Abstract
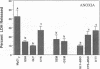
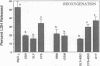
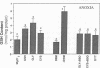
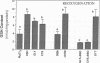
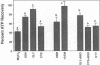
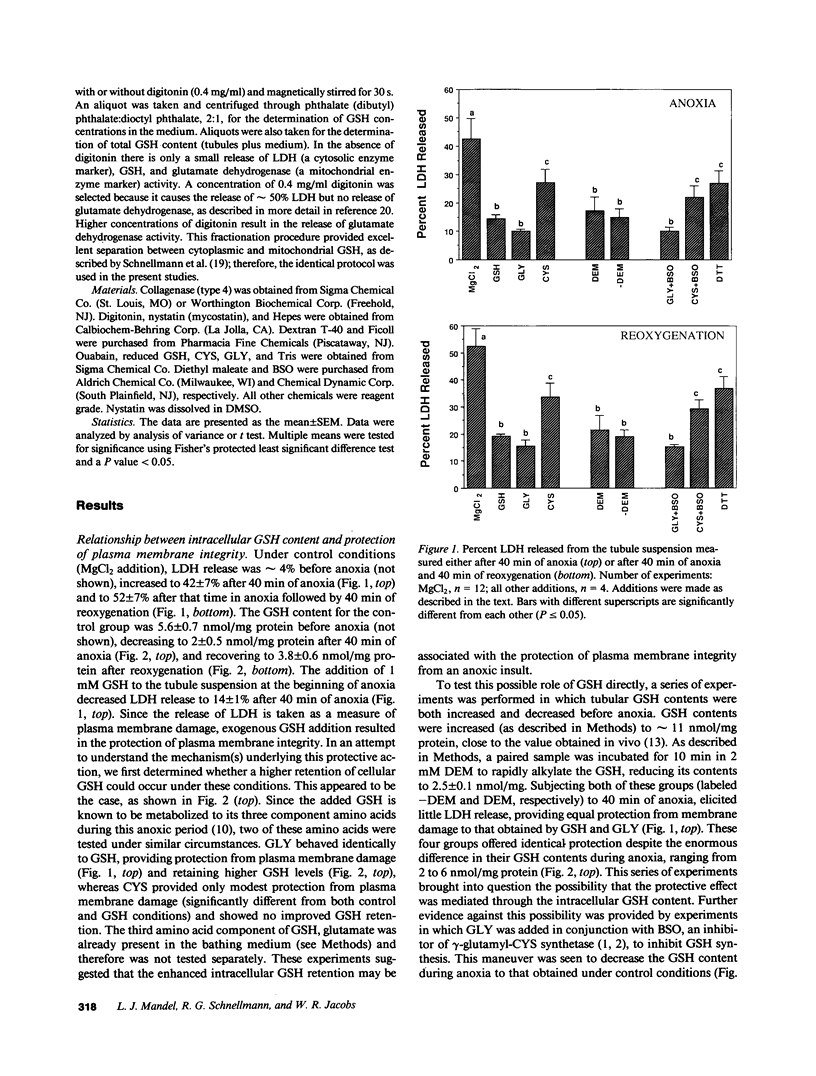
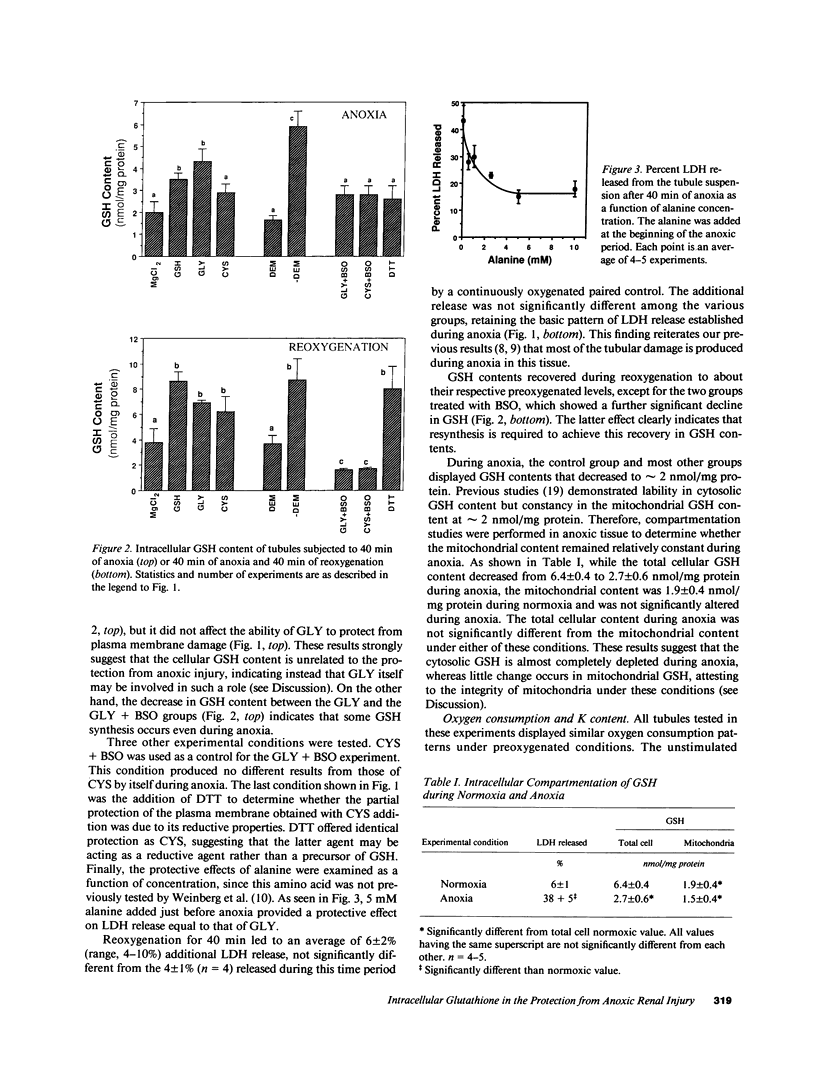
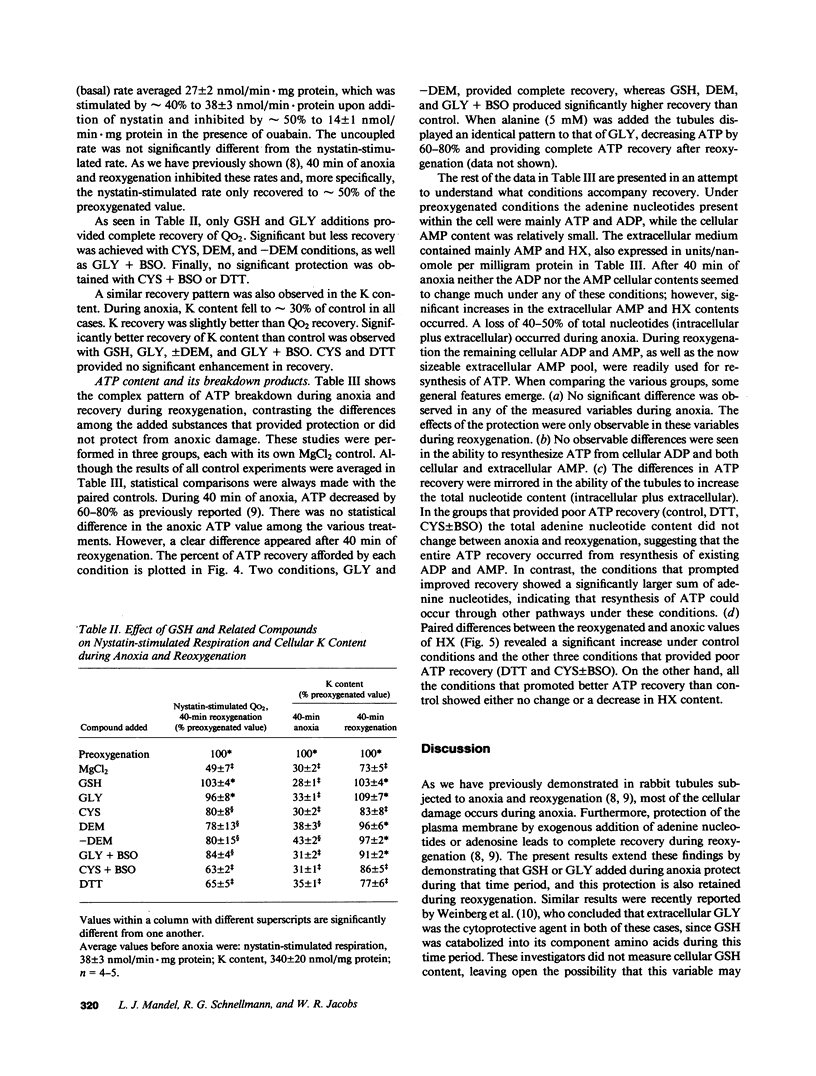
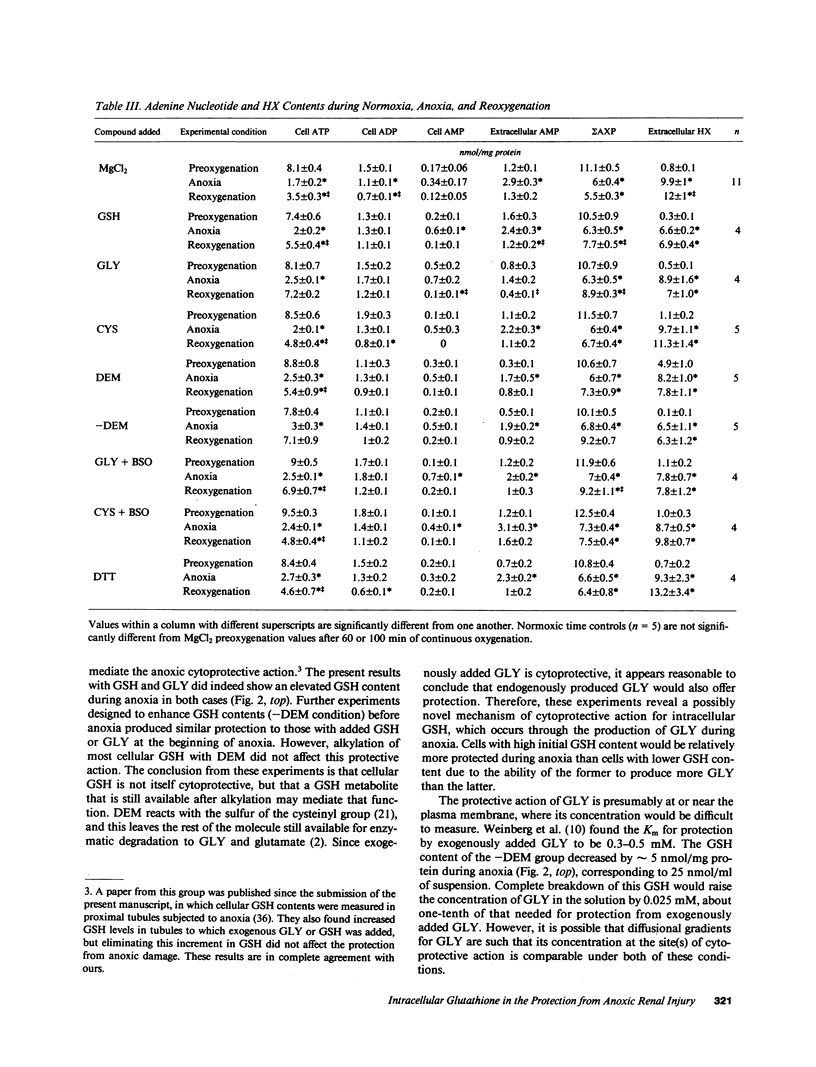
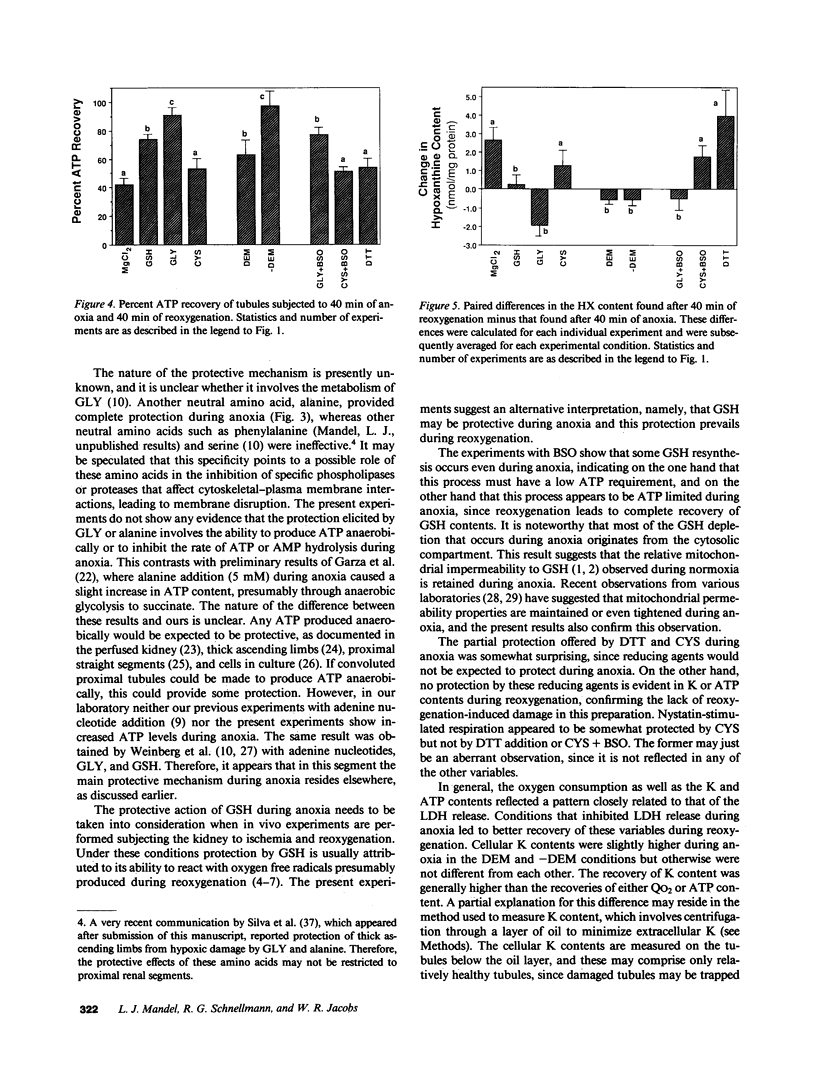
Previous results (Weinberg, J. M., J. A. David, M. Abarzua, and T. Rajan. 1987. J. Clin. Invest. 80:1446-1454) have shown that GSH and glycine (GLY) are cytoprotective during anoxia when added extracellularly. The present studies investigate the role that intracellular GSH plays in this cytoprotection. Proximal renal tubules in suspension prepared with either high (11 +/- 1 nmol/mg protein) or low (6 +/- 1 nmol/mg protein) GSH contents were subjected to 40 min of anoxia and 40 min of reoxygenation. Low GSH tubules were protected from plasma membrane damage during anoxia by exogenous addition of 1 mM GSH or GLY, reducing lactate dehydrogenase (LDH) release from 42 +/- 7 to 14 +/- 1 and 10 +/- 1%, respectively. High GSH tubules were equally protected from anoxic damage without exogenous additions. Since the high GSH content approximates the in vivo values, it may be concluded that GSH may be cytoprotective during anoxia in vivo. However, it is not the intracellular GSH itself that is cytoprotective; rather, this protection resides in the ability to produce GLY, which appears to be the cytoprotective agent. Alanine was also shown to have similar cytoprotective properties, although higher concentrations were required. Sulfhydryl reducing agents such as cysteine and dithiothreitol offered less, but significant protection from anoxic damage. Protection by GSH, GLY, or alanine was not associated with higher ATP levels during anoxia. Tubules that were protected from membrane damage during anoxia recovered oxygen consumption and K and ATP contents significantly better during reoxygenation than unprotected tubules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B. S., Aw T. Y., Jones D. P. Mitochondrial transmembrane potential and pH gradient during anoxia. Am J Physiol. 1987 Apr;252(4 Pt 1):C349–C355. doi: 10.1152/ajpcell.1987.252.4.C349. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Soltoff S. P., Storey J. M., Mandel L. J. Improved renal cortical tubule suspension: spectrophotometric study of O2 delivery. Am J Physiol. 1980 Jan;238(1):F50–F59. doi: 10.1152/ajprenal.1980.238.1.F50. [DOI] [PubMed] [Google Scholar]
- Buhl M. R. The predictive value of 5'-adenine nucleotide depletion and replenishment in ischaemic rabbit kidney tissue. Int Urol Nephrol. 1979;11(4):325–333. doi: 10.1007/BF02086820. [DOI] [PubMed] [Google Scholar]
- Busch E. W., von Borcke I. M., Martinez B. Abbauwege und Abbaumuster der Purinnucleotide in Herz-, Leber-, und Nierengewebe von Kaninchen nach Kreislaufstillstand. Biochim Biophys Acta. 1968 Sep 24;166(2):547–556. [PubMed] [Google Scholar]
- Chamberlin M. E., Mandel L. J. Na+-K+-ATPase activity in medullary thick ascending limb during short-term anoxia. Am J Physiol. 1987 May;252(5 Pt 2):F838–F843. doi: 10.1152/ajprenal.1987.252.5.F838. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Gerlach E., Marko P., Zimmer H. G., Pechan I., Trendelenburg C. Different response of adenine nucleotide synthesis de novo in kidney and brain during aerobic recovery from anoxia and ischemia. Experientia. 1971 Aug;27(8):876–878. doi: 10.1007/BF02135716. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Harris S. I., Balaban R. S., Barrett L., Mandel L. J. Mitochondrial respiratory capacity and Na+- and K+-dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J Biol Chem. 1981 Oct 25;256(20):10319–10328. [PubMed] [Google Scholar]
- Hull-Ryde E. A., Cummings R. G., Lowe J. E. Improved method for high energy nucleotide analysis of canine cardiac muscle using reversed-phase high-performance liquid chromatography. J Chromatogr. 1983 Jul 8;275(2):411–417. doi: 10.1016/s0378-4347(00)84388-1. [DOI] [PubMed] [Google Scholar]
- Jennische E. Possible influence of glutathione on postischemic liver injury. Acta Pathol Microbiol Immunol Scand A. 1984 Jan;92(1):55–64. doi: 10.1111/j.1699-0463.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Takano T., Soltoff S. P., Murdaugh S. Mechanisms whereby exogenous adenine nucleotides improve rabbit renal proximal function during and after anoxia. J Clin Invest. 1988 Apr;81(4):1255–1264. doi: 10.1172/JCI113443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy R. N., Hill K. E., Ayon M. A., Stein J. H., Burk R. F. Oxidant stress following renal ischemia: changes in the glutathione redox ratio. Kidney Int. 1988 Apr;33(4):812–817. doi: 10.1038/ki.1988.72. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Paller M. S. Hypothyroidism protects against free radical damage in ischemic acute renal failure. Kidney Int. 1986 Jun;29(6):1162–1166. doi: 10.1038/ki.1986.122. [DOI] [PubMed] [Google Scholar]
- Paller M. S. Renal work, glutathione and susceptibility to free radical-mediated postischemic injury. Kidney Int. 1988 Apr;33(4):843–849. doi: 10.1038/ki.1988.75. [DOI] [PubMed] [Google Scholar]
- Scaduto R. C., Jr, Gattone V. H., 2nd, Grotyohann L. W., Wertz J., Martin L. F. Effect of an altered glutathione content on renal ischemic injury. Am J Physiol. 1988 Nov;255(5 Pt 2):F911–F921. doi: 10.1152/ajprenal.1988.255.5.F911. [DOI] [PubMed] [Google Scholar]
- Schnellmann R. G., Gilchrist S. M., Mandel L. J. Intracellular distribution and depletion of glutathione in rabbit renal proximal tubules. Kidney Int. 1988 Aug;34(2):229–233. doi: 10.1038/ki.1988.169. [DOI] [PubMed] [Google Scholar]
- Schnellmann R. G., Lock E. A., Mandel L. J. A mechanism of S-(1,2,3,4,4-pentachloro-1,3-butadienyl)-L-cysteine toxicity to rabbit renal proximal tubules. Toxicol Appl Pharmacol. 1987 Sep 30;90(3):513–521. doi: 10.1016/0041-008x(87)90143-8. [DOI] [PubMed] [Google Scholar]
- Schnellmann R. G., Mandel L. J. Intracellular compartmentation of glutathione in rabbit renal proximal tubules. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1001–1005. doi: 10.1016/0006-291x(85)91235-5. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Active ion transport in the renal proximal tubule. I. Transport and metabolic studies. J Gen Physiol. 1984 Oct;84(4):601–622. doi: 10.1085/jgp.84.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromski M. E., Cooper K., Thulin G., Gaudio K. M., Siegel N. J., Shulman R. G. Chemical and functional correlates of postischemic renal ATP levels. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6142–6145. doi: 10.1073/pnas.83.16.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromski M. E., van Waarde A., Avison M. J., Thulin G., Gaudio K. M., Kashgarian M., Shulman R. G., Siegel N. J. Metabolic and functional consequences of inhibiting adenosine deaminase during renal ischemia in rats. J Clin Invest. 1988 Nov;82(5):1694–1699. doi: 10.1172/JCI113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Soltoff S. P., Murdaugh S., Mandel L. J. Intracellular respiratory dysfunction and cell injury in short-term anoxia of rabbit renal proximal tubules. J Clin Invest. 1985 Dec;76(6):2377–2384. doi: 10.1172/JCI112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Patel Y. J., Kreisberg J. I., Weinberg J. M. Energy thresholds that determine membrane integrity and injury in a renal epithelial cell line (LLC-PK1). Relationships to phospholipid degradation and unesterified fatty acid accumulation. J Clin Invest. 1988 Mar;81(3):745–758. doi: 10.1172/JCI113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Kiani T. Relationship between cell adenosine triphosphate and glutathione content and protection by glycine against hypoxic proximal tubule cell injury. J Lab Clin Med. 1989 May;113(5):612–622. [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest. 1987 Nov;80(5):1446–1454. doi: 10.1172/JCI113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Lawton A., Abarzua M. Modulation of cell nucleotide levels of isolated kidney tubules. Am J Physiol. 1988 Mar;254(3 Pt 2):F311–F322. doi: 10.1152/ajprenal.1988.254.3.F311. [DOI] [PubMed] [Google Scholar]