Abstract
O6-alkylG adducts are highly mutagenic due to their capacity to efficiently form O6-alkylG:T mispairs during replication, thus triggering G→A transitions. Mutagenesis is largely prevented by repair strategies such as reversal by alkyltransferases or excision by nucleotide excision repair (NER). Moreover, methyl-directed mismatch repair (MMR) is known to trigger sensitivity to methylating agents via a mechanism that involves recognition by MutS of the O6-mG:T replication intermediates. We wanted to investigate the mechanism by which MMR controls the genotoxicity of environmentally relevant O6-alkylG adducts formed by ethylene oxide and propylene oxide. Recently, the alkyltransferase-like gene ybaZ (eATL) was shown to enhance repair of these slightly larger O6-alkylG adducts by NER. We analyzed the toxicity and mutagenesis induced by these O6-alkylG adducts using single-adducted plasmid probes. We show that the eATL gene product prevents MMR-mediated attack of the O6-alkylG:T replication intermediate for the larger alkyl groups but not for methyl. In vivo data are compatible with the occurrence of repeated cycles of MMR attack of the O6-alkylG:T intermediate. In addition, in vitro, the eATL protein efficiently prevents binding of MutS to the O6-alkylG:T mispairs formed by the larger alkyl groups but not by methyl. In conclusion, eATL not only enhances the efficiency of repair of these larger adducts by NER, it also shields these adducts from MMR-mediated toxicity.
Keywords: futile mismatch repair cycling, interference between repair pathways, nucleotide excision repair, ybaZ gene
Alkylating agents react covalently with DNA, producing a large variety of adducts. Despite their low abundance, O6-alkylguanine adducts are responsible for most biological consequences induced by alkylating agents, both in terms of mutagenesis and toxicity. Whereas mutagenesis results from the capacity of these adducts to mispair efficiently with T during replication (1–4), the way these adducts induce toxicity is more cumbersome. Indeed, cytotoxicity involves the mismatch repair (MMR) system that recognizes the mutagenic O6-methylguanine:T (mG:T) replication intermediates. There is strong evidence from in vitro experiments that MutS proteins recognize mG:T mispairs more efficiently than mG:C pairs (5–9). In vivo data also show that mG:T mispairs are processed by MMR more efficiently than mG:C pairs (10). Similarly, MMR recognizes UV lesion-containing mismatches and may thus explain a decrease in UV-induced mutagenesis (11). The mechanism by which processing by MMR of O6-mG:T mispairs leads to cytotoxicity and apoptosis is still not fully understood. In eukaryotes, two models are currently proposed: Either MutS binding triggers apoptosis directly in the absence of any further MMR processing activity via the ATM/ATR DNA damage signaling cascade (7, 12) or MMR provokes cycles of futile processing of the newly replicated T-containing strand that may lead to strand breaks [see commentary by Karran (13)]. Recent work has shown the involvement of Exo1 function together with MutSα recognition as an important modulator of the mammalian cellular response to carcinogenic and chemotherapeutic agents that induce O6-mG adducts (14).
Both mutagenesis and toxicity triggered by O6-mG lesions involve replication. Thus, to reduce the genotoxic effect of these lesions, cells possess efficient error-free repair mechanisms that remove these adducts before replication. Efficient repair is mediated either by direct reversal of the lesion by alkyltransferase proteins (AT), a pathway that is particularly efficient for O6-mG adducts, or via nucleotide excision repair (NER) for the larger alkyl adducts (15, 16). Recently, we have shown that the ybaZ gene in Escherichia coli, a member of the alkyltransferase-like protein (eATL) family (for a recent review, see ref. 17), stimulates the repair by NER of O6-alkylguanine adducts (16). Whereas eATL binds to O6-alkylguanine adducts, it is devoid of any alkyltransferase activity (18, 19) and thus, unlike Ada or Ogt, does not act as a repair factor per se. eATL merely promotes repair via an “enhanced NER” pathway by facilitating the recruitment of the NER factors to the O6-alkylguanine lesion sites that are otherwise poor substrates (16), akin to the stimulation of photoproduct excision by the binding of photolyase in the absence of light (20). Similarly, the Atl1 gene in Schizosaccharomyces pombe stimulates alkylating agent repair by the NER pathway (21, 22).
In the present paper, we have investigated the modulation by MMR and eATL of the genotoxicity of defined O6-alkylguanine adducts in E. coli produced by ethylene oxide and propylene oxide, which together with their precursors ethylene and propylene are important raw materials and chemical intermediates (23, 24). Adducts under investigation here are O6-hydroxyethylguanine (heG), O6-1-hydroxypropylguanine (1hpG), and its isomer O6-2-hydroxypropylguanine (2hpG). Natural sources and endogenous metabolic processes such as lipid peroxidation and oxidation of methionine can also lead to the formation of such adducts (25). The toxicity and mutation frequency induced by the different adducts were determined in strains proficient or deficient in MMR and eATL. We show that the eATL gene product precludes MMR-mediated toxicity of O6-hydroxyethyl and O6-propylguanine adducts but not for methyl. We also provide evidence that MMR-mediated toxicity of O6-mG adducts requires mutH function, suggesting that toxicity involves MMR processing steps beyond MutS binding. In addition, in vitro, the eATL protein efficiently prevents binding of MutS to the O6-alkylG:T mispairs formed by these alkyl groups but not by methyl. In conclusion, eATL acts at the crossroads of NER and MMR; it decreases the genotoxicity of these larger O6-alkylguanine adducts at two stages: (i) by enhancing the efficiency of repair by NER (16) and (ii) by preventing MMR-mediated toxicity.
Results and Discussion
Overview and Strategy of the Work.
In the present work, we wanted to investigate how mismatch repair (mutS) and eATL (ybaZ) modulate the toxicity and mutagenesis of various O6-alkylguanine adducts. The alkyl residues under investigation here are O6-hydroxyethyl (heG) and the O6-hydroxypropyl isomers (1hpG and 2hpG) that are formed by the reaction with DNA of ethylene and propylene oxides, respectively (Fig. 1A) (23, 24). These larger adducts are compared with the more extensively studied O6-methylguanine (mG) adduct (1–4). Double- or single-stranded plasmids carrying single O6-alkylguanine adducts are introduced by transformation into bacteria; the transformed colonies are selected on the basis of their resistance to ampicillin. When introduced into bacteria, the adduct-bearing constructs are potentially subject to repair and replication. It should be noted that the plasmid constructs are fully methylated at their GATC sequences and will thus be subject to methyl-directed mismatch repair following replication, as evidenced in the following sections.
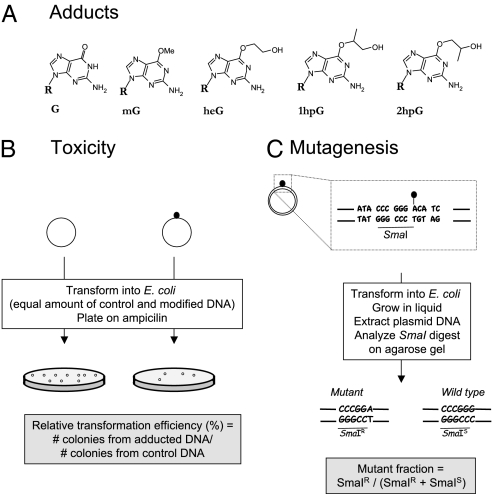
Fig. 1.
Experimental outline. (A) O6-alkylguanine adducts used in the present study: O6-methylguanine (mG), O6-hydroxyethylguanine (heG), O6-1-hydroxypropylguanine (1hpG), and O6-2-hydroxypropylguanine (2hpG). (B) The relative transformation efficiency characterizes the toxicity of a single adduct during replication. For this purpose, single-stranded plasmid constructs are transformed into E. coli cells. Transformants are selected on ampicillin-containing plates. The relative transformation efficiency is determined as the ratio of colonies produced by a given amount of adduct-carrying plasmid DNA over the same amount of control plasmid DNA. (C) Determination of the induced mutant fraction. A 14-mer oligonucleotide (dotted rectangle) that carries the O6-alkylguanine adduct on the third G within an SmaI restriction site (underlined) was inserted by ligation into a gapped-duplex structure as described in Materials and Methods. Following introduction into E. coli, the transformation mixture is cultivated in ampicillin-containing LB medium and the plasmid pool is extracted. During replication, the O6-alkylguanine adduct will mostly mispair with T and yield SmaI-resistant plasmid progeny (SmaIR). In contrast, accurate repair before replication or occasional C insertion during replication will yield SmaI-sensitive plasmid progeny (SmaIS). In addition, with double-stranded constructs, replication of the undamaged strand yields SmaIS plasmid progeny. The mutant fraction can be quantified following agarose gel electrophoresis as the ratio of the intensities SmaIR/(SmaIS + SmaIR).
The toxicity of a given adduct can be quantified by the “relative transformation efficiency” (RTE), that is, the efficiency of the single-stranded plasmid carrying a given adduct to form colonies compared with the colony-forming efficiency of the same quantity of lesion-free control construct (Fig. 1B). Only the single-stranded constructs are sensitive probes for assessing the intrinsic toxicity of a given adduct. Indeed, with double-stranded probes, potential repair of the adduct before replication as well as preferential replication of the undamaged strand masks the potential replication delay imposed by the lesion-containing strand (26).
The induced “mutant fraction” is determined, using either single- or double-stranded DNA probes, by plasmid pool restriction analysis, as described previously (Fig. 1C) (16). Briefly, upon introduction into E. coli cells, the O6-alkylguanine adduct located within the unique SmaI restriction site contained in the plasmid is processed by repair pathways and/or undergoes replication. Due to the high miscoding capacity of these adducts (1–4), unrepaired adducts will yield plasmid progeny containing a majority of G→A transitions at the adduct site, thus inactivating the SmaI restriction site. With single-stranded constructs, the mutant fraction may reach 100% if the adduct is fully miscoding. In contrast, with double-stranded constructs, as replication of the undamaged strand yields wild-type progeny, the maximal theoretical mutant fraction is 50% provided no repair occurs before replication.
eATL Prevents MMR-Mediated Toxicity.
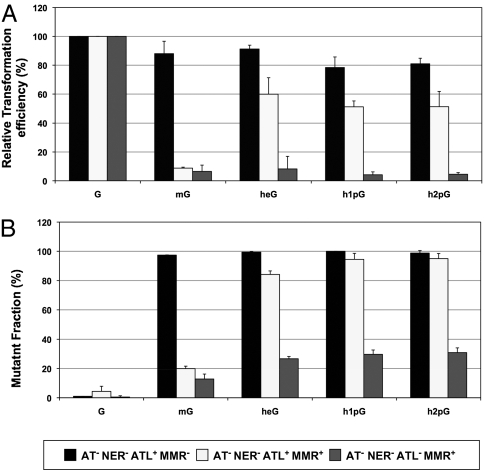
Single-stranded plasmid vectors carrying a single O6-alkylguanine adduct were introduced into various E. coli strains by transformation. To investigate the potential interplay between eATL and MMR in the absence of repair, all strains are defective in AT (ada, ogt) and NER (uvrA) repair and carry additional mutations in either mutS (MMR−) or ybaZ (ATL−). The RTE is determined as outlined (Fig. 1B). When MMR is inactivated in a background that is already defective in both AT and NER pathways, all adducts exhibit an RTE close to 80%, indicating that these adducts display low if any replication-hindering properties (Fig. 2A). In an MMR-proficient strain, the RTE corresponding to the mG adduct is strongly reduced (<10%), as documented previously (27). In contrast, the RTE values remain high for all other adducts (50–60%) despite the presence of an active MMR system. Interestingly, inactivation of ybaZ (ATL− strain) strongly sensitizes all of these adducts for attack by MMR, leading to low RTE values (<10%). These data highlight the protective effect of eATL against the toxic processing by the MMR system of the larger O6-alkylguanine:T replication intermediates. Indeed, eATL protection is effective for heG and hpG adducts but not for mG, suggesting a stronger affinity of eATL for heG and hpG compared with mG. This inference is supported by biochemical data presented below. The data also show that, in the absence of protection by eATL, MMR not only targets mG:T mispairs but also heG:T and hpG:T mispairs with similar efficiencies. Surprisingly, there are reports mentioning that O6-ethylguanine (eG) adducts are not processed by MutS (5, 28), in contrast to what is found here for heG. Moreover, eG, unlike heG, is not repaired by the alkyltransferase pathway (28). These surprising differences between the apparently similar eG and heG adducts may be explained by the recent finding that these two adducts exhibit a drastically different partition between the syn and anti conformation of their alkyl moiety (29). Therefore, apparently minor chemical changes appear to trigger major biological consequences.
Fig. 2.
eATL protects O6-alkylguanine:T mispairs from MMR-mediated toxic processing. Single-stranded plasmid vectors carrying a single O6-alkylguanine adduct were introduced into various E. coli strains by transformation. All strains used are defective in both AT (ada, ogt) and NER (uvrA) repair and additionally carry a mutation in mutS (MMR−) or ybaZ (ATL−). (A) The RTE is determined as the efficiency of the single-stranded plasmid carrying a given adduct to form colonies on ampicillin plates over the efficiency of the same quantity of lesion-free control construct. In an MMR− strain, all adducts exhibit an RTE of ≈80%, illustrating the low replication-hindering capacity of these adducts. In an MMR-proficient strain, survival of the mG adduct is strongly reduced (<10%), whereas it remains high for all other adducts (50–60%). Interestingly, inactivation of ybaZ (ATL−) strain strongly sensitizes all adducts to levels < 10%. It can thus be concluded that eATL efficiently protects all adducts (except mG) from the attack by MMR of the O6-alkylguanine:T replication intermediate. (B) Determination of the mutant fraction induced by the O6-alkylguanine adducts as described in Materials and Methods. In an MMR-defective background, all adducts exhibit mutant fractions close to 100%. When MMR processing is proficient (AT−NER−ATL+MMR+), the mutant fraction is strongly decreased for mG but not for the other adducts. Further inactivation of eATL sensitizes all adducts to MMR (AT−NER−ATL−MMR+).
Sequencing of individual mutant colonies reveals that all mutations are G→A transitions at the position carrying the O6-alkylguanine adduct. In the absence of repair by AT and NER and in the absence of MMR, all adducts exhibit a mutant fraction close to 100%, indicating an almost absolute preference for T insertion across the O6-alkylguanine adducts by the replicative polymerase (Fig. 2B). When MMR processing is proficient, the mutant fraction is strongly decreased for mG but not for the other adducts. However, upon inactivation of eATL (ybaZ strain), the mutant fraction for all adducts severely drops from 80–90% to ≈25%, showing that MMR specifically mediates killing of the mutagenic replication intermediates (thus acting as a mutation avoidance strategy). Indeed, it is noteworthy that MMR specifically targets the G*:T intermediates and not the G*:C intermediates, as confirmed in vitro by the binding of MutS to G*:T- but not the G*:C-containing oligonucleotides (see below).
MMR-Mediated Toxicity of O6-mG Adducts in E. coli Involves Mismatch Repair Steps Beyond MutS Binding.
Whereas it is clear that MMR-mediated toxicity requires binding of MutS protein to O6-alkylG:T replication intermediates, it is not known whether mismatch repair steps beyond MutS binding are necessary. To investigate this possibility, we determined the relative transformation efficiency and mutant fraction of single-stranded plasmids carrying a single mG adduct in mut+, mutH, and mutS strains. These experiments were performed in an AT− (ada, ogt) but otherwise NER+ background, as mG lesions are only minimally susceptible to excision repair (16). The single mG lesion exhibits an elevated level of relative transformation efficiency and a high mutant fraction in both mutS and mutH strains compared with the mut+ strain (Table 1). These data show that toxicity of O6-mG adducts is strongly dependent upon the function of both MutS and MutH proteins, strongly suggesting that toxicity requires mismatch repair steps downstream from MutS binding.
Table 1.
Involvement of both mutH and mutS functions in MMR-mediated toxicity of O6-methylG adducts in E. coli
| RTE (%) |
MF (%) |
|
| Strain/adduct | O6-methyl-G adduct | |
| Mut+ | 15.4 (±4.9) | 8.4 (±4.0) |
| mutS | 69.9 (±2.7) | 95.2 (±2.4) |
| mutH | 65.6 (±5.3) | 93.9 (±0.9) |
The RTE (relative transformation efficiency) and MF (mutant fraction) were determined as described in Materials and Methods. All strains are AB1157 derivatives defective for ada and ogt. Only the MMR genotype is indicated. The average value and SD of three experiments are indicated.
Evidence for Multiple Cycles of Mismatch Repair Processing.
For the O6-mG construct, in a strain defective in MMR, we determined the RTE and mutant fraction to be 88% and 97.4%, respectively (Fig. 2). From these values, we can estimate the amounts of error-free and mutagenic replication product to be ≈2.6% and 85.7%, respectively. In an MMR+ strain, the observed RTE and mutant fraction values are equal to 8.8% and 20%, respectively. The amounts of error-free and mutagenic replication product derived from these values are thus 7% and 1.8%, respectively. The comparison between the figures obtained in the MMR− and MMR+ strains shows a dramatic decrease in mutagenic replication product (from 85.7% to 1.8%) and a concomitant threefold increase in error-free replication product (from 2.3% to 7%). We interpret these data as follows: (i) they illustrate a massive attack by MMR of the mutagenic replication intermediate and (ii) the 2.3% value of error-free replication in the MMR− background is an estimation of the amount of correct C insertion mediated by the replicative polymerase during a single cycle of synthesis across the lesion. We propose that the threefold increase in error-free replication product observed in the MMR+ background results from a minimum of three cycles of excision synthesis across the lesion. For all other lesions, there is a similar enrichment in the fraction of error-free replication product when comparing the strain in which MMR is proficient to the MMR-deficient strain. These repeated cycles of massive attack of the G*:T replication intermediates are reflected by the high toxicity (low relative transformation efficiency) observed for these adducts in the mismatch repair-proficient strain (AT−NER−ATL−MMR+; Fig. 2A).
Mutagenesis Induced in Double-Stranded Plasmids.
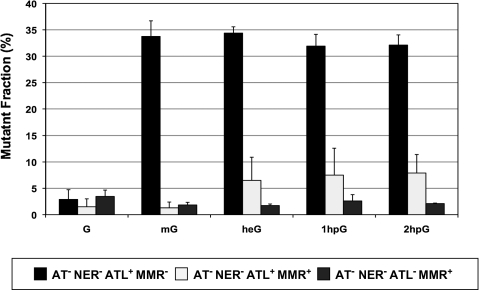
With double-stranded probes, there is no observable decrease in colony-forming ability (compared with an undamaged construct), as uncoupled replication of the undamaged strand will compensate for any replication hindrance in the lesion-containing strand (26). In contrast, monitoring the mutant fraction can yield important clues, although the effects will be less pronounced than with single-stranded DNA due to dilution of the mutagenic replication products by error-free replication products of the undamaged strand. Even in the absence of both AT and nucleotide excision repair pathways, when MMR is functional the induced mutant fraction is greatly reduced (to less than 10%) for all adducts compared with the values measured in the MMR-deficient background (≈30–35%) (Fig. 3). However, a more careful examination of the data reveals that the mutant fraction is particularly low for mG adducts (≈1–2%) compared with the slightly bulkier heG and hpG adducts (≈7%). When eATL is inactivated in the AT−NER− background, the mutant fraction for the heG and hpG adducts further decreases to reach the 1–2% value determined for mG (Fig. 3). Although the effects are quantitatively less pronounced using double-stranded versus single-stranded probes, they lead to the same conclusion, namely that eATL protects the G*:T mispairs formed by heG and hpG but not by mG against MMR processing. All conclusions drawn from the analysis of the single-stranded DNA data are thus confirmed using double-stranded probes.
Fig. 3.
Modulation by eATL and MMR of the induced mutant fraction using double-stranded DNA probes. The elevated mutant fraction (30–35%) induced by all adducts in the MMR-defective strain is severely reduced when MMR is proficient. This reduction is less pronounced for heG and hpG adducts compared with mG except when eATL is also inactivated. Processing by MMR of the mutagenic G*:T replication intermediates is thus counteracted by eATL for heG and hpG but not mG, reflecting the differential affinity of eATL for the various adducts in vitro.
Competition Between MutS and eATL (YbaZ) for Binding to O6-Alkylguanine Adducts.
We conducted electrophoretic mobility shift assays to monitor the binding of eATL or MutS proteins to double-stranded oligonucleotide substrates containing either a single G*:C or G*:T base pair, G* being unmodified G, mG, heG, or hpG (Table S1). eATL protein was purified (Fig. S1, lane 3) and found to trigger a clear band shift for all substrates tested except for the normal G-containing oligonucleotides in the absence of cold competitor DNA (Fig. S2). The concentration of eATL that causes a band shift of 50% of the input oligonucleotide is taken as an indication of the relative binding affinity (Table S2). The affinity of eATL decreases in the order hpG > heG > mG (Fig. S2), in good agreement with the inferences derived from the genetic data (Fig. 2). In addition, for a given adduct the binding affinity is similar whether the adduct faces C or T in the complementary strand, suggesting that eATL essentially recognizes the O6-alkylG residue with little influence from the pairing base.
Next, we conducted band shift assays with purified His6-MutS protein (a gift from W. Yang, National Institutes of Health, Bethesda, MD) using the same substrates (Fig. S3). The gel shift assays that involve MutS protein include cold competitor DNA to minimize the nonspecific binding of MutS to double-stranded DNA. Under these conditions, eATL is still able to induce a clear gel shift of the hpG-containing substrates but not the mG-containing substrates, whereas the heG substrates exhibit a weak smear (Fig. S4). MutS protein binds with the highest affinity to the unmodified G:T mispair (50% band shift at ≈125 nM) and with slightly lower but similar affinities to all O6-alkylG:T mispairs (50% band shift at ≈250 nM). Nonspecific binding of MutS to all G*:C constructs can be observed at high MutS concentration (Fig. S3).
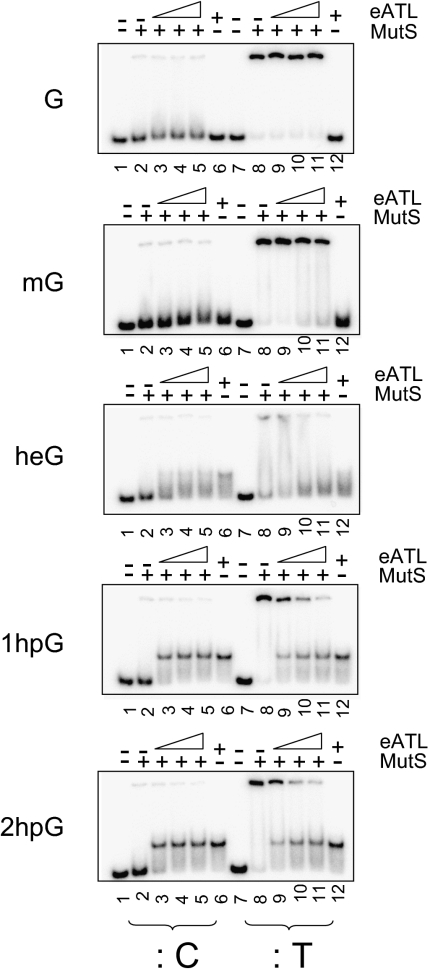
Finally, we performed competitive binding experiments between eATL and MutS protein to G*:T substrate oligonucleotides in the presence of cold competitor DNA (Fig. 4). The substrates are first incubated with increasing concentrations of eATL protein (0–500 nM) and then challenged with a constant amount of MutS protein (1 μM) before gel electrophoresis. The data show that eATL efficiently interferes with MutS protein binding to both 1hpG:T and 2hpG:T substrates, whereas eATL is unable to prevent MutS binding to G:T and mG:T substrates at any of the eATL concentrations tested. The response for the heG:T substrates falls in between, reflecting the following order for eATL binding affinities: mG < heG < hpG. Addition of eATL and MutS in the reverse order yielded the same results. These in vitro data are in good agreement with the observation that eATL prevents MMR-dependent toxicity of the slightly larger O6-alkylguanine adducts and strongly suggest that eATL is able to shield such adducts from MMR processing in vivo (Fig. 2). During MMR processing of the G*T replication intermediates, MutS protein recognizes the G*:T mispairs and allows subsequent incision of the newly synthesized T-containing strand. Recognition by MutS of the G*:T mispairs is prevented by binding of eATL to the G*-containing strand for the heG and hpG adducts.
Fig. 4.
eATL prevents binding of MutS to O6-alkylguanine:T mispairs. The incubation buffer for MutS binding assays contains cold competitor DNA (1 kb ladder; New England Biolabs) at a concentration of 10 ng/mL to minimize unspecific binding of MutS to double-stranded DNA. The DNA probes are 32P-radiolabeled oligoduplexes containing G, mG, heG, 1hpG, and 2hpG, paired to C (:C) or to T (:T). Oligonucleotides were first incubated with eATL at 0 nM (lanes 2 and 8), 125 nM (lanes 3 and 9), 250 nM (lanes 4 and 10), and 500 nM (lanes 5, 6, 11, and 12). Reactions were next supplemented with MutS at a final concentration of 1 μM (lanes 2–5 and 8–11). eATL interferes with MutS binding for all adducts except mG. The same results are observed when the order of addition of eATL and MutS is reversed.
Conclusion
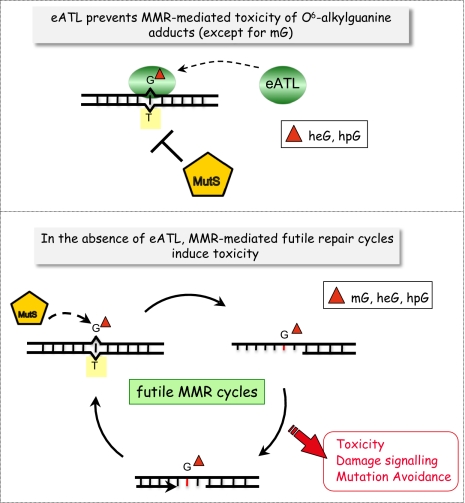
O6-alkylguanine adducts represent a unique and unusually hazardous class of adducts, as they are both highly mutagenic and cytotoxic. Whereas the high mutational potency of O6-methylguanine is related to its capacity to mispair efficiently with T during replication, its toxicity is indirect as it is mediated by the MMR system. In mammalian cells, the O6-methylguanine:T mispairs generated during replication have been shown to be a strong signal for apoptosis mediated by MutSα and MutLα (30–33). O6-alkylguanine adducts are induced by a variety of SN1-alkylating agents, including several drugs used for cancer chemotherapy (34). Another drug used in chemotherapy, 6-thioguanine, also produces G adducts that signal apoptosis via MMR (9, 35). Consequently, many tumors deficient in MMR become resistant to these drugs, presumably because they no longer signal apoptosis following therapy-induced DNA damage. The mechanism by which MMR triggers apoptosis is still under debate. Either MutSα binding triggers apoptosis directly in the absence of any further MMR processing activity via the ATM/ATR DNA damage signaling cascade (7, 12) or MMR provokes cycles of futile processing of the newly replicated T-containing strand that may lead to strand breaks [see commentary by Karran (13)]. Indeed, following recognition by MutS of the O6-alkylguanine:T mispair generated during replication, the MMR machinery generates a repair gap in the newly synthesized strand. The DNA polymerase that fills in the gap will predominantly reinsert a T residue opposite the O6-alkylguanine adduct. It is likely that repeated cycles of excision and resynthesis (futile repair) will ultimately trigger double-strand breaks and cell death (Fig. 5). By acting at G*:T mispairs, MMR does not accomplish a genuine repair function, as the adduct per se stays intact in the template strand. By acting at the T-containing strand of the mutagenic G*:T replication intermediate, MMR acts as a mutation avoidance pathway at the expense of toxicity. The present work provides compelling evidence that, in E. coli, using a plasmid-based probe, the toxicity of O6-alkylguanine adducts is caused by repeated cycles of MMR attack of the T-containing strand in the G*:T replication intermediate.
Fig. 5.
How eATL interferes with MMR-mediated processing of O6-alkylG:T replication intermediates. eATL binds to O6-alkylguanine-containing oligonucleotides and thus inhibits MutS protein binding to the G*:T mispair. As a consequence, eATL prevents MMR-mediated processing of the G*:T replication intermediates. Data presented in the present paper show that MMR-mediated toxicity involves MutH functions rather than just MutS binding and suggest that repeated cycles of processing trigger toxicity in E. coli. The effect of eATL is observed for the larger O6-alkylG adducts (hydroxyethyl and hydroxypropyl) but not for methyl, in good agreement with the respective binding affinities of eATL for the different adducts.
Our work also highlights the biological roles of eATL in the processing of O6-alkylguanine adducts in E. coli cells. The O6-alkylguanine adducts under scrutiny here are heG and hpG adducts that form upon reaction of ethylene and propylene oxides with DNA. It turns out that eATL interferes with the processing of these O6-alkylguanine lesions at two levels: First, it enhances NER, thus diminishing both mutagenesis and toxicity (16), and second, it inhibits the action of the MMR system on O6-alkylguanine:T intermediates, thus lowering toxicity at the cost of mutagenesis. Although ATL proteins are present in all three kingdoms of life, no ATL homolog in higher eukaryotes or plants has yet been found (17, 18, 22). One may speculate that the DNA damage binding protein complex UV-DDB (36) that recognizes lesions such as UV-induced cyclobutane dimers, AP sites (apurinic/apyrimidinic sites), and so forth may act as a functional homolog of ATL. It will be of interest to see whether such proteins also modulate the genotoxicity of alkylating agents by acting at the crossroads of NER and MMR pathways, thus providing insights into the mode of action of this important class of chemotherapeutic drugs. A deep understanding of how cells deal with the genotoxicity of alkylating agents is critical to the development and improvement of cancer therapy.
Materials and Methods
Synthesis of Oligonucleotides Containing DNA Adducts.
The 14-mer oligonucleotides (5′-ATA CCCGGG ACA TC-3′) carrying the various O6-alkylguanine adducts at the underlined G within an SmaI restriction site (in italics) were chemically synthesized following phosphoramidite methodology by using a versatile O6-sulfonyl-2′-deoxynucleoside intermediate. Fully protected 2′-deoxynucleosides-3′-phosphoramidites (dT, dAPAc, dCPac, dGPac, and 06-MedGibu) (Glen Research) were dissolved in dry acetonitrile (0.1 M solutions) and then assembled with an Applied Biosystems model 392 DNA synthesizer using standard coupling times (1 μmol scale). The oligonucleotides containing O6-hydroxyalkylguanine nucleoside were deprotected by a final treatment with 30% aqueous ammonia solution at 20 °C over 4 h. After evaporation of the solvent under vacuum, all of the oligonucleotides were purified by reverse-phase HPLC, quantified by UV measurements at 260 nm, and lyophilized. Their purity was checked by analytical RP-HPLC and denaturing PAGE after 32P labeling. The purity and the integrity of the oligonucleotides were confirmed by MALDI-TOF mass spectrometry measurements.
Plasmid Constructions.
Single-adducted plasmids were constructed by ligation of the lesion-containing oligonucleotide into a “gapped-duplex” plasmid as previously described (37, 38). The single-stranded DNA plasmids were derived from the corresponding double-stranded constructs using a procedure described previously (38). In brief, this procedure involves the construction of double-stranded plasmids carrying a single lesion in one strand and randomly distributed uracil residues in the complementary strand. The uracil-containing strand is selectively degraded using the following enzymatic mixture: uracil-DNA-glycosylase to remove the uracil residues, incision of the resulting AP sites by exonuclease III, and full digestion of the nicked strand by the 3′-5′ exonuclease activity associated with T7 DNA polymerase (38). The resulting single-stranded plasmids are analyzed and quantified by agarose gel electrophoresis. All restriction enzymes and other enzymes were obtained from New England Biolabs and were used as recommended by the manufacturer in the provided buffers.
Strains and in Vivo Manipulations.
All strains are derivatives of AB1157 [F-, thr-1, ara14, leuB6, Δ(gpt-proA)62, lacY1, tsx-33, supE44, galK2, l-, rac-, hisG4, rfbD1, mgl-51, rpsL31, kdgK51, xyl-5, mtl-1, argE3, thi-1, qsr-]. The following alleles were introduced into the AB1157 or AB1886 (AB1157, uvrA6) background by P1 transduction: Δada25::Cm and ogt1::Kan (39); mutS::Tc; ybaZ::cat (16); mutH::Tc (a generous gift from M. Marinus, University of Massachusetts Medical School, Worcester, MA).
Determination of the Toxicity and Mutant Fractions.
The toxicity of a given adduct can be characterized as the relative transformation efficiency, that is, the efficiency of the single-stranded plasmid carrying the given adduct to form colonies on ampicillin plates compared with the efficiency to form colonies of the same quantity of lesion-free control construct. With single-stranded constructs, we typically transform 20 ng of plasmid into CaCl2-competent cells and plate appropriate dilutions on LB plates containing 50 μg/mL of ampicillin (38). Determination of the relative transformation efficiency involves at least three independent transformations.
The mutant fraction induced by a given adduct is determined as follows, using either single- or double-stranded plasmid constructs. The single O6-alkylguanine adducts are located within a unique SmaI restriction site. Mutagenic replication can be monitored quantitatively as the inactivation of this restriction site (Fig. 1B). We use CaCl2-competent cells for experiments with single-stranded plasmids and electrocompetent cells for experiments with double-stranded plasmids (38). The transformation mixture is used to inoculate 100 mL of LB medium containing 50 μg/mL of ampicillin, and cultured overnight. Plasmid DNA is extracted from the pool of transformants. The plasmid pool DNA preparation is subjected to digestion by SmaI or EcoRI as a control. An equivalent sample is incubated in the absence of restriction enzyme as a control. The bands resistant to SmaI (covalently closed circular + relaxed forms) and sensitive to SmaI (linear form) were quantified following agarose gel electrophoresis using Bio-Capt software (Vilber Lourmat) to acquire the image and Multi Gauge software (Multi Gauge v2.3; Fuji) to quantify the intensity of the DNA bands. The mutant fraction was determined as the ratio SmaIR/(SmaIS + SmaIR).
Electrophoretic Mobility Shift Assays.
Double-stranded 37-mer oligonucleotide substrates containing the different O6-alkylguanine adducts opposite a C or a T residue were constructed by ligation as outlined in SI Materials and Methods. eATL was produced and purified as described in SI Materials and Methods (Fig. S1). eATL and MutS protein stocks were diluted in 20 mM Tris·HCl (pH 8), 2 mM DTT, 100 μg/mL acetylated BSA, 10% glycerol, and 300 mM KCl. For eATL DNA binding reactions (10 μL), radiolabeled double-stranded 37-mer oligonucleotide substrates (1.25 nM) were incubated with the protein at the indicated concentrations in 20 mM Tris·HCl (pH 8), 1 mM DTT, 50 μg/mL acetylated BSA, 5% glycerol, and 30 mM KCl for 20 min at room temperature. The reaction mixtures were fractionated by electrophoresis on a 10% nondenaturing PAGE using standard Tris-acetate-EDTA (TAE) buffer run at 100 V for 50 min at room temperature using a Mini-PROTEAN (Bio-Rad). Gels were dried on Whatman 3MM paper and radioactive signals were collected and quantified by phosphorimaging using an FLA-5100 scanner and Multi Gauge software (Fuji). For MutS binding, conditions were as described above for eATL except that the binding buffer was supplemented with 5 mM MgCl2 and 10 ng/mL of cold competitor DNA (1 kb ladder; New England Biolabs), and that the gel and running buffer were supplemented with 6 mM MgCl2. Experiments involving eATL and MutS proteins were performed under the same conditions as for MutS alone. eATL (or MutS) was added first to the DNA substrate and incubated for 10 min at room temperature. MutS (or eATL) was added next and further incubated for 20 min at room temperature.
Supplementary Material
Acknowledgments
MutS protein is a generous gift of Dr. Wei Yang, National Institutes of Health (Bethesda, MD). We thank P. Karran for critical reading of this work. This work was partly funded by the Olefins Panel Ethylene/Propylene Work Group of the American Chemistry Council, the Propylene Oxide and Glycols Sector Group of Cefic, the Ethylene Oxide and Derivatives Sector Group of Cefic, and the Lower Olefins Sector Group of Cefic. M.M. is supported by the Association for International Cancer Research (AICR).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008635107/-/DCSupplemental.
References
- 1.Bhanot OS, Ray A. The in vivo mutagenic frequency and specificity of O6-methylguanine in ϕX174 replicative form DNA. Proc Natl Acad Sci USA. 1986;83:7348–7352. doi: 10.1073/pnas.83.19.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delaney JC, Essigmann JM. Effect of sequence context on O(6)-methylguanine repair and replication in vivo. Biochemistry. 2001;40:14968–14975. doi: 10.1021/bi015578f. [DOI] [PubMed] [Google Scholar]
- 3.Hill-Perkins M, Jones MD, Karran P. Site-specific mutagenesis in vivo by single methylated or deaminated purine bases. Mutat Res. 1986;162:153–163. doi: 10.1016/0027-5107(86)90081-3. [DOI] [PubMed] [Google Scholar]
- 4.Loechler EL, Green CL, Essigmann JM. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci USA. 1984;81:6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taira K, et al. Binding of MutS protein to oligonucleotides containing a methylated or an ethylated guanine residue, and correlation with mutation frequency. Mutat Res. 2008;640:107–112. doi: 10.1016/j.mrfmmm.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen LJ, Samson L. The Escherichia coli MutS DNA mismatch binding protein specifically binds O(6)-methylguanine DNA lesions. Carcinogenesis. 1996;17:2085–2088. doi: 10.1093/carcin/17.9.2085. [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka K, Yoshioka Y, Hsieh P. ATR kinase activation mediated by MutSα and MutLα in response to cytotoxic O6-methylguanine adducts. Mol Cell. 2006;22:501–510. doi: 10.1016/j.molcel.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin S, Branch P, Xu YZ, Karran P. DNA mismatch binding and incision at modified guanine bases by extracts of mammalian cells: Implications for tolerance to DNA methylation damage. Biochemistry. 1994;33:4787–4793. doi: 10.1021/bi00182a006. [DOI] [PubMed] [Google Scholar]
- 9.Waters TR, Swann PF. Cytotoxic mechanism of 6-thioguanine: hMutSα, the human mismatch binding heterodimer, binds to DNA containing S6-methylthioguanine. Biochemistry. 1997;36:2501–2506. doi: 10.1021/bi9621573. [DOI] [PubMed] [Google Scholar]
- 10.Pauly GT, Hughes SH, Moschel RC. Response of repair-competent and repair-deficient Escherichia coli to three O6-substituted guanines and involvement of methyl-directed mismatch repair in the processing of O6-methylguanine residues. Biochemistry. 1994;33:9169–9177. doi: 10.1021/bi00197a020. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman PD, et al. Binding of MutS mismatch repair protein to DNA containing UV photoproducts, “mismatched” opposite Watson–Crick and novel nucleotides, in different DNA sequence contexts. DNA Repair (Amst) 2005;4:983–993. doi: 10.1016/j.dnarep.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 13.Karran P. Mechanisms of tolerance to DNA damaging therapeutic drugs. Carcinogenesis. 2001;22:1931–1937. doi: 10.1093/carcin/22.12.1931. [DOI] [PubMed] [Google Scholar]
- 14.Klapacz J, et al. O6-methylguanine-induced cell death involves exonuclease 1 as well as DNA mismatch recognition in vivo. Proc Natl Acad Sci USA. 2009;106:576–581. doi: 10.1073/pnas.0811991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samson L, Thomale J, Rajewsky MF. Alternative pathways for the in vivo repair of O6-alkylguanine and O4-alkylthymine in Escherichia coli: The adaptive response and nucleotide excision repair. EMBO J. 1988;7:2261–2267. doi: 10.1002/j.1460-2075.1988.tb03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazon G, Philippin G, Cadet J, Gasparutto D, Fuchs RP. The alkyltransferase-like ybaZ gene product enhances nucleotide excision repair of O(6)-alkylguanine adducts in E. coli. DNA Repair (Amst) 2009;8:697–703. doi: 10.1016/j.dnarep.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Tubbs JL, Tainer JA. Alkyltransferase-like proteins: Molecular switches between DNA repair pathways. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0405-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margison GP, et al. Alkyltransferase-like proteins. DNA Repair (Amst) 2007;6:1222–1228. doi: 10.1016/j.dnarep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Pearson SJ, Ferguson J, Santibanez-Koref M, Margison GP. Inhibition of O6-methylguanine-DNA methyltransferase by an alkyltransferase-like protein from Escherichia coli. Nucleic Acids Res. 2005;33:3837–3844. doi: 10.1093/nar/gki696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozer Z, Reardon JT, Hsu DS, Malhotra K, Sancar A. The other function of DNA photolyase: Stimulation of excision repair of chemical damage to DNA. Biochemistry. 1995;34:15886–15889. doi: 10.1021/bi00049a002. [DOI] [PubMed] [Google Scholar]
- 21.Pearson SJ, et al. A novel DNA damage recognition protein in Schizosaccharomyces pombe. Nucleic Acids Res. 2006;34:2347–2354. doi: 10.1093/nar/gkl270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tubbs JL, et al. Flipping of alkylated DNA damage bridges base and nucleotide excision repair. Nature. 2009;459:808–813. doi: 10.1038/nature08076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolman A, Chovanec M, Osterman-Golkar S. Genotoxic effects of ethylene oxide, propylene oxide and epichlorohydrin in humans: Update review (1990–2001) Mutat Res. 2002;512:173–194. doi: 10.1016/s1383-5742(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 24.Rusyn I, et al. Effects of ethylene oxide and ethylene inhalation on DNA adducts, apurinic/apyrimidinic sites and expression of base excision DNA repair genes in rat brain, spleen, and liver. DNA Repair (Amst) 2005;4:1099–1110. doi: 10.1016/j.dnarep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Törnqvist M, et al. Unsaturated lipids and intestinal bacteria as sources of endogenous production of ethene and ethylene oxide. Carcinogenesis. 1989;10:39–41. doi: 10.1093/carcin/10.1.39. [DOI] [PubMed] [Google Scholar]
- 26.Pagès V, Fuchs RP. Uncoupling of leading- and lagging-strand DNA replication during lesion bypass in vivo. Science. 2003;300:1300–1303. doi: 10.1126/science.1083964. [DOI] [PubMed] [Google Scholar]
- 27.Karran P, Marinus MG. Mismatch correction at O6-methylguanine residues in E. coli DNA. Nature. 1982;296:868–869. doi: 10.1038/296868a0. [DOI] [PubMed] [Google Scholar]
- 28.Pauly GT, Hughes SH, Moschel RC. Mutagenesis in Escherichia coli by three O6-substituted guanines in double-stranded or gapped plasmids. Biochemistry. 1995;34:8924–8930. doi: 10.1021/bi00027a045. [DOI] [PubMed] [Google Scholar]
- 29.Coulter R, et al. Differences in the rate of repair of O6-alkylguanines in different sequence contexts by O6-alkylguanine-DNA alkyltransferase. Chem Res Toxicol. 2007;20:1966–1971. doi: 10.1021/tx700271j. [DOI] [PubMed] [Google Scholar]
- 30.Hickman MJ, Samson LD. Apoptotic signaling in response to a single type of DNA lesion, O(6)-methylguanine. Mol Cell. 2004;14:105–116. doi: 10.1016/s1097-2765(04)00162-5. [DOI] [PubMed] [Google Scholar]
- 31.Duckett DR, Bronstein SM, Taya Y, Modrich P. hMutSα- and hMutLα-dependent phosphorylation of p53 in response to DNA methylator damage. Proc Natl Acad Sci USA. 1999;96:12384–12388. doi: 10.1073/pnas.96.22.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takagi Y, et al. Roles of MGMT and MLH1 proteins in alkylation-induced apoptosis and mutagenesis. DNA Repair (Amst) 2003;2:1135–1146. doi: 10.1016/s1568-7864(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 33.Roos WP, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 34.Roth RB, Samson LD. Gene transfer to suppress bone marrow alkylation sensitivity. Mutat Res. 2000;462:107–120. doi: 10.1016/s1383-5742(00)00021-1. [DOI] [PubMed] [Google Scholar]
- 35.Swann PF, et al. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996;273:1109–1111. doi: 10.1126/science.273.5278.1109. [DOI] [PubMed] [Google Scholar]
- 36.Hirschfeld S, Levine AS, Ozato K, Protić M. A constitutive damage-specific DNA-binding protein is synthesized at higher levels in UV-irradiated primate cells. Mol Cell Biol. 1990;10:2041–2048. doi: 10.1128/mcb.10.5.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koehl P, Burnouf D, Fuchs RPP. Construction of plasmids containing a unique acetylaminofluorene adduct located within a mutation hot spot. A new probe for frameshift mutagenesis. J Mol Biol. 1989;207:355–364. doi: 10.1016/0022-2836(89)90259-3. [DOI] [PubMed] [Google Scholar]
- 38.Napolitano RL, Fuchs RPP. New strategy for the construction of single-stranded plasmids with single mutagenic lesions. Chem Res Toxicol. 1997;10:667–671. doi: 10.1021/tx970018w. [DOI] [PubMed] [Google Scholar]
- 39.Rebeck GW, Samson L. Increased spontaneous mutation and alkylation sensitivity of Escherichia coli strains lacking the ogt O6-methylguanine DNA repair methyltransferase. J Bacteriol. 1991;173:2068–2076. doi: 10.1128/jb.173.6.2068-2076.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.