Abstract
Rett syndrome (RTT) is an autism spectrum disorder caused by mutations in the X-linked gene that encodes the transcription factor methyl-CpG-binding protein 2 (MeCP2). A major debilitating phenotype in affected females is frequent apneas, and heterozygous Mecp2-deficient female mice mimic the human respiratory disorder. GABA defects have been demonstrated in the brainstem of Mecp2-deficient mice. Here, using an intact respiratory network, we show that apnea in RTT mice is characterized by excessive excitatory activity in expiratory cranial and spinal nerves. Augmenting GABA markedly improves the respiratory phenotype. In addition, a serotonin 1a receptor agonist that depresses expiratory neuron activity also reduces apnea, corrects the irregular breathing pattern, and prolongs survival in MeCP2 null males. Combining a GABA reuptake blocker with a serotonin 1a agonist in heterozygous females completely corrects their respiratory defects. The results indicate that GABA and serotonin 1a receptor activity are candidates for treatment of the respiratory disorders in Rett syndrome.
Keywords: apnea, GABA, respiration, serotonin 1a receptor
Rett syndrome (RTT) is an autism spectrum disorder that is caused by mutations in the X-linked gene that encodes methyl-CpG- binding protein 2 (MeCP2) (1). The role of this transcription factor is incompletely understood. Until recently, on the basis of animal studies primarily in embryonic or early neonatal brain, Mecp2 was considered a translational repressor (2, 3). Recently, however, Skene et al. (4) found in neuronal nuclei of mature (6–8 wk) WT littermates of Mecp2 null males that Mecp2 expression is ≈fivefold greater than that at birth. The amount of Mecp2 bound to DNA was proportional to the methylation density of CpG sequences. Importantly, in adult mice, it has been shown that restoring Mecp2 reverses the abnormalities in mobility, gait, hind limb clasping, tremor, breathing, and general condition that they developed during the period that the transcription factor was deficient (5). Thus, Mecp2 deficiency does not cause neuronal degeneration, nor is it necessary for the correct development of neuronal networks. Strategies aimed at pharmacological corrections of symptoms are therefore essential in treating RTT.
Respiratory disorders are prominent and one of the most disturbing features of RTT (6, 7). These abnormalities are faithfully mimicked in mouse models of RTT (8). In Mecp2 null male animals studied in situ phrenic (PN) apneas were characterized by prolonged postinspiratory (post-I) activity in the central vagus nerve (9). Post-I activity is linked to the control of laryngeal adductors that control expiratory airflow and protect the lower airways from aspiration during swallowing. Active breath-hold with laryngeal closure is a common feature of RTT (6, 7, 10).
This raises the possibility that the apneas are due to overactive brainstem expiratory neurons in Mecp2 heterozygote mice, perhaps consequent to a lack of synaptic inhibitory control. In this regard, examination of GABA synaptic inhibition in the ventrolateral medulla of Mecp2 null male mice revealed that it was markedly reduced compared with WT (11). These findings led us to hypothesize that insufficient synaptic inhibition of expiratory neurons underlies the respiratory disturbances in RTT.
Using a combination of in situ studies in which PN, hypoglossal (HN), central vagus (cVN), and abdominal (AbN) nerves were recorded simultaneously from adult Mecp2-deficient female mice and separately monitored pleural pressure in awake, freely moving animals, we have performed a detailed characterization of respiratory motor pattern and examined the effects of blocking GABA reuptake and of allosteric modulation of its type A receptor. Because serotonin 1a receptor agonists have been shown to both inhibit expiratory neurons (12) and reinstate breathing after opioid-induced central apneas (13, 14), we tested whether 8-hydroxy-dipropyl-aminotetralin (8-OH-DPAT) (a serotonin 1a receptor agonist) could depress the respiratory apneas in Mecp2 heterozygote mice. Our findings reveal that both treatments significantly reduced apneas and periodic breathing in Mecp2-deficient mice and restored regularity to their cycle intervals. Combining GABA reuptake block with 8-OH-DPAT resulted in a respiratory pattern similar to that in WT.
Results
Characterization of Apnea in Heterozygous Mecp2-Deficient Female Mice (Mecp2−/+) Studied in Situ.
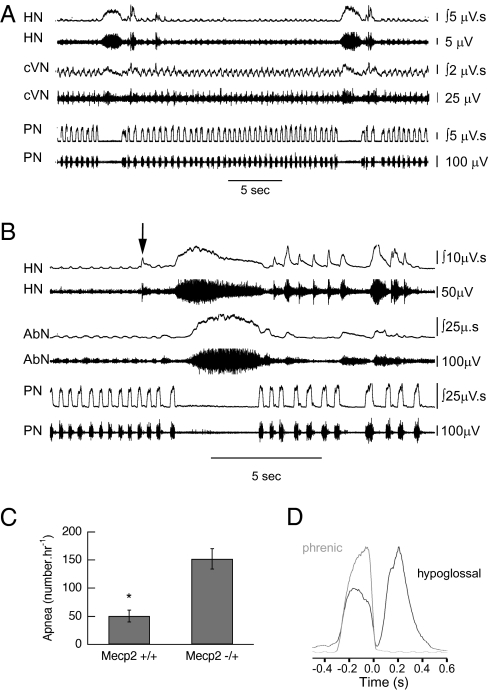
Studies were performed in WT and heterozygous Mecp2-deficient mice between 4 and 14 mo of age. Apneas were significantly more common in Mecp2−/+ (152 ± 18 · h−1, n = 17) compared with Mecp2+/+ (50 ± 10 · h−1, n = 16) (P ≤ 0.0001, unpaired t test) (Fig. 1C). The incidence of apnea was not affected by age (Fig. S1). The arrest of PN activity was characterized by both a prolonged post-I phase of cVN restricted to the initial portion of the apnea and tonic discharge of HN throughout the apnea. AbN activity was obtained in seven Mecp−/+ mice. Tonic AbN associated with PN apnea was seen in some of the apneas in all seven, and in four animals it exceeded 10% (range, 12.3–47.9%) (Fig. 1B). The temporal relationship for the onset of tonic AbN relative to that of HN was random: 29.9% ± 3.2% concurrent, 26.5% ± 6.6% preceded HN, and 43.5% ± 4.9% followed HN. In a number of eupneic respiratory cycles HN was biphasic with a distinct early expiratory component (Fig. 1 B, arrow, and D). The association of tonic HN with that in AbN, an expiratory nerve (15), and with postinspiration in cVN suggests that it is the expiratory component of HN that tonically discharges in PN apnea. The duration of apnea in Mecp2−/+ (2.82 ± 0.16 s) exceeded that in WT (1.96 ± 0.12 s) (P = 0.0004, unpaired t test).
Fig. 1.
Characterization of apnea in Mecp2−/+ mice studied in situ. (A) Representative traces show increased post-I activity in cVN that terminates before end of PN apnea and marked increase in HN that persists throughout PN apnea. (B) Example of tonic HN accompanied by tonic AbN during PN apnea. Note expiratory activity in one HN burst before the apnea (arrowed) and in several after PN apnea. (C) Incidence of apneas in Mecp2+/+ (n = 16) and Mecp2−/+ (n = 17) mice studied in situ. *P ≤ 0.0001 (unpaired t test). (D) Cycle triggered average of 20 consecutive cycles for integrated HN activity superimposed on integrated PN activity recorded in situ. There is significant HN activity during postinspiration.
Heterozygous Mecp2-deficient mice studied in situ differed from WT by their more frequent and especially longer episodes of periodic breathing (Fig. S2). Episodes were seen in 8 of 17 animals. Animals that showed periodic breathing were not older (8.6 ± 1.6 mo) than those that did not (8.0 ± 0.9 mo) (P = 0.75, unpaired t test). The duration of periodic breathing in Mecp2-deficient mice studied in situ was not evenly distributed: mean ± SEM, 12.9 ± 3.5 min; median, 6.7 min; range, 1.2–64.5 min. In contrast to Mecp2−/+ mice only 3 of 16 Mecp2+/+ animals had periodic breathing, and these episodes were shorter: 2.5 ± 1.6 min.
Effect of Augmenting GABA on Respiratory Disorders in Conscious Mecp2-Deficient Mice.
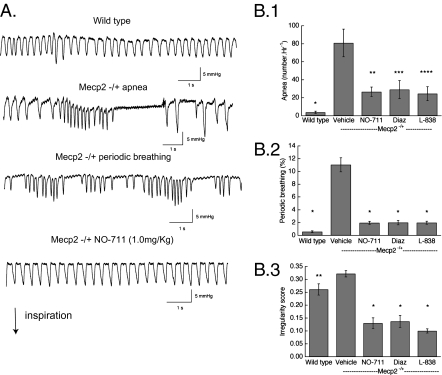
The tonic activity in expiratory nerves seen during PN apnea and periodic breathing suggested that an increase in inhibition could modulate the respiratory disturbances. Experiments were performed in Mecp2−/+ mice between 9.8 and 14.5 mo of age. Mecp2−/+ animals had frequent episodes of periodic breathing (Fig. 2A): 11.0 ± 1.1% (n = 8) of the 3-h study period for deficient females compared with 0.56% ± 0.14% for WT (n = 9) (Fig. 2B.2) (P < 0.001, two-way repeated-measures ANOVA with strain and treatment as the two factors). Isolated apneas (81 ± 15/h) exceed those in Mecp2+/+ (3.8 ± 1.2) (Fig. 2B.1) (P ≤ 0.001). The duration of apnea in Mecp2−/+ was longer (1.33 ± 0.03 s) than in WT (1.19 ± 0.04 s) (P = 0.018, unpaired t test). The respiratory cycle was more irregular (irregularity score: 0.32 ± 0.01) in Mecp2−/+ than that in Mecp2+/+ (0.26 ± 0.02) (Fig. 2B.3) (P = 0.022).
Fig. 2.
Effect of augmenting GABA on respiratory disorders in conscious, freely moving Mecp2−/+ mice. (A) Representative pleural pressure records by telemetry for WT and Mecp2−/+ showing isolated apnea (breath interval ≥ 1.0 s), periodic breathing, and effect of the GABA transporter blocker NO-711; inspiration is downward. (B) Effect of NO-711, diazepam (Diaz, a broad-spectrum GABAA receptor allosteric modulator), and L-838,417 (L-838, a derivative that is relatively specific for receptors that contain α2, α3, and α5 receptor subunits). (B.1) Apnea. WT. *P = 0.002 vs. Mecp2−/+ vehicle; **P = 0.008 vs., Mecp2−/+ vehicle; ***P = 0.015 vs. Mecp2−/+ vehicle; ****P = 0.028 vs. Mecp2−/+ vehicle (n = 9 for WT, n = 8 for vehicle, n = 5 for treatments) (two-way repeated-measures ANOVA with strain and treatment as the two factors). (B.2) Periodic breathing. *P = <0.001 vs. Mecp2−/+ vehicle. (B.3) Irregularity score. *P = <0.001 vs. Mecp2−/+ vehicle; **P = 0.022 vs. Mecp2−/+ vehicle.
Blocking GABA reuptake with NO-711 (1 mg/kg i.p.) significantly decreased the number of apneas to 26.6 ± 5.4/h (P = 0.008) and the incidence of periodic breathing to 1.97% ± 0.23% (P ≤ 0.001) (Fig. 2 B.1 and B.2). After NO-711 the irregularity score reverted to 0.13 ± 0.02 (Fig. 2B.3) (P ≤ 0.001). NO-711 (1.0 mg/kg) resulted in a slight increase in the incidence of apnea in Mecp2+/+ animals studied in situ (Fig. S3A.1) but did not affect the irregularity of their breath cycle (Fig. S3A.2).
The broad-spectrum benzodiazepine diazepam (1.0 mg/kg i.p.) reduced apneas (to 29.2 ± 10.1/h) (P = 0.013), the incidence of periodic breathing (to 2.0% ± 0.35%) (P = 0.001), and irregularity (14 ± 0.03) (Fig. 2 B.1–B.3). Diazepam, through its actions at the α1 subunit of GABAA receptors, causes sedation (16) and is addictive (17). Therefore, we examined the effects of a benzodiazepine derivative, L-838,417, that is relatively specific as a potentiating modulator for GABAA receptors that contain the α2, α3, and α5 subunits (18) This ligand (20 mg/kg i.p.) reduced apneas to 24.5 ± 7.8/h (P = 0.025), the incidence of periodic breathing to 1.98% ± 0.24% (P ≤ 0.0001), and irregularity score to 0.10 ± 0.01 (P ≤ 0.0001) (Fig. 2 B.1–B.3). All three drugs used to augment GABA inhibition significantly reduced respiratory frequency in Mecp2−/+ females: vehicle 239 ± 18 breaths/min, NO-711 157 ± 7, diazepam 153 ± 21, and L-838,417 164 ± 4 (P < 0.001, ANOVA). In contrast, NO-711 did not affect frequency in Mecp2+/+ mice: vehicle 224 ± 11 breaths/min, drug 211 ± 4 (P = 0.24, n = 5, paired t test). The corrective effects of augmenting GABA inhibition were not due to a significant reduction in animal activity (Fig. S4).
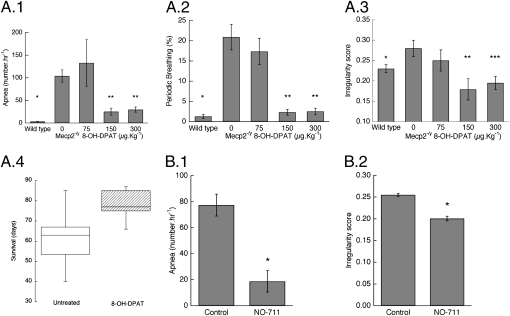
NO-711 (5 mg/kg i.p.) also reduced the incidence of apnea and corrected irregular breathing in Mecp2-/y males studied with plethysmography (Fig. 4 B.1 and B.2). Respiratory frequency (247 ± 37 breaths/min), however, was not affected in null male mice (246 ± 25).
Fig. 4.
Effect of blocking GABA reuptake and of the serotonin 1a receptor agonist 8-OH-DPAT on respiratory disorders in Mecp2 null male mice. (A) Dose-dependent effect of 8-OH-DPAT on apnea, periodic breathing, and irregularity score. (A.1) Apnea. *P = <0.001 vs. Mecp2-/y 0 mg/kg; **P = 0.046 and 0.02 vs. Mecp2-/y 0 mg/kg (n = 9 for WT, 10 for Mecp2-/y 0 and 300 μg/kg, and 4 for Mecp2-/y 75 and 150 μg/kg) (two-way repeated-measures ANOVA with strain and treatment as the two factors). (A.2) Periodic breathing. *P ≤ 0.001 vs. Mecp2-/y 0 mg/kg; **P = 0.003 and <0.001 vs. Mecp2-/y 0 mg/kg. (A.3) Irregularity score. *P = 0.001 vs. Mecp2-/y 0 mg/kg; **P = 0.048 vs. Mecp2-/y 0 mg/kg; ***P = 0.009 vs. Mecp2-/y 0 mg/kg. (A.4) Box plot for untreated (n = 22) and Mecp2 null males treated with 8-OH-DPAT osmotic pump. P = 0.035 (n = 9) (Kaplan–Meier log–rank test). (B) Effect of NO-711 (5 mg/kg i.p.) on apnea and irregularity score. (B.1) Apnea. *P = 0.001 (n = 4) (paired t test). (B.2) Irregularity score. *P = 0.004.
Effect of Blocking GABA Reuptake on HN and AbN Activity in the Residual PN Apneas.
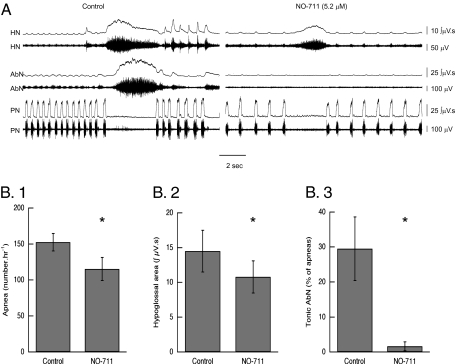
To confirm that augmenting GABA suppresses expiratory nerve activity, the effects of blocking reuptake were studied in Mecp2−/+ in situ. In six Mecp2−/+ mice studied with simultaneous recording of activity in PN, HN, and AbN the effects of NO-711 (5.2 μM in the perfusate) were examined. The number of apneas was reduced from 152 ± 12/h to 115 ± 36/h (P = 0.009, paired t test) (Fig. 3B.1). The reuptake block had a significant effect on HN and AbN activity in the PN apneas that remained after it was introduced. The area of the burst in tonic HN decreased from 14.5 ± 3.0 μV · s to 10.8 ± 2.4 μV · s (Fig. 3B.2) (P = 0.005, paired t test), and the number of apneas that contained tonic AbN activity fell from 29.5% ± 9.1% to 1.6% ± 1.3% (P = 0.04) (Fig. 3B.3). Experiments were continued for 46.9 ± 6.7 min after the reuptake blocker was placed in the perfusate. In that time frame there was no change in the reduction of HN amplitude, nor did tonic bursts in AbN return. This suggests that GABAA activity does not decline over time. NO-711 did not affect the duration of apnea (3.0 ± 0.1 s vs. 3.0 ± 0.2 s).
Fig. 3.
Effect of NO-711 on residual apneas in Mecp2−/+ mice studied in situ. (A) HN, AbN, and PN traces before (control) and after adding NO-711 (5.2 μM) to the perfusate. (B.1–B.3) Effect of the GABA reuptake blocker on (B.1) the incidence of apnea (*P = 0.009, n = 5, paired t test), (B.2) the HN burst envelope in residual PN apneas (*P = 0.005, n = 5), and (B.3) the incidence of apneas that contained tonic AbN activity (*P = 0.04, n = 4).
Effect of Serotonin 1a Agonist on Respiratory Disorders in Mecp2-Deficient Mice.
Although augmenting GABA inhibition significantly reduced isolated apneas and periodic breathing in Mecp2−/+ animals, it did not result in a pattern that was similar to that of Mecp2+/+ mice. Because serotonin 1a agonists have been shown to reduce the respiratory depressant effects of opioids (14), we examined the dose-dependent effects of a 1a agonist on respiratory pattern in Mecp2 null males (Mecp2-/y) and in heterozygous females, as well as the long-term effects on survival in null males. Mecp2-/y animals examined with body plethysmography showed abnormal breathing similar to freely moving heterozygous female mice (Fig. S5). Their incidence of apnea (104 ± 14/h, n = 10) exceeded that in WT (Mecp2+/y) (3.2 ± 0.6, n = 5) (P < 0.001), as did periodic breathing (P < 0.001) and the irregularity score (P = 0.001) (Fig. 4 A.1–A.3). 8-OH-DPAT significantly reduced the incidence of apnea (P = 0.02) and periodic breathing (P < 0.001) and irregularity (P = 0.009). 8-OH-DPAT in null males did not affect frequency: control, 213 ± 10 breaths/min; DPAT 300 μg/kg, 236 ± 19 breaths/min.
8-OH-DPAT (50 μg/kg i.p.) reduced the incidence of apnea in heterozygous female mice (Fig. S3B.1) and restored regularity (Fig. S3B.2). The serotonin 1a agonist did not affect respiratory frequency in Mecp2−/+ animals. Mecp2+/+ mice when studied in situ have a number of apneas (Fig. 1C). These were significantly reduced by the serotonin 1a agonist (0.25–0.5 μM in the perfusate) (Fig. S3A.1). The irregularity score, however, was not changed (Fig. S3A.2).
To determine the long-term effects of a serotonin 1a agonist, Mecp2 null males were implanted with osmotic pumps to provide a slow release of 8-OH-DPAT. On the basis of animal weight at the time the pumps were placed, the dose of 8-OH-DPAT ranged between 43 and 52 μg per kg per h. The median survival of treated mice (77 d) exceeded that of untreated animals (63.5 d) (P = 0.035, Kaplan–Meier log–rank test) (Fig. 4A.4). This represents a lower estimate of the serotonin 1a agonist's effect on survival because the drug was only present for 28 d.
Effect of Combined GABA Reuptake Block and Serotonin 1a Agonist on Apnea and Irregularity in Mecp2-Deficient Mice.
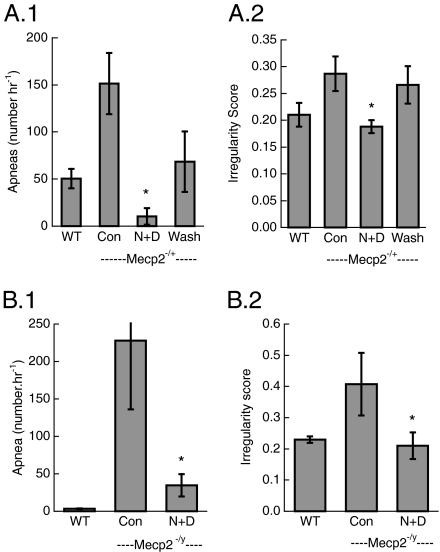
Because neither augmenting GABA synaptic inhibition nor serotonin stimulation at 1a receptors reduced apnea to the incidence seen in WT animals, and because their postulated modes of action are different, we investigated the effects of combining these treatments. NO-711 (5.2 μM in perfusate) followed 10 min later by the addition of 8-OH-DPAT (0.1 μM) reduced the occurrence of apnea in Mecp2−/+ animals studied in situ to that of Mecp2+/+ mice. In four of six Mecp2−/+ animals apnea was eliminated with the combined treatment (Fig. 5A). In one mouse apnea was reduced from 164/h to 36/h. The sixth animal showed periodic breathing, and the serotonin agonist reduced this pattern from 56.3% to 17.1%. The combined treatment restored breath cycle regularity. In Mecp2 null males the combination of NO-711 (1.0 mg/kg) and 8-OH-DPAT (150 μg/kg) reduced the incidence of apnea (Fig. 5C). The magnitude of reduced apnea (82.0% ± 5.5%) was larger than that seen with 300 μg/kg 8-OH-DPAT alone (71.3% ± 7.5%).
Fig. 5.
Effect of combined GABA reuptake block and serotonin 1a agonist on apnea and irregularity score in Mecp2−/+ studied in situ and conscious Mecp2-/y mice. (A.1) Apnea in Mecp2−/+ female mice treated with combined NO-711 (5.2 μM) and 8-OH-DPAT (0.1 μM). *P = 0.01 (n = 6) (ANOVA). (A.2) Irregularity score. *P = 0.001. (B.1) Apnea in Mecp2-/y males treated with combined NO-711 (1.0 mg/kg) and 8-OH-DPAT (150 μg/kg). *P = 0.016 (n = 5) (paired t test). (B.2) Irregularity score. *P = 0.05.
Locomotor activity was greater in heterozygous Mecp2-deficient females aged 6.4–7.8 mo after treatment with NO-711 (1.0 mg/kg) combined with 8-OH-DPAT (50 μg/kg); 3.4 ± 0.4 min · 5 min−1 than after vehicle injection, 1.9 ± 0.4 min · 5 min−1 (P = 0.002) (two-way repeated-measures ANOVA with strain and treatment as the two factors). In addition, whereas in the control condition Mecp2−/+ mice were less active than Mecp2+/+, after combined treatment there was no difference: Mecp2−/+ 3.3 ± 0.4, Mecp2+/+ 3.5 ± 0.3 (P = 0.68) (Fig. S6A). Vertical rearing movements were not significantly less in Mecp2−/+ animals compared with Mecp2+/+ (P values between 0.06 and 0.55) (Fig. S6B).
As with Mecp2-deficient females, locomotor activity was decreased in Mecp2-/y mice compared with WT. Combined treatment did not affect locomotor activity in either strain (Fig. S7A). It reduced vertical rearing activity in WT; however, a similar effect was seen with vehicle injections. Vertical rearing was not significantly affected in Mecp2-/y animals (Fig. S7B).
Discussion
The present study confirms, in heterozygous female mice, previous observations in Mecp2 null males that showed prolonged discharge in the post-I portion of cVN at the onset of PN apnea (9). Our characterization of respiratory disorders extended this earlier study by showing (i) all PN apneas are associated with large-amplitude tonic activity in HN that persists throughout the PN silence; (ii) a number of apneas are accompanied with tonic activity in AbN; (iii) augmenting GABAA inhibition significantly decreases the incidence of apnea and periodic breathing and corrects cycle irregularity; (iv) the serotonin 1a agonist 8-OH-DPAT reduces apnea and periodic breathing in a dose-dependent manner, induces regular breathing in Mecp2 null males, and when given chronically extends their survival; and (v) the combination of blocking GABA reuptake and 8-OH-DPAT reduces apnea to the level seen in WT.
Tonic Activity in Expiratory Neurons Causes Apnea in Mecp2-Deficient Mice.
The increased amplitude and extended duration of cVN post-I bursts coupled with tonic activity in AbN strongly suggest that arrest of PN activity is associated with a high excitability state of expiratory neurons. This may trigger enhanced levels of inhibitory synaptic influences from population of expiratory neurons into the respiratory pattern generator. The tonic activity seen in HN is also likely to be expiratory in origin. Although HN is often described as having solely inspiratory activity, particularly from in vitro preparations, there is evidence that the output of this cranial nerve also contains expiratory motor activity especially in vivo (19–21). Thus, the in situ characterization of PN apnea in Mecp2−/+ female mice reveals that three expiratory outputs—post-I in cVN, AbN, and expiratory component of HN—are tonic.
Insufficient GABAA Inhibition Underlies Tonic Activity in Expiratory Neurons.
The present experiments do not determine whether phasic or tonic GABAA receptor-mediated signaling account for the effects of NO-711. In the hippocampus this reuptake blocker induced a tonic conductance in pyramidal cells (22). The L-838,417 protocol does not answer this question because the compound potentiates activity in receptors with α2 and α3 (synaptic) and α5 (extrasynaptic) subunits (18). L-838,417, however, established an important clinically relevant finding, namely that modulation of the α1 subunit is not necessary for achieving beneficial effects on respiration. The α1 subunit mediates the sedative (18) and addictive (17) properties of benzodiazepines. Blocking GABA reuptake in the present studies could act through GABAA and/or GABAB receptors. GABAB agonists have been shown to depress respiratory rate (23, 24). Thus the reduction in frequency observed in Mecp2−/+ mice treated with NO-711 may have involved GABAB receptors. This effect, however, was not seen in null males. Potentiation of GABAA receptor activation through allosteric benzodiazepines was equally effective in correcting respiratory disorders in Mecp2−/+ animals. This suggests that the correction of respiratory disorders involves primarily GABAA receptors. The significant amelioration of apnea and periodic breathing, both of which were associated with tonic activity in HN, by augmenting GABA indicates that insufficient inhibition is the underlying mechanism.
Possible Expiratory Neuronal Groups That Are Tonic During Apnea.
Two observations in the present study suggest that expiratory neurons reflected by the expiratory portion of HN and by AbN, which are tonic during PN apnea, do not arise from a single population: (i) the temporal relationship between onset of tonic discharge in AbN relative to that in HN, when both were present, was equally distributed between concurrent, earlier, and later; and (ii) the effect of blocking GABA reuptake with NO-711 on these two nerves was markedly different. AbN tonic activity was virtually abolished in the apneas that remained after reuptake block, whereas it generally persisted in HN with a decrease in burst amplitude.
The finding of increased and prolonged discharge in post-I activity of cVN raises the question of where in the brainstem this activity originates. Recent evidence supports a role for the pons: microtransection studies in rat that eliminated the pons abolished post-I recorded from cVN (25, 26). Moreover, glutamate stimulation of Kölliker-Fuse (KF) neurons caused a prolongation of post-I recorded from the recurrent laryngeal nerve of juvenile rats in situ, whereas GABA receptor activation at the identical injection sites completely suppressed post-I activity (27). In addition, there is evidence that medullary sites also play a role. Bilateral microinjections of the GABAA agonist isoguvacine into retrotrapezoid nucleus (RTN) reduced post-I in cVN (15). This indicates that RTN either through afferents to KF or as a relay from KF to post-I neurons in the Bötzinger complex plays an important role in the generation of post-I expression.
It is also likely that stage 2 or late expiratory (augmenting neurons) are driven during the respiratory apneas of the Mecp2-deficient mice. Recently, expiratory activity in the AbN has been well characterized in neonatal and juvenile rats examined with the in situ preparation (15). Under baseline conditions (5% CO2) only post-I discharge was present. Hypercapnia (7% or 10% CO2), however, induced late expiratory (late-E) bursts that were characterized by an incrementing shape and abrupt termination with the onset of PN activity. The tonic AbN seen in Mecp2−/+ mice during PN apnea has a similar, albeit prolonged, pattern. Either pontine transection or bilateral isoguvacine injections into RTN abolished late-E AbN activity (15), and on the basis of this we propose that these regions could generate the pathological discharge in the abdominal motor outflow in Mecp2−/+ mice.
To date, the location and characterization of GABA interneurons whose insufficiency may underlie the respiratory disorders in Mecp2-deficient mice are not known. However, Medrihan et al. (11) examined GABA synapses in the ventrolateral medulla of Mecp2 null males at postnatal day 7. Neither the discharge pattern relative to PN nor markers to more clearly define the area under study were used. Neurokinin-1 receptor [a marker of pre-Bötzinger neurons (28)] immunoreactive cells receive both GABAergic and glycinergic boutons onto their soma and dendrires (29). More recently, glutamic acid decarboxylase-green fluorescence protein (GAD67-GFP) knockin mice have been used to examine inhibition in respiratory related areas. Recordings from GFP-positive neurons in medullary slices that contained the pre-Bötzinger complex showed that they fired concurrently with the integrated rootlet of HN (30). The spatial projections of these interneurons were not reported.
Mechanism of Serotonin 1a Agonist Induced Inhibition of Expiratory Activity.
Serotonin 1a receptors are located presynaptically, where they act as heteroreceptors and modulate neurotransmitter release (reviewed in ref. 13). At this site they attenuate GABA release. Because insufficient GABA underlies the respiratory disorders in Mecp2-deficient mice, it is unlikely that the effects observed with 8-OH-DPAT are due to presynaptic serotonin 1a receptor activation. Postsynaptic 1a receptors by coupling to Gαi subunits activate G protein-dependent inward rectifying K+ channels with resultant hyperpolarization. This inhibitory effect has been demonstrated in late-E neurons of anesthetized cats (12) and most likely contributes to the effects seen with 8-OH-DPAT. More recently it has been shown that the glycinergic inhibitor strychnine abolishes the effects of 8-OH-DPAT in rats studied in situ (14). Thus glycine-mediated inhibition may also add to the correction of respiratory disturbances seen with the serotonin 1a agonist.
8-OH-DPAT also exerts effects through serotonin 7 receptors, albeit at concentrations 100-fold higher than that required at 1a receptors (31). Systemic administration of a serotonin 7 agonist in rats studied in situ, however, reduced respiratory frequency (32). In contrast, we observe no change in frequency with 8-OH-DPAT, suggesting that its primary site of action is mediated by serotonin 1a receptors.
In summary, we have demonstrated that either alone or especially in combination, augmenting GABAA inhibition or a serotonin 1a agonist markedly reduces the incidence of apnea and periodic breathing and restores breath cycle regularity in Mecp2-deficient mice. The treatments did not adversely affect animal behavior, and in Mecp2−/+ mice the combination increased activity over that seen with vehicle, to a level the same as in WT. Taken together with the demonstration that activation of Mecp2 in mature symptomatic mice reverses their phenotype (5), the results indicate that the respiratory network is intact in Mecp2-deficient mice. Inasmuch as drugs that act in this manner are currently approved to treat human disorders, although not specifically for respiratory disorders in RTT, the results have significant clinical relevance.
Materials and Methods
Both heterozygous female and null male mice and their WT littermates were of the B6.129P2(C)-Mecp2tm1.1Bird strain. Mice were genotyped using a published method (33). In situ arterially perfused brainstem–spinal cord studies were performed as previously described (34). Telemetry recording of pleural pressure was obtained as previously described (35, 36). Respiratory pattern determined with body plethysmography was performed as previously described (37, 38). For chronic pharmacological treatment, respiratory pattern was examined at weekly intervals in Mecp2-/y males until they developed an abnormal pattern (approximately postnatal day 40). Under general anesthesia (1.5% isoflurane in oxygen) a 100-μL volume osmotic pump (Alzet model 1004) was placed in the peritoneal cavity through a midline abdominal incision. The pump contained 8-OH-DPAT (20 mM or 6.25 μg/μL) and delivered 0.11 μL/h for 28 d. A full description of methods is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Sharon Knopp for technical assistance and Victoria Jenkins and Caitlin Monaghan for genotyping mice. This work was supported by March of Dimes Foundation Grant 6-FY06-314 (to J.M.B.), International Rett Syndrome Foundation Grant 0802 (to J.M.B. and J.F.R.P.), and National Institutes of Health Grant NS057815 (to J.F.R.P.). J.F.R.P. was in receipt of a Royal Society Wolfson Research Merit Award. J.M.B. received a Benjamin Meaker visiting fellowship from the University of Bristol.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012104107/-/DCSupplemental.
References
- 1.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 2.Bird A. The methyl-CpG-binding protein MeCP2 and neurological disease. Biochem Soc Trans. 2008;36:575–583. doi: 10.1042/BST0360575. [DOI] [PubMed] [Google Scholar]
- 3.Chahrour M, Zoghbi HY. The story of Rett syndrome: From clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Skene PJ, et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Southall DP, et al. Hyperventilation in the awake state: Potentially treatable component of Rett syndrome. Arch Dis Child. 1988;63:1039–1048. doi: 10.1136/adc.63.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weese-Mayer DE, et al. Autonomic nervous system dysregulation: Breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60:443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- 8.Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: Progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol. 2009;168:101–108. doi: 10.1016/j.resp.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stettner GM, et al. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stettner GM, Huppke P, Gärtner J, Richter DW, Dutschmann M. Disturbances of breathing in Rett syndrome: Results from patients and animal models. Adv Exp Med Biol. 2008;605:503–507. doi: 10.1007/978-0-387-73693-8_88. [DOI] [PubMed] [Google Scholar]
- 11.Medrihan L, et al. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J Neurophysiol. 2008;99:112–121. doi: 10.1152/jn.00826.2007. [DOI] [PubMed] [Google Scholar]
- 12.Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol. 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- 13.Manzke T, et al. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- 14.Dutschmann M, et al. The potency of different serotonergic agonists in counteracting opioid evoked cardiorespiratory disturbances. Philos Trans R Soc Lond B Biol Sci. 2009;364:2611–2623. doi: 10.1098/rstb.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: Patterns, origins and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudolph U, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 17.Tan KR, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKernan RM, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 19.Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience. 1988;25:1041–1051. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]
- 20.Roda F, Gestreau C, Bianchi AL. Discharge patterns of hypoglossal motoneurons during fictive breathing, coughing, and swallowing. J Neurophysiol. 2002;87:1703–1711. doi: 10.1152/jn.00347.2001. [DOI] [PubMed] [Google Scholar]
- 21.Saito Y, Ezure K, Tanaka I. Difference between hypoglossal and phrenic activities during lung inflation and swallowing in the rat. J Physiol. 2002;544:183–193. doi: 10.1113/jphysiol.2002.022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: Role of Gi/o protein-mediated mechanisms. J Appl Physiol. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- 24.Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: A hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- 28.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YY, et al. GABAergic and glycinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Bötzinger complex of rats: Light and electron microscopic studies. Eur J Neurosci. 2002;16:1058–1066. doi: 10.1046/j.1460-9568.2002.02163.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuwana S, et al. Electrophysiological and morphological characteristics of GABAergic respiratory neurons in the mouse pre-Bötzinger complex. Eur J Neurosci. 2006;23:667–674. doi: 10.1111/j.1460-9568.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- 31.Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A. 8-OH-DPAT as a 5-HT7 agonist: Phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology. 2004;46:52–62. doi: 10.1016/j.neuropharm.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Manzke T, et al. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos Trans R Soc Lond B Biol Sci. 2009;364:2589–2602. doi: 10.1098/rstb.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miralvès J, Magdeleine E, Joly E. Design of an improved set of oligonucleotide primers for genotyping MeCP2tm1.1Bird KO mice by PCR. Mol Neurodegener. 2007;2:16. doi: 10.1186/1750-1326-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 35.Murphy DJ, Renninger JP, Gossett KA. A novel method for chronic measurement of pleural pressure in conscious rats. J Pharmacol Toxicol Methods. 1998;39:137–141. doi: 10.1016/s1056-8719(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 36.Bissonnette JM, Knopp SJ. Effect of inspired oxygen on periodic breathing in methy-CpG-binding protein 2 (Mecp2) deficient mice. J Appl Physiol. 2008;104:198–204. doi: 10.1152/japplphysiol.00843.2007. [DOI] [PubMed] [Google Scholar]
- 37.Mortola JP, Noworaj A. Two-sidearm tracheal cannula for respiratory airflow measurements in small animals. J Appl Physiol. 1983;55:250–253. doi: 10.1152/jappl.1983.55.1.250. [DOI] [PubMed] [Google Scholar]
- 38.Bissonnette JM, Knopp SJ. Separate respiratory phenotypes in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Pediatr Res. 2006;59:513–518. doi: 10.1203/01.pdr.0000203157.31924.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.