted increase in Nox4. Nox4-transgenic mice had significantly increased Nox4 protein levels (Fig. 3A and Fig. S2A). Nox4 heterodimerizes with p22phox, with the two proteins stabilizing each other (8, 16), and increased Nox4 expression was accompanied by a twofold increase in p22phox levels. Nox2 levels by contrast were similar between strains. Nox4-transgenic mice had a modest elevation of myocardial H2O2 production (Fig. 3B) but did not show increased O2− levels as assessed by electron paramagnetic resonance spectroscopy (EPR) (Fig. S2B), which is in line with data that suggest that Nox4 generates predominantly H2O2 rather than O2− (17–20). Consistent with this, myocardial nitrotyrosine levels—as a readout of nitrosative stress resulting from interaction of O2− and NO—were unaltered in Nox4-transgenic mice, which also had unaltered endothelial NO synthase levels (Fig. 3A and Fig. S2A). Overexpressed Nox4 protein in transgenic cardiomyocytes was found in a perinuclear location, similar to the location of endogenous Nox4 in normal cardiomyocytes or after myocyte transfection (Fig. S3), and as reported in other cell types (21–23). Nox4-transgenic mice were grossly normal and showed no cardiac dysfunction up to 12 mo of age, although the older animals had a slightly increased cardiac mass compared with wild-type littermates (Table S2). There was no evidence of increased fibrosis or apoptosis in the hearts of Nox4-transgenic mice (Fig. S2 C and D). These results show that an increase in myocardial Nox4 levels in the absence of stress has no significant detrimental consequences.
Fig. 3.
Cardiomyocyte-targeted Nox4 overexpression protects against load-induced dysfunction. (A) Protein expression in hearts of Nox4-transgenic mice (TG) and wild-type littermates (WT). (B) H2O2 production in TG compared with WT myocardium. **P < 0.01; n = 6/group. (C) Contractile assessment of pressure-overloaded Nox4-transgenic and WT mice by LV pressure–volume analysis. End-systolic pressure volume relation (ESPVR) and preload-recruitable stroke work (PRSW) are measures of systolic function. LV end-diastolic pressure (LVEDP) and dP/dtmin are measures of diastolic function. Representative pressure–volume curves are shown at the right. **P < 0.01; *P < 0.05 for band vs. respective sham; ##P < 0.01; #P < 0.05 for TG band vs. WT band; n = 15/group. (D–F) Mean data for heart hypertrophy (n > 20/group), cardiomyocyte cross-sectional area (n = 6/group), and interstitial fibrosis (n = 6/group), respectively. Statistics in D–F as in C.
Cardiomyocyte-Targeted Nox4-Transgenic Mice Are Protected Against Load-Induced Stress.
We next subjected Nox4-transgenic mice and wild-type littermates to chronic pressure overload. Quantification of in vivo left ventricular (LV) pressure–volume relations revealed that both systolic and diastolic function were better preserved in Nox4-transgenic mice than in wild type after pressure overload for 9 wk (Fig. 3C). The protective effect of Nox4 was confirmed by echocardiography (Fig. S4A) as well as in a second independent transgenic line (Fig. S5). Nox4-transgenic mice developed less cardiac hypertrophy after pressure overload than wild-type littermates and also had significantly less interstitial fibrosis (Fig. 3 D–F and Fig. S4 B–D). Taken together, the data obtained so far using both loss-of-function and gain-of-function approaches indicate that an increase in myocardial Nox4 expression is protective against chronic pressure overload-induced cardiac dysfunction.
Nox4-Dependent Enhancement of Myocardial Capillary Density During Pressure Overload.
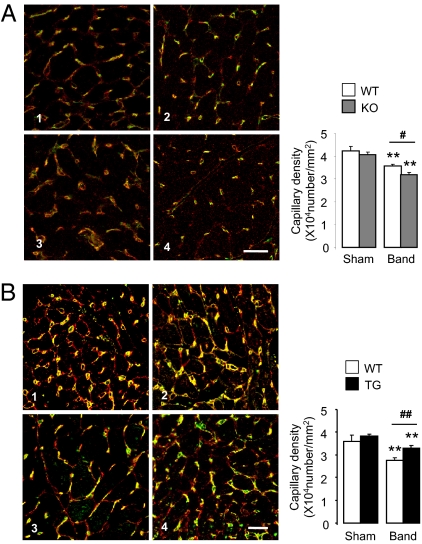
Signaling effectors of the cardiac response to chronic pressure overload include various protein kinases that are potentially redox-sensitive (3, 5). We undertook an immunoblotting-based profiling screen (Kinexus Bioinformatics) that encompasses a broad range of signaling pathways in Nox4-transgenic and wild-type mice subjected to pressure overload. The only protein with a more than twofold difference in phosphorylation between groups was Akt1. Quantitative immunoblotting showed that phosphorylated Akt (S473) levels were modestly elevated in Nox4-transgenic hearts, but there was no difference between Nox4-null mice and wild-type mice subjected to pressure overload (Fig. S5C). Levels of myocardial apoptosis after pressure overload were also unaffected either by Nox4 deletion or by cardiomyocyte-specific overexpression [WT band: 7.2 ± 1.1; KO band: 6.7 ± 1.1; transgenic band: 6.0 ± 0.9/105 nuclei; n = 6–9/group; P = NS]. A key determinant of functional cardiac compensation during chronic pressure overload has recently been recognized as the extent of myocardial capillarization, with insufficient angiogenesis being a driver of heart failure (24–27). Quantification of myocardial capillary density in LV sections of Nox4-null mice, Nox4-transgenic mice, and respective wild-type littermate controls showed that although there were no differences between groups at baseline, after imposition of pressure overload, capillary density was significantly lower in Nox4-null mice compared with wild type (Fig. 4A). By contrast, Nox4-transgenic mice had significantly higher myocardial capillary density than wild-type littermates after pressure overload (Fig. 4B). These results suggest that Nox4 up-regulation during cardiac stress is required to protect against load-induced cardiac dysfunction by controlling the compensatory increase in myocardial capillary density.
Fig. 4.
Nox4-dependent maintenance of myocardial capillary density. (A) Representative LV sections from Nox4-null mice and WT littermates stained with isolectin B4 to label myocardial capillaries (yellow) and WGA to outline cardiomyocytes (red). 1, WT sham; 2, KO sham; 3, WT band; 4, KO band. Mean data at the right (n = 7–12/group). (B) Increased myocardial capillary density in Nox4-transgenic mice. Representative LV sections at the left. 1, WT sham; 2, TG sham; 3, WT band; 4, TG band. Mean data to the right (n = 6/group). **P < 0.01 for band vs. respective sham; ##P < 0.01; #P < 0.05 for TG or KO band vs. WT band. Scale bars: 20 μm.
Nox4 Enhances Cardiomyocyte Hif1α and VEGF.
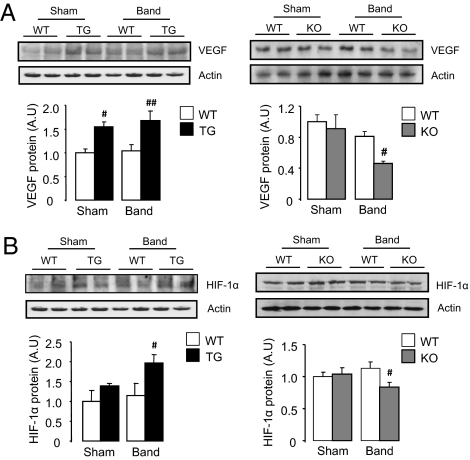
Previous work shows that a central mechanism underpinning myocardial stress-induced angiogenesis is the release of angiogenic factors, notably VEGF (25–27). We found that cardiac VEGF-A protein levels were significantly increased in Nox4-transgenic mice whereas Nox4-null mice had markedly lower levels than wild-type after pressure overload (Fig. 5A). Immunostaining for VEGF in heart demonstrated a significant increase at cardiomyocyte membranes and in vessels in Nox4-transgenic mice after aortic banding whereas Nox4-null animals showed very little staining (Fig. S6A). An important upstream transcriptional regulator of VEGF during load-induced stress is Hif1 (26), which is known to be redox-regulated (28). Hif1α protein levels were significantly higher in Nox4-transgenic hearts compared with wild type after pressure overload (Fig. 5B). By contrast, Hif1α levels in Nox4-null mice were significantly lower than in wild-type littermates after aortic banding.
Fig. 5.
Nox4-dependent increase in VEGF and Hif1α levels in heart. (A) Western blots for VEGF in LV of Nox4-transgenic mice (Upper left) and Nox4-null mice (Upper right) and respective WT littermates subjected to pressure overload or control surgery. Mean data are shown below. ##P < 0.01; #P < 0.05 for TG or KO vs. respective WT; n = 4/group. (B) Hif1α protein levels in LV of Nox4-transgenic mice (Upper left) or Nox4-null mice (Upper right) compared with respective WT. #P < 0.05 for TG vs. WT band; n = 4/group. #P < 0.05 for KO vs. WT band; n = 8/group.
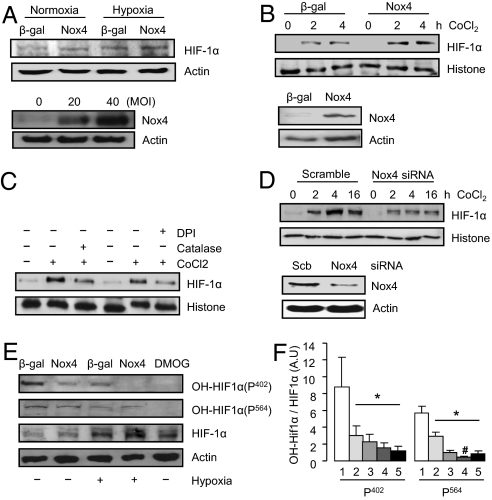
To more directly investigate the relationship between cardiomyocyte Nox4, Hif1α, and VEGF, we studied cultured cardiac cells. Overexpression of Nox4 in cultured cardiomyocytes increased H2O2 levels and slightly increased Hif1α protein levels during normoxia but substantially enhanced them during hypoxia (Fig. 6A and Fig. S6 B and C). A similar Nox4-dependent augmentation of Hif1α levels was observed in H9c2 cardiomyoblasts after treatment with cobalt chloride, a chemical hypoxia mimetic (Fig. 6B). Hypoxia-induced Hif1α accumulation in control cells was significantly reduced by the potent but nonspecific Nox inhibitor, diphenylene iodonium, or by catalase (Fig. 6C). Importantly, similar effects were observed after specific down-regulation of endogenous Nox4 by siRNA-mediated knockdown (Fig. 6D).
Fig. 6.
Enhancement of cardiomyocyte Hif1α and VEGF by Nox4. Nox4-dependent regulation of Hif1α protein levels in (A) cardiomyocytes and (B–D) nuclear extracts of H9c2 cells. Doses were CoCl2 (200 μmol/L), diphenylene iodonium (DPI; 20 μmol/L), and PEG-catalase (catalase; 125 U/mL). Scb: scrambled siRNA. (E) Effect of β-gal control, Nox4, and a Hif1 prolyl hydroxylase inhibitor, dimethyloxalylglycine (DMOG; 1 mmol/L) on Hif1α hydroxylation using anti-hydroxylated-Pro402 and -Pro564 antibodies. Representative blots at the left and mean data at the right. 1, β-gal; 2, Nox4; 3, β-gal hypoxia; 4, Nox4 hypoxia; 5, DMOG. *P < 0.05 for all groups vs. β-gal control; #P < 0.05 vs. β-gal hypoxia. Hydroxylated Hif1α was expressed relative to total HIF1α. All blots are representative of three or more independent experiments.
Both transcriptional and posttranslational mechanisms could potentially be involved in Hif1α up-regulation (28). We found that Hif1α mRNA expression in vivo was significantly higher in Nox4-transgenic than in wild-type mice, but there was no significant difference in levels between Nox4-null mice and wild-type littermates (Fig. S6 D and E). Notably, Hif1α protein levels in Nox4-transgenic mice were not elevated at baseline despite increased mRNA levels, suggesting that an effect at the protein level was more important. Increasing Nox4 in cultured cardiomyocytes did not affect Hif1α mRNA levels during either normoxia or hypoxia (Fig. S6F). Because Hif1α protein stability is regulated by hydroxylation by specific prolyl hydroxylases, leading to targeting for proteosomal degradation (28), we assessed the effect of Nox4 on levels of hydroxylated Hif1α. Nox4 overexpression in cardiomyocytes led to a substantial decrease in hydroxylated Hif1α levels as assessed by two different antibodies, an effect potentiated by hypoxia (Fig. 6 E and F). This was similar to the effect of a specific prolyl hydroxylase inhibitor, dimethyloxalylglycine, in reducing hydroxylated Hif1α and in increasing total Hif1α levels (Fig. 6E). These results suggest that the inhibition of prolyl hydroxylase activity may be a major mechanism by which Nox4 increases cardiomyocyte Hif1α levels.
Paracrine Angiogenic Activity Mediated by Nox4.
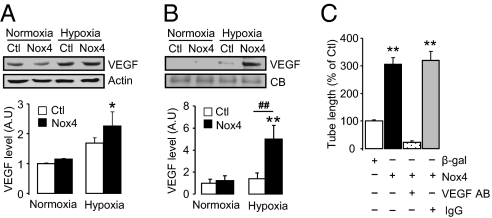
We found that Nox4-induced increases in cultured cardiomyocyte Hif1α during hypoxia were accompanied by an increase in VEGF levels in the cells and even more so in their conditioned medium, indicative of extracellular release (Fig. 7 A and B). To directly test the potential angiogenic effects of Nox4-dependent changes in factors secreted by cardiomyocytes, we undertook in vitro endothelial cell tube formation assays. The conditioned medium of Nox4-overexpressing cardiomyocytes subjected to hypoxia markedly enhanced endothelial tube formation as compared with that of hypoxic cardiomyocytes overexpressing a β-galactosidase (β-gal) control gene (Fig. 7C and Fig. S6G). The angiogenic effect of cardiomyocyte-conditioned medium was almost fully inhibited by a VEGF-blocking antibody but was unaltered by nonspecific IgG. These results indicate that the paracrine release of VEGF is central to the proangiogenic effects of cardiomyocyte Nox4.
Fig. 7.
Nox4-dependent enhancement of angiogenic response. Immunoblotting for VEGF in (A) cardiomyocytes and (B) culture supernatants of cardiomyocytes overexpressing Nox4 or a β-gal control (Ctl). Actin and Coomassie blue (CB) staining were used to confirm equal protein loading. Mean data are shown below. **P < 0.01; *P < 0.05 for Nox4 hypoxia vs. normoxia; ##P < 0.01 for Nox4 vs. β-gal; n = 3/group. (C) Mean data showing effects of conditioned media on tube formation. **P < 0.01 vs. β-gal control (Ctl) or vs. Nox4 overexpression + VEGF antibody; n = 4/group.
Discussion
This study provides definitive data on the in vivo function of Nox4 in the heart with the use of complementary loss-of-function and gain-of-function models. We present a previously unrecognized and unexpected protective role of an endogenous ROS-generating enzyme in the cardiac response to load-induced stress, involving an enhancement of myocardial capillary density and functional cardiac compensation. The proangiogenic role of Nox4 involves a paracrine mechanism in which Nox4 up-regulation in cardiomyocytes leads to enhanced Hif1 activation and increased release of VEGF, which in turn promotes capillarization. The beneficial effects of Nox4 contrast markedly with those of other ROS sources in the remodeling heart, such as mitochondria, which have been found to be detrimental and have formed the basis for the testing of antioxidant therapies in human heart failure (29, 30). Our findings, however, indicate that therapeutic strategies may need to be directed toward specific ROS sources and pathways rather than the nonspecific targeting of ROS.
Nox enzymes differ from most other ROS sources in that ROS generation is their primary function (8, 9). Among the Nox enzymes expressed in the cardiovascular system, Nox4 is unique in that its activity is regulated mainly by its protein level whereas Nox1/Nox2 activation are under control of posttranslational mechanisms such as agonist-dependent phosphorylation of regulatory subunits (8, 16–18). Nox4 appears to be largely stress-inducible with low expression in the healthy adult heart and most other tissues apart from kidney (31), but with significantly increased levels after stresses such as pressure overload or hypoxia. Our findings that Nox4-null mice have no obvious abnormalities in the absence of stress are consistent with the notion of stress inducibility. Nox4 differs from Nox2 in two other important respects: First, its subcellular location in cardiomyocytes is in or associated with the perinuclear endoplasmic reticulum whereas activated Nox2 is found predominantly at the cell membrane (32). Second, several recent independent reports indicate that Nox4 generates predominantly H2O2 (as further confirmed in the current study) whereas Nox2 primarily generates O2− (refs. 17–20). These differences in regulation, activation mechanism, subcellular location, and ROS generation translate into isoform-specific actions in isolated cellular models (9, 19, 22, 33). In the heart, previous studies in mouse models of defective Nox2 activation or its deletion showed that Nox2 is detrimental during remodeling, causing increased hypertrophy, apoptosis, and contractile dysfunction (10–15). The current results on Nox4, taken together with previous data on Nox2, indicate that the two isoforms contrast markedly in their effects on cardiac remodeling, with Nox2 being detrimental and Nox4 beneficial.
The major mechanism underlying the beneficial effects of Nox4 during load-induced stress is an increase in myocardial capillaries, with capillary density being impaired in Nox4-null animals but better preserved in Nox4-transgenic mice compared with wild type. Myocardial angiogenesis is tightly coupled to cardiomyocyte growth during heart development (34) and is also an important determinant of the response to disease-causing stresses such as pressure overload (24–27). Stress-induced angiogenesis has been shown to be underpinned by the release of VEGF from cardiomyocytes to exert paracrine effects on adjacent vessels (25). Myocardial VEGF production is under the control of transcription factors such as Hif1 and GATA4 (26, 27), but the upstream signals regulating activation of these factors during cardiac stress remain unclear. The results reported here suggest that stress-induced increase in Nox4 levels is a key mechanism that enhances cardiomyocyte Hif1α levels during overload, in turn leading to VEGF release and an increase in angiogenic capacity. This facilitates functional compensation of the heart, which is manifested as better-preserved contractile function and a reduced extent of cardiac hypertrophy, fibrosis, and dilatation. Hif1α levels are the primary regulator of Hif1 transcriptional activity through dimerization with Hif1β and the recruitment of coactivators (28). Although Hif1α levels may be influenced by changes in mRNA expression, the major regulatory mechanism is via oxygen-dependent hydroxylation of Hif1α protein by prolyl hydroxylases, which results in its targeting for proteosomal degradation. Hydroxylase activity is inhibited during hypoxia, leading to increased Hif1α levels. Although we found an increase in Hif1α mRNA levels in Nox4-transgenic mice, the dominant mechanism by which Nox4 increased Hif1α appeared to be at the protein level. Assessment of Hif1α hydroxylation indicated that Nox4 may act by inhibition of prolyl hydroxylase activity, thereby stabilizing Hif1α and increasing protein levels. Interestingly, previous studies have reported that ROS may increase Hif1α mRNA expression in vascular cells (35) as well as inhibit Hif1 prolyl hydroxylases in tumor cells (36).
In contrast to load-induced cardiac stress or ischemia, angiogenesis is detrimental in cancer by promoting tumor growth, and anti-angiogenic therapies are considered a promising strategy. Additionally, Nox4 reportedly has prosurvival effects in certain tumors and is thought to be a suitable therapeutic target (37, 38). Our results suggest, however, that caution should be exercised in using Nox4-targeted cancer therapy in patients with cardiac overload (e.g., hypertension) to avoid cardiotoxicity. In this study, we used a mouse model with global Nox4 deficiency so that part of the detrimental effect observed in pressure overloaded Nox4-null mice could potentially be due to loss of Nox4 in noncardiomyocytes—in, for example, other cardiac cell types or organs such as the kidneys—although the data in cardiomyocyte-specific Nox4-transgenics argue against this. Regardless of this point, the current results with global Nox4-null mice may be predictive of potential side effects with systemic Nox4-inhibitor therapy.
Despite treatments such as beta blockers and angiotensin-converting enzyme inhibitors that decrease mortality in heart failure patients, prognosis remains poor. New therapeutic strategies that can impact on disease mechanisms are therefore needed. ROS imbalance has long been recognized as potentially important in the remodeling and failing heart, but its therapeutic targeting has proven elusive. The present study indicates that ROS have not only detrimental but also beneficial effects in the remodeling heart depending upon the source and suggests that specific targeting of an individual ROS source linked to an adaptive and potentially disease-preventing pathway (i.e., myocardial angiogenesis) may be a useful approach. Our results also have wider relevance to the use of nonspecific antioxidant approaches in human diseases and may provide an explanation for the failure of such strategies in many settings.
Methods
Detailed methods are provided in SI Methods.
Gene-Modified Mice.
Nox4-null mice were generated by targeted deletion of the translation initiation site and exons 1 and 2 of the gene (Fig. S1B). Cardiomyocyte-targeted Nox4-transgenic mice were generated using the mouse Nox4 cDNA downstream of the mouse α-myosin heavy chain promoter. All lines were backcrossed >10 generations onto a C57BL/6 background.
Animal Studies.
Procedures were performed in accordance with the Guidance on the Operation of the Animals (Scientific Procedures) Act, 1986 (United Kingdom). Aortic constriction was induced by suprarenal banding (11).
Histology.
FITC-conjugated wheat germ agglutinin (WGA) was used to outline cardiomyocytes and Picrosirius red staining to assess fibrosis (15). Capillaries were immunostained with isolectin B4 (39).
Cell Studies.
Neonatal rat cardiomyocytes were subjected to hypoxia using a 95% N2/4% CO2/1% O2 mixture. Human umbilical vein endothelial cells seeded on Matrigel-coated slides were used for tube formation assays.
Detection of ROS.
H2O2 levels were detected with a homovanillic acid assay (22). EPR was used to measure O2− generation by heart particulate fractions, using a 5,5-dimethylpyrroline-N-oxide (DMPO, 50 mmol/L) spin trap (40).
Statistics.
All data are presented as mean ± SEM. Comparisons were undertaken by Student's t test or one-way ANOVA, as appropriate, followed by a post hoc Tukey's test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank M. Mayr for advice on tube formation assays. This study was supported by the British Heart Foundation (Grants RG/08/011/25922, CH/99001, and RE/08/003), the Leducq Foundation, by EUGeneHeart (EU FP6 Grant LSHM-CT-2005-018833), by the German Search Foundation (Grants SFB815 TPA1, SFB834 TPA2), and by the Excellence Cluster Cardio-Pulmonary System and Goethe University.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009700107/-/DCSupplemental.
References
- 1.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–928. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 2.Catalucci D, Latronico MV, Ellingsen O, Condorelli G. Physiological myocardial hypertrophy: How and why? Front Biosci. 2008;13:312–324. doi: 10.2741/2681. [DOI] [PubMed] [Google Scholar]
- 3.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 4.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anilkumar N, Sirker A, Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–3187. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 6.Rhee SG, et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Saini HK, Machackova J, Dhalla NS. Role of reactive oxygen species in ischemic preconditioning of subcellular organelles in the heart. Antioxid Redox Signal. 2004;6:393–404. doi: 10.1089/152308604322899468. [DOI] [PubMed] [Google Scholar]
- 8.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 9.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 11.Byrne JA, et al. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 12.Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2003;35:851–859. doi: 10.1016/s0022-2828(03)00145-7. [DOI] [PubMed] [Google Scholar]
- 13.Satoh M, et al. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doerries C, et al. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 15.Looi YH, et al. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 16.Ambasta RK, et al. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 17.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Serrander L, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikalov SI, et al. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 23.Helmcke I, Heumüller S, Tikkanen R, Schröder K, Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal. 2009;11:1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 24.Hilfiker-Kleiner D, et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 25.Shiojima I, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano M, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 27.Heineke J, et al. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1391–1396. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- 29.Ide T, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 30.Schriner SE, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 31.Geiszt M, Kopp JB, Várnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heymes C, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 33.Wu RF, Ma Z, Myers DP, Terada LS. HIV-1 Tat activates dual Nox pathways leading to independent activation of ERK and JNK MAP kinases. J Biol Chem. 2007;282:37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]
- 34.Giordano FJ, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci USA. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonello S, et al. Reactive oxygen species activate the HIF-1α promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 36.Gerald D, et al. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 38.Mochizuki T, et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 39.Hilfiker-Kleiner D, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 40.Janiszewski M, et al. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem. 2005;280:40813–40819. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.