Abstract
Heparanase is involved in tumor growth and metastasis. Because of its unique cleavage of heparan sulfate, which binds cytokines, chemokines and proteases, we hypothesized that heparanase is also involved in regulation of early stages of hematopoiesis. We report reduced numbers of maturing leukocytes but elevated levels of undifferentiated Sca-1+/c-Kit+/Lin− cells in the bone marrow (BM) of mice overexpressing heparanase (hpa-Tg). This resulted from increased proliferation and retention of the primitive cells in the BM microenvironment, manifested in increased SDF-1 turnover. Furthermore, heparanase overexpression in mice was accompanied by reduced protease activity of MMP-9, elastase, and cathepsin K, which regulate stem and progenitor cell mobilization. Moreover, increased retention of the progenitor cells also resulted from up-regulated levels of stem cell factor (SCF) in the BM, in particular in the stem cell–rich endosteum and endothelial regions. Increased SCF-induced adhesion of primitive Sca-1+/c-Kit+/Lin− cells to osteoblasts was also the result of elevation of the receptor c-Kit. Regulation of these phenomena is mediated by hyperphosphorylation of c-Myc in hematopoietic progenitors of hpa-Tg mice or after exogenous heparanase addition to wildtype BM cells in vitro. Altogether, our data suggest that heparanase modification of the BM microenvironment regulates the retention and proliferation of hematopoietic progenitor cells.
Introduction
Hematopoiesis, the development of blood cells, is a highly orchestrated process that originates from a small population of primitive stem cells with high proliferation and differentiation potential. Regulation of the ongoing hematopoietic process is based on strict relationships and interactions between hematopoietic stem and progenitor cells (HSPCs) with their bone marrow (BM) microenvironment.1,2
The hematopoietic microenvironment, composed of hematopoietic stem cells (HSCs), immature and maturing leukocytes, stromal cells, and the extracellular matrix (ECM), bears the essential function of simultaneously maintaining the size of the stem cell pool and supplying the required number of mature blood cells throughout life. Alterations in the structure and/or function of some of these components may contribute to the development of hematologic disorders.3,4
The survival and proliferation of HSCs in vivo are dependent on close association with the BM stroma. This provides HSCs with a rich, but complex, milieu composed of many cell types and a vast array of intrinsic molecules that they produce.5,6 The distribution of HSPCs within the extravascular space is not random, and the majority of HSCs are located within the BM endosteal region7–9 and adjacent to blood vessel endothelial and reticular cells.10,11 A direct role for the involvement of endosteal osteoblasts in HSC regulation and maintenance in vivo has been established12 and osteoclastosteoblast interactions regulate egress and mobilization of hematopoietic progenitor cells.13
The stromal factors that impact on stem cell maintenance, propagation, homing, and homeostasis include growth factors and hematopoietic cytokines, such as stem cell factor (SCF)14 and stromal cell-derived factor-1 (SDF-1; also known as CXCL12).15 They are produced by a variety of stromal cells, including osteoblasts and reticular and endothelial cells.11,16 HSCs express the SCF receptor c-Kit.17,18 Mutations in SCF or c-Kit produce various defects, including impairment of hematopoiesis,19 suggesting that SCF and its receptor play a key role in maintaining and reconstituting the stem cell pool in adult mice.20 SCF was shown to stimulate adherence of stem cells as well as endosteal lodgment by murine stem cells,21 and is cleaved by the proteolytic enzymes cathepsin K and matrix metalloproteinase-9 (MMP-9). Furthermore, cleavage of membrane bound SCF by MMP-9 induces stem cell mobilization.13,22
Currently, SDF-1 is the only chemotactic factor known to induce high levels of directional migration of both human CD34+/CD38− and murine Sca-1+/c-Kit+/Lin− HSC-enriched populations.23,24 SDF-1 is produced by various cell types, including osteoblasts and reticular and endothelial cells within the BM11,15,16,25 and, together with its receptor CXCR4, is crucial for homing and long-term repopulation of human stem cells in transplanted NOD/SCID mice.23,26 Unlike proinflammatory chemokines, BM SDF-1 is constitutively expressed, pointing to a major role of SDF-1 in the maintenance of steady-state homeostatic processes, such as leukocyte trafficking and retention of stem and progenitor cells in the BM.16,25
The ECM serves as a reservoir for many biologic factors; consequently, cells can respond to changes in their environment through the effects of such factors.27 Heparan sulfate proteoglycans are ubiquitous macromolecules associated with the ECM and cell surface of nearly all cell types.28,29 Heparan sulfate chains are unique in their ability to bind a wide variety of biologic mediators, including growth factors, chemokines, and proteolytic enzymes, thereby affecting the bioavailability and functionality of these molecules.28–30 Cleavage of heparan sulfate proteoglycans releases the bound molecules, thus representing an additional mechanism by which cells can respond to changes in their microenvironment. At present, only a single heparan sulfate degrading enzyme, heparanase, has been identified in vertebrates.31,32
Expression of heparanase by human tumor cells correlates with tumorigenicity, whereas heparanase gene silencing or treatment with heparanase inhibitors markedly reduces tumor growth and the incidence of metastases in experimental animal models.33–35 These observations were attributed to heparanase-mediated release of active molecules from the ECM.36,37 The involvement of heparanase in these pathologic processes prompted us to investigate the potential roles of heparanase in hematopoiesis, specifically in the retention and proliferation of the stem cell enriched Sca-1+/c-Kit+/Lin− population in the BM.
Methods
Mice
All experiments were approved by the Weizmann Institutional Animal Care and Use Committee. Heparanase overexpressing transgenic mice (hpa-Tg) were previously described in detail.38 In brief, full-length human heparanase cDNA (hpa) was microinjected into fertilized eggs of C57BL/6 x Balb/c origin to produce transgenic mice overexpressing the hpa cDNA. Founder mice were mated with C57BL/6 mice to create F1 mice and those were mated among themselves to create F2 mice (bearing a mixed genetic background). F2-negative mice from the same littermate served as wildtype controls (WT) in all the experiments. In experiments testing survival of mice, C57BL/6 recipient mice (pure genetic background) were irradiated with a lethal dose (900 cGy) from a cesium source. Twenty-four hours later, recipient mice were intravenously injected with total BM containing 2.5 × 105 or 5 × 105 white blood cells (WBCs; counted after red cell lysis) obtained from hpa-Tg or control WT mice, which have a mixed genetic background (C57BL/6 x Balb/c). NOD/SCID mice (NOD/LtSz PrKdcscid/PrKdcscid) were irradiated with a sub lethal (350 cGy) dose from a cesium source 24 hours before death and evaluation of heparanase concentration and activity. Blood and BM cell analyses were performed by ADVIA 120 (Bayer, Emeryville, CA). Hematoxylin and eosin (H&E) staining of bone sections was done according to standard procedure. Megakaryocytes were quantified according to morphologic criteria as counted in 9 random fields, from 2 different sections of a femur, per mouse.
Cells
Murine stromal cell line MS-5 cells were grown in RPMI-1640, supplemented with 10% fetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel), l-glutamine (Biological Industries), 50 nM β-mercaptoethanol, penicillin, and streptomycin (Invitrogen, Carlsbad, CA). Primary osteoblasts were obtained and grown as previously described.13 Human cord blood was collected after informed consent was obtained in accordance with the Declaration of Helsinki and procedures approved by the Human Ethics Committee of the Weizmann Institute of Science. CD34+ cells were purified as previously described.39
Colony forming unit assay
Semisolid cultures were performed as previously described.40 Briefly, murine spleen (5 × 105 cells/plate) mononuclear cells or peripheral blood (50 μL/plate) were plated in 0.9% methylcellulose (Sigma-Aldrich, St Louis, MO), 30% fetal calf serum (Biological Industries), 50 ng/mL SCF, 5 ng/mL IL-3, 5 ng/mL GM-CSF (PeproTech, Rocky Hill, NJ), and 2 U/mL of erythropoietin (Orto Bio Tech, Don Mills, ON). The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2 and scored 7 days later according to morphologic criteria.
Chemotaxis assay
Chemotaxis experiments were performed in Costar transwells (6.5 mm/diameter, 5 μm/pore; Corning, Corning, NY). Conditioned medium from MS-5 cells or BM supernatant from WT or hpa-Tg mice cells were placed in the lower chamber. CD34+ cells (105) were added in the upper chamber and kept in 37°C, 5% CO2. Migrating cells were counted after 4 hours using FACSCalibur (BD Biosciences, San Jose, CA).
Real time reverse-transcribed polymerase chain reaction
Total RNA was isolated using TRI-Reagent (Sigma-Aldrich, Rehovot, Israel) according to the manufacturer's protocol. An aliquot of 2 μg of total RNA was reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI) and oligo-dT primers (Promega). Quantitative reverse transcribed–polymerase chain reaction (qRT-PCR) was done using the ABI 7000 machine (Applied Biosystems, Foster City, CA) with SYBR Green PCR Master Mix (Applied Biosystems). Comparative quantization of transcripts was assessed relative to hypoxanthine phosphoribosyl transferase (HPRT) levels and amplified with appropriate primers. Primer sequences used were as follows: murine SCF forward 5′-GTCATTGTTGGCTACGAGATA-3′; reverse 5′-AACACGAGGTCATCCACTATT-3′; murine SDF-1 forward 5′-GGACGCCAAGGTCGTCGCCGTG-3′; reverse 5′-TTGCATCTCCCACGGATGTCAG-3′; murine TIMP-1 forward 5′-GCAGATATCCGGTACGCCTACA-3′; reverse 5′-GGCGGCCCGTGATGA-3′; murine HPRT forward 5′-GCAGTACAGCCCCAAAATGG-3′; reverse 5′-GGTCCTTTTCACCAGCAAGCT-3′.
Protease activity
Gelatin zymography was performed as previously described39 with the following modification: BM supernatant (0.5 μg protein measured by Bradford assay) was loaded on 10% SDS-PAGE containing 1 mg/mL gelatin. Conditioned media from HT-1080 cells secreting MMP-9 served as control. Elastase activity was determined by cleavage of a specific chromogenic substrate (Calbiochem, Darmstadt, Germany) according to the manufacturer's instructions. Cathepsin K activity was determined by hydrolysis of the fluorogenic peptide substrate ZFR-AMC (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Recombinant heparanase
Single-chain GS3 active heparanase gene construct, composed of the 8 kDa and 50 kDa heparanase subunits, was kindly provided by Dr Christian Steinkuhler (IRMB/Merck Research Laboratories, Pomezia, Italy), and the protein was purified from the conditioned medium of baculovirus infected cells, as described.41 BM mononuclear cells (MNCs; 0.5-1 × 106 cells in 500 μL) were incubated overnight with or without recombinant heparanase (3.5 μg/well), and phosphorylated c-Myc expression or c-Kit receptor levels on c-Kit+/Lin− cells were detected by flow cytometry.
Enzyme-linked immunosorbent assay
Detection of SDF-1 levels was performed as previously described.40 To detect biotinylated-SDF-1 (bSDF-1), the assay was modified and the second biotinylated capture antibody was not added. Inhibition of bSDF-1 proteolysis by BM supernatant-derived proteases was obtained by incubating the samples (30-60 minutes, 37°C) with 1% protease inhibitor cocktail (Sigma-Aldrich). SCF enzyme-linked immunosorbent assay (ELISA) kit (DuoSet kit, R&D Systems) was used according to manufacturer's instructions. Heparanase ELISA was performed as previously described.42 Results were normalized to total milligrams of protein in each sample, as measured by the Bradford assay.
Immunohistochemistry
Immunohistochemical staining was performed as previously described,16 on formalin-fixed, paraffin-embedded murine bone sections, using goat antimouse SCF (limiting dilutions 2.5-10 μg/mL; R&D Systems). Immunoreactivity was detected using biotinylated anti goat and LSAB2 avidin-biotin-DAB detection kit (Dako Denmark, Glostrup, Denmark) according to the manufacturer's instructions.
Adhesion assay
BM MNCs were plated for 2 hours on flat-bottomed plates precoated with monolayer of primary osteoblasts. Nonadherent cells were washed twice with phosphate-buffered saline. Adherent cells were removed with 0.5 mM EDTA in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline containing 0.01% sodium azide and 1% fetal calf serum). Cells were stained for the immature Sca-1+/c-Kit+/Lin− population, and the number of adherent cells was determined by flow cytometry. In some experiments, the hematopoietic cells were pretreated with neutralizing anti-CD117 (c-Kit) antibodies (ACK45 clone; BD PharMingen, San Diego, CA). This clone did not interfere with Sca-1+/c-Kit+/Lin− staining.
Heparanase activity assay
Cell lysate or BM supernatant from NOD/SCID mice was analyzed for heparanase activity, as described.32,43 Briefly, samples were incubated (16 hours, 37°C) on sulfate-labeled, extracellular matrix (ECM)-coated, 35-mm dishes in 1 mL heparanase reaction buffer (20 mM phosphate-citrate, pH 5.8, 1 mM dithiothreitol, 1 mM CaCl2, 50 mM NaCl). The incubation medium containing sulfate-labeled degradation fragments released from ECM was subjected to gel filtration on a Sepharose CL-6B column. Intact heparan sulfate proteoglycans were eluted just after the void volume (Kav < 0.2, fractions 1-10); heparan sulfate degradation fragments were eluted toward the Vt of the column (0.5 < Kav < 0.8, fractions 15–35).
Flow cytometric analysis
Phenotypes of murine cells were examined by immunostaining, followed by flow cytometric analysis on FACSCalibur (BD Biosciences) with CellQuest software. Single cell suspension was prepared in FACS buffer. Mouse IgG was used to block murine Fc-receptors. Staining was performed at 4°C for 30 minutes. Murine BM cells were triple stained with fluorescein isothiocyanate (FITC)-conjugated antibody (Ab) indicating lineage positive phenotype (CD4, NK, CD8, B220, CD11b, Gr-1; eBiosciences, San Diego, CA), together with anti Sca-1 phycoerythrin (PE; BD PharMingen) and anti c-Kit allophycocyanin (APC; eBiosciences) and were used to determine the levels of Sca-1+/c-Kit+/Lin− stem cell–enriched populations. Number of Sca-1+/c-Kit+/Lin− in 1 femur was counted using flow cytometry. In some experiments, progenitor cells were triple stained with FITC-conjugated Ab indicating lineage positive phenotype (CD4, NK, CD8, B220, CD11b, Gr-1; eBiosciences), anti-c-Kit APC (eBiosciences) and anti-PE-conjugated bromodeoxyuridine (BrdU; Cell Signaling Technology, Danvers, MA) or anti-c-Myc (Cell Signaling Technology), subsequently stained with secondary goat antirabbit antibodies). The expression level of c-Myc or BrdU was analyzed on the progenitor c-Kit+/Lin− cell population.
BrdU incorporation
WT or hpa-Tg mice were administered 200 μL of 10 mg/mL BrdU (BD Biosciences) by intraperitoneal injection 12 and 24 hours before death. BM cells were flushed and stained with FITC-conjugated Ab indicating lineage-positive phenotype (CD4, NK, CD8 B220, CD11b, Gr-1; eBiosciences), anti-c-Kit APC (eBiosciences). After extracellular staining, cells were permeabilized using cytofix/cytoperm kit (BD Biosciences). BrdU incorporation was measured using PE-anti-BrdU monoclonal antibody (BD Biosciences) according to the manufacturer's instructions.
Statistics
Results of experimental points are reported as means plus or minus SE. Statistical significance was determined by Student t test.
Results
Heparanase overexpression alters hematopoietic cell development and leads to increased retention of primitive cells in the bone marrow
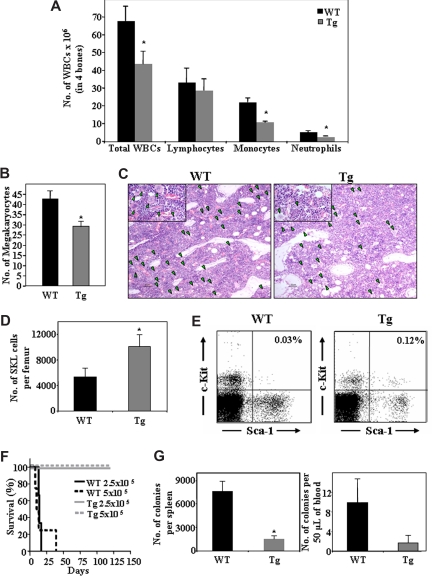
Transgenic mice overexpressing the heparanase gene (hpa-Tg) served as a model system to study the role of the enzyme under conditions of steady-state homeostasis. As shown in Figure 1, the levels of WBCs in the BM of hpa-Tg mice were significantly lower than in WT control mice. This phenomenon was not uniform among the different cell lineages. Although no difference in the number of lymphoid cells was detected, a significant decrease in the number of myeloid cells, such as monocytes and neutrophils (Figure 1A), as well as megakaryocytes (Figure 1B,C) was found in the BM of hpa-Tg mice. Furthermore, whereas the peripheral WBC count in hpa-Tg mice was similar to that of the WT, the proportion of cell types was different (Table 1). Altogether, these results indicate involvement of heparanase in steady-state homeostasis, suggesting a role for the enzyme in hematopoiesis.
Figure 1.
Heparanase overexpression alters hematopoietic development and leads to increased retention of stem cells in the BM. Cells were flushed from the BM of wildtype (WT) or hpa-Tg (Tg) mice. (A) The number of WBC, lymphocytes, monocytes, and neutrophils in 4 bones was assessed. Data are means plus or minus SE; n ≥ 10 mice. (B) Number of megakaryocytes in bone sections of WT or hpa-Tg mice, determined by counting of 9 random fields per bone section. Data are means plus or minus SE; n = 3 mice, 2 bone sections per mouse. (C) Representative figure showing hematoxylin and eosin staining of femoral bone in WT and hpa-Tg mice (original magnification ×10). Inset: original magnification ×40. Green arrowheads point at megakaryocytes. Micrographs were acquired by staining with H&E, viewed with a light microscope (Eclipse E800M) fitted with a 10×/Plan-Apoobjective, and photographed with a digital camera (DXm1200), and image acquisition software (ACT-1, v. 2.63, all from Nikon, Tokyo, Japan). Images were processed by Adobe Photoshop, v. 7.0 (San Jose, CA). (D) Number of primitive undifferentiated Sca-1+/c-Kit+/Lin− cells in one femur of WT or hpa-Tg mice, as counted by flow cytometry. Data are means plus or minus SE; n = 7 mice. (E) A representative dot plot of Sca-1+/c-Kit+ (gated from Lin− population) derived from the BM of WT or hpa-Tg mice (F) Survival of lethally irradiated C57/BL6 mice injected with the indicated number of BM cells from WT or hpa-Tg donors. (G) Number of progenitor cells in the peripheral blood and spleen of WT and hpa-Tg mice, determined by colony assay. Data are means plus or minus SE; n ≥ 4 mice (*P < .05).
Table 1.
Lineage populations in the peripheral blood
| WT | hpa-Tg | |
|---|---|---|
| RBCs, ×106 | 8.72 ± 0.16 | 9.12 ± 0.11 |
| WBCs | 8760 ± 835 | 8080 ± 1030 |
| Platelets, ×106 | 1.1 ± 0.02 | 0.9 ± 0.06* |
| Neutrophils | 300 ± 35 | 320 ± 40 |
| Lymphocytes | 5470 ± 680 | 6050 ± 790 |
| Monocytes | 1930 ± 350 | 940 ± 60* |
Data are means plus or minus SE of number of cells/μL blood from at least 3 independent experiments; n > 8 mice per group.
RBCs indicates red blood cells; and WBCs, white blood cells.
P < .05.
Having shown altered BM and blood cell levels in hpa-Tg mice, we focused on the effect of heparanase overexpression on the primitive progenitor cell population. We detected a 2.5-fold increase in the number of Sca-1+/c-Kit+/Lin− stem cell–enriched population in the BM of hpa-Tg, compared with WT control mice (Figure 1D,E). This was further substantiated in a functional assay, showing that a minimal dose of 2.5 × 105 WBCs from the BM was sufficient to rescue lethally irradiated C57BL/6 recipients. None of the mice that were injected either with an equivalent dose or even a double amount of BM cells from WT mice survived 6 weeks after irradiation, suggesting that a higher number of repopulating cells exists in the BM of hpa-Tg mice (Figure 1F).
Considering the higher proportions of undifferentiated cells detected in the BM, we investigated the effect of heparanase overexpression on additional compartments inhabited by immature hematopoietic progenitor cells in the hpa-Tg mice, namely, the spleen and the peripheral blood. The number of colony-forming unit-cells (CFU-C) in the spleen and peripheral blood was assessed. As shown in Figure 1G, the levels of CFU-C in the peripheral blood of hpa-Tg mice were markedly lower than in their WT counterparts. We also observed smaller spleens in the hpa-Tg mice (data not shown), which taken together with the reduced frequency of CFU-C lead to an overall profound reduction in the number of CFU-C in the spleen of hpa-Tg mice (Figure 1G).
Altogether, these results imply that the increase in the primitive stem and progenitor cell population may be the result of enhanced retention of these cells within their BM microenvironment at the expense of their release into the periphery, indicating an important role for heparanase in the regulation of stem and progenitor cell retention.
Heparanase overexpression elevates SDF-1 turnover in the bone marrow
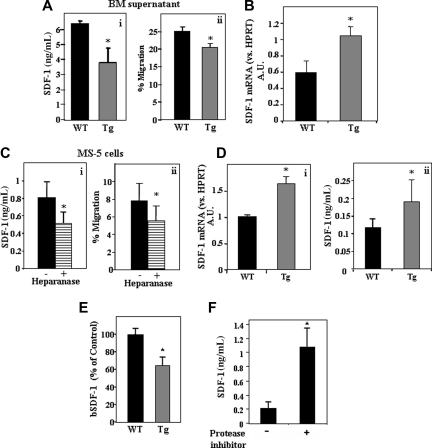
Increased levels of SDF-1 and the subsequent increase in MMP-9 activity have been shown to regulate stem cell exit from the BM.22 We hypothesized that the increased retention of primitive cells within the BM of hpa-Tg mice results from an opposite situation, namely, reduction in SDF-1 and MMP-9 levels. Indeed, SDF-1 levels in the BM of hpa-Tg mice were significantly reduced compared with control WT mice (Figure 2Ai). This result was corroborated in a functional assay showing decreased migration of immature human CD34+-enriched cells (for which SDF-1 is the most potent chemoattractant) toward the BM supernatant obtained from hpa-Tg mice (Figure 2Aii). In contrast to the reduced amount of protein, increased levels of SDF-1 mRNA were detected in the BM of hpa-Tg mice, suggesting that the reduction in protein results from post-translational modifications (Figure 2B). We next tested for an effect of heparanase in an in vitro model system where stromal cell-line MS-5 cells were treated with recombinant heparanase exogenously. The results pointed to decreased SDF-1 levels in the conditioned medium of these cells, as determined by ELISA (Figure 2Ci), as well as decreased migration of human CD34+ cells toward the conditioned medium (Figure 2Cii). Of interest, although lower levels of SDF-1 were found in the BM supernatant, this was not the case in the context of hpa-Tg osteoblasts. SDF-1 mRNA (Figure 2Di) as well as protein secreted by the osteoblasts (detected by ELISA of cell supernatant) were up-regulated in osteoblasts isolated from hpa-Tg mice (Figure 2Dii), suggesting that the indirect effect of heparanase on SDF-1 stability varies among different cell types found in the BM. These results imply that SDF-1 secreted in the BM microenvironment is degraded. To substantiate our claim regarding enhanced SDF-1 degradation in the BM, we tested the chemokine degradation by BM supernatant from hpa-Tg mice (compared with WT mice). Indeed, lower SDF-1 protein was detected following its incubation with supernatant from BM of hpa-Tg mice (Figure 2E). However, this SDF-1 degradation could be prevented by preincubation of the BM supernatant with a broad range protease inhibitor (Figure 2F), demonstrating SDF-1 proteolysis by the hpa-Tg BM.
Figure 2.
Heparanase increases SDF-1 turnover in the BM.(A) SDF-1 levels in the BM of WT or hpa-Tg mice, detected by ELISA (i). Migration of cord blood CD34+ cells toward RPMI supplemented with supernatant from BM of WT or hpa-Tg mice placed in the lower chamber (ii). Data are means plus or minus SE; n ≥ 9 samples of BM supernatants. (B) mRNA levels of SDF-1 in BM cells from WT or hpa-Tg mice. Data are means plus or minus SE; n ≥ 4. (C) SDF-1 levels in the conditioned medium of MS-5 cells with or without pretreatment of the cells with heparanase, detected by ELISA (i). Migration of cord blood CD34+ cells toward the conditioned medium of MS-5 cells placed in the lower chamber of a transwell (ii). Data are means plus or minus SE; n ≥ 10 samples of conditioned medium. (D) (i) mRNA levels of SDF-1 (vs HPRT) in osteoblasts from WT or hpa-Tg mice. Data are means plus or minus SE; n ≥ 4. (ii) SDF-1 levels in the conditioned medium of osteoblasts obtained from WT or hpa-Tg mice, detected by ELISA. Data are mean plus or minus SE; n = 9. (E) Levels of biotinylated SDF-1 added exogenously to the BM supernatant of WT or hpa-Tg mice, detected by ELISA. Data are means plus or minus SE; n ≥ 8 samples of BM supernatants. (F) Levels of biotinylated SDF-1 added exogenously to the BM supernatant of hpa-Tg mice with or without pretreatment with a broad range protease inhibitor, detected by ELISA. Data are means plus or minus SE; n = 3 samples of BM supernatants (*P < .05).
These results collectively suggest that heparanase overexpression indirectly regulates increased SDF-1 turnover in the BM, whereas more SDF-1 is secreted in the BM microenvironment, and an increased degradation of this ligand in the BM is observed.
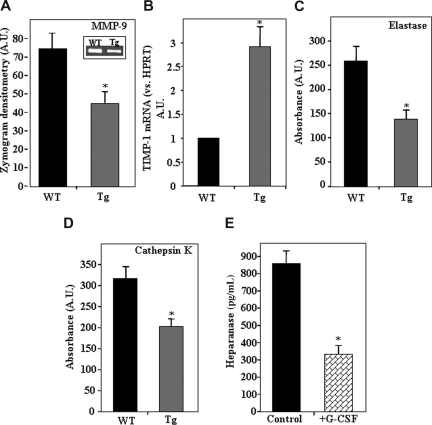
Reduced activity of proteolytic enzymes in the bone marrow of hpa-Tg mice
Because heparanase overexpression led to reduced levels of SDF-1 in the BM, we next tested whether the activity of the proteolytic enzyme MMP-9, which is secreted in response to SDF-122,44,45 and involved in mobilization of hematopoietic progenitors, was also affected. As demonstrated in Figure 3A, a significantly lower level of MMP-9 activity was found in the BM supernatant of hpa-Tg compared with WT mice. Because low levels of MMP-9 activity may be a result of an indirect pathway rather than attenuated secretion, we tested the expression levels of tissue inhibitors of metalloproteinases (TIMPs) in the BM. Indeed, a 3-fold increase in mRNA levels of TIMP-1, a potent inhibitor of MMP-9,46 was detected in the BM of hpa-Tg mice (Figure 3B). This up-regulation was specific because no difference was noted in TIMP-2 mRNA (data not shown). We further tested the activity of the proteolytic enzymes elastase and cathepsin K that are also known to promote hematopoietic stem and progenitor cell mobilization into the blood.13,40 A significant reduction in the activity of both these enzymes was detected in the BM supernatants of hpa-Tg mice (Figure 3C,D). Altogether, these results indicate that heparanase overexpression leads to down-regulation of enzymes that facilitate mobilization of progenitor cells.
Figure 3.
Decreased protease activity resulting from heparanase overexpression.(A) Average levels of MMP-9 activity in the BM supernatant of WT (control) and hpa-Tg mice. Inset shows a representative gelatin zymography experiment. Data are means plus or minus SE; n = 6 samples. (B) mRNA levels of TIMP-1 in BM cells from WT or hpa-Tg mice. Data are means plus or minus SE; n = 3. (C) Levels of elastase activity in the BM supernatant of WT and hpa-Tg mice. Average absorbance (arbitrary units) is shown. Data are means plus or minus SE; n = 6 samples. (D) Levels of cathepsin K activity in the BM supernatant of WT and hpa-Tg mice. Average absorbance (arbitrary units) is shown. Data are means plus or minus SE; n = 12 samples. (E) Heparanase levels in BM supernatant of mice treated with G-CSF (compared with control nontreated mice), determined by ELISA. Data are means plus or minus SE; n = 3 mice (*P < .05).
Next we tested whether a reciprocal effect exists in stress-induced conditions that lead to mobilization. To this end, we tested heparanase levels in the BM supernatant of hpa-Tg mice after treatment with the mobilizing agent granulocyte colony-stimulating factor (G-CSF). As shown in Figure 3E, a 60% decrease in heparanase levels was noted after treatment with G-CSF. A similar reduction in heparanase levels in the BM was detected after treatment with CXCR4 antagonists (data not shown). In all, these results suggest that stress-induced mobilization is facilitated by down-regulation of heparanase levels.
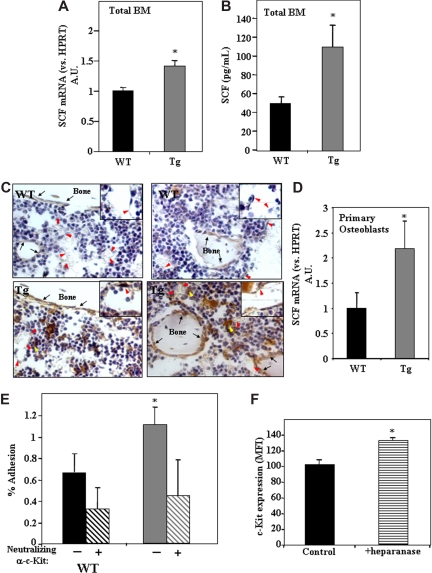
Higher levels of SCF in the BM of hpa-Tg mice are associated with increased adhesion of progenitor Sca-1+/c-Kit+/Lin− cells to osteoblasts
The increased retention of primitive Sca-1+/c-Kit+/Lin− cells within the BM in the presence of high levels of heparanase expression may result not only from down-regulation of cell egress but also from potent anchorage of the cells to their microenvironment. This can be an outcome of the modification by heparanase of both the microenvironment as well as the immature hematopoietic cells. Because SCF plays a key role in adhesion of stem cells to stromal cells in the endosteum,21 we checked whether heparanase is involved in this process through regulation of SCF levels and subsequent cell adhesion. We thus determined the levels of SCF in hpa-Tg mice. As shown in Figure 4A, SCF mRNA levels were 40% higher in BM cells obtained from hpa-Tg compared with WT control mice. Furthermore, protein levels of SCF were found to be 80% higher in the BM supernatant of hpa-Tg mice than in WT mice (Figure 4B). Interestingly, immunohistochemical staining of bone sections revealed a marked increase in SCF expression in the stem cell–rich regions, that is, endothelial and surrounding cells as well as endosteal bone lining osteoblasts located along the bone shaft and trabecules in hpa-Tg mice (Figure 4C). This result was further corroborated by our finding showing increased levels of SCF mRNA in osteoblasts obtained from hpa-Tg mice (Figure 4D).
Figure 4.
Increased levels of SCF in the BM of hpa-Tg mice stimulate adhesion of primitive cells to osteoblasts.(A) SCF mRNA levels in BM cells from WT or hpa-Tg mice, detected by real-time RT-PCR. Data are means plus or minus SE; n = 9 samples. (B) SCF levels in the BM of WT or hpa-Tg mice, detected by ELISA. Data are means plus or minus SE; n = 5 samples. (C) SCF staining (brown) of femoral bone from WT or hpa-Tg mice. Inset shows enlargement of sinusoidal endothelial region. Black arrows point at bone lining osteoblasts. Red arrowheads point at endothelial cells. Yellow arrowheads point at cells that highly express SCF and are in close proximity to sinusoids. Micrographs were acquired by staining with goat anti–mouse stem cell factor (R&D systems) and processed as in Figure 1C, except with a 40×/Plan-Apo objective. (D) mRNA levels of SCF in primary osteoblasts derived from WT or hpa-Tg mice. Data are means plus or minus SE; n = 4 experiments. (E) Percentage adhesion to primary osteoblasts of BM MNC Sca-1+/c-Kit+/Lin− cells derived from hpa-Tg compared with control (WT) mice. In some experiments, leukocytes were pretreated with neutralizing anti-c-Kit antibodies before adhesion. Data are means plus or minus SE; n ≥ 4 experiments. (F) c-Kit receptor expression in the primitive c-Kit+/Lin− cells following exogenous in vitro treatment with heparanase. Data are means plus or minus SE; n = 3 experiments (*P < .05).
Our results show that the endothelial and endosteal regions in hpa-Tg mice differ from those of the WT controls. We next tested whether heparanase also affects the hematopoietic progenitor cell population, namely, whether adhesion of stem cell– enriched Sca-1+/cKit+/Lin− cells to osteoblasts is also affected by heparanase. For this purpose, BM MNCs from hpa-Tg and WT mice were incubated with primary murine osteoblasts, and percentage adhesion of the Sca-1+/c-Kit+/Lin− population was determined. As shown in Figure 4E, hpa-Tg mice displayed a 2-fold increase in adhesion of Sca-1+/c-Kit+/Lin− cells to primary osteoblasts. This adhesion was mediated by SCF because pretreatment of the cells with neutralizing anti-c-Kit antibodies resulted in decreased adhesion (Figure 4E). Furthermore, to corroborate the direct effect of heparanase on the cells, we tested the effect of exogenous addition of the enzyme to BM MNCs. After overnight incubation with heparanase, a 30% increase in the expression of the SCF receptor, c-Kit, was noted in the progenitor c-Kit+/Lin− population (Figure 4F). Altogether, these results indicate that heparanase is involved in SCF-mediated stem cell adhesion by regulating both SCF expression in the BM microenvironment and adhesive properties of the primitive cells.
Increased proliferation and c-Myc phosphorylation in hpa-Tg mice
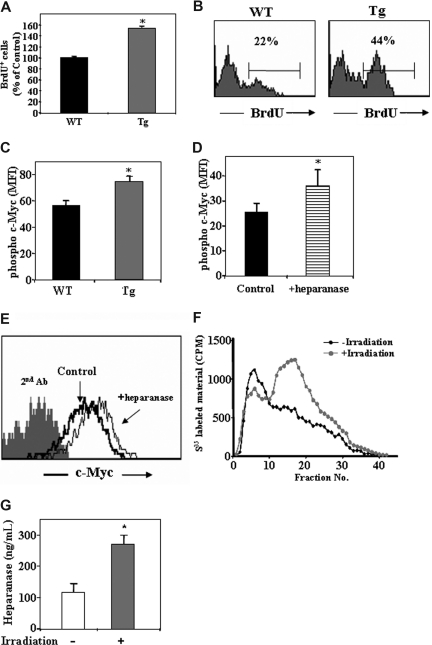
The increase in number of primitive Sca-1+/c-Kit+/Lin− cells may result not only from the increased retention of the cells in the BM, as we have shown. An additional option may be higher proliferation rate of the cells. To test this, we injected control WT and hpa-Tg mice with BrdU and checked for its incorporation in the primitive c-Kit+/Lin− cells in the BM. Indeed, a higher percentage of the cells were BrdU+ among this progenitor population in the hpa-Tg mice compared with the control WT mice (Figure 5A,B).
Figure 5.
Heparanase is involved in progenitor cell proliferation.(A) BrdU uptake in the primitive c-Kit+/Lin− cells obtained from control WT or hpa-Tg mice, as detected by flow cytometry. Data are means plus or minus SE; n = 4 samples. (B) Representative histogram plot of BrdU+ cells among the c-Kit+/Lin− population obtained from WT or hpa-Tg mice. (C) Phosphorylated c-Myc expression in the primitive cKit+/Lin− BM cells obtained from the BM of WT or hpa-Tg mice. (D) Phosphorylated c-Myc expression in the primitive c-Kit+/Lin− cells following exogenous in vitro treatment with heparanase (compared with control nontreated cells). Data are means plus or minus SE; n = 3 samples. (E) Representative histogram plot of phosphorylated c-Myc expression levels among the primitive c-Kit+/Lin− cells following in vitro treatment with or without exogenous heparanase. (F) Heparanase activity (determined by the ability to degrade sulfate-labeled heparan-sulfate) in lysates of cells collected from the BM of irradiated (24 hours after irradiation) or nonirradiated mice. Data are means plus or minus SE; n = 3 mice. (G) Heparanase levels in BM supernatant of WT and hpa-Tg mice as determined by ELISA. Data are means plus or minus SE; n = 7 mice (*P < .05).
Next we wanted to decipher the molecular mechanism underlying the increased proliferation of the progenitor c-Kit+/Lin− cells. To this end, we tested for intracellular levels of phosphorylated c-Myc, which has been shown to be involved in hematopoietic stem and progenitor cell proliferation. As shown in Figure 5C, a 30% increase in the levels of phosphorylated c-Myc was detected in c-Kit+/Lin− cells in hpa-Tg mice compared with WT control. Furthermore, a similar increase in phosphorylated c-Myc was obtained in the primitive c-Kit+/Lin− cells when BM mononuclear cells were treated in vitro with recombinant heparanase (Figure 5D,E). These results suggest that heparanase regulates progenitor cell proliferation, and this correlates with its effect on c-Myc–mediated signaling.
Having shown a role for heparanase in proliferation of the progenitor cells in steady-state homeostasis, we next tested whether heparanase is involved in nonhomeostatic conditions. To this end, we tested heparanase levels in the BM after total body irradiation. The results showed a significant increase in heparan-sulfate degradation, indicating heparanase activity (Figure 5F). This result was further verified by ELISA, pointing to an increase in heparanase levels in the BM supernatant in response to irradiation (Figure 5G). This implies a role for heparanase in regulation of the stem cell pool also in stress-induced conditions, facilitating hematopoietic reconstitution, which requires stem and progenitor cell proliferation and expansion.
Discussion
Heparanase activity has been researched mainly in relation to inflammation and cancer progression.27 The objective of our study was to investigate the possible roles of heparanase in hematopoiesis, focusing on interactions between primitive SKL cells and their BM microenvironment. Indeed, we revealed a role for heparanase in steady-state conditions of hematopoiesis, affecting developmental processes in the BM reservoir of immature and maturing leukocytes.
Our results show that development of murine hematopoietic cells is significantly modified by alterations in heparanase expression. Hence, heparanase-overexpressing mice exhibited decreased numbers of myeloid cells in the BM as well as a reduction in the platelet and monocyte counts in the peripheral blood. In contrast to the reduction in the overall leukocyte cellularity in the BM of hpa-Tg mice, an increased number of hematopoietic progenitor cells was noted. This was determined by both flow cytometric staining for Sca-1+/c-Kit+/Lin− cells and the functional rescue of lethally irradiated mice. These findings point to an important role of heparanase in hematopoietic progenitor cell regulation, implying its involvement in the control of the primitive cell pool size. The up-regulated heparanase activity detected in the BM of mice after total body irradiation shows that heparanase facilitates hematopoietic reconstitution, which requires stem cell proliferation and expansion. This suggests that heparanase overexpression may provide a mechanism for expansion of stem and progenitor cells in stress-induced conditions.
Maturation and development of leukocytes involve their translocation from the endosteal region.4,6,47,48 Therefore, the increased retention of the primitive cells presumably within the endosteal and endothelial niches may explain the reduced numbers of maturing leukocytes detected in the BM and periphery of heparanase-overexpressing mice. Of note, we suggest that the increase in the primitive cell numbers results from both increased retention and higher proliferation rate in progenitor cells from hpa-Tg mice as demonstrated by increased BrdU uptake. These phenomena may be explained by our results showing elevated levels of phosphorylated c-Myc in progenitor c-Kit+/Lin− cells in the BM of hpa-Tg mice as well as following in vitro treatment with recombinant heparanase. This is in line with previous reports showing that c-Myc acts as a “gate-keeper” between progenitor proliferation and differentiation. Elevated levels of c-Myc correlate with proliferation of hematopoietic progenitor cells, and overexpression of c-Myc prevents differentiation of the immature cells.49 Indeed, we detected accumulation of progenitor cells (manifested in increased retention and proliferation) and lack of differentiation to mature hematopoietic cells, yielding a reduction in overall leukocyte cellularity.
During steady-state hematopoiesis, most BM HSCs reside in the endosteum region and adjacent to blood vessels in close proximity to SDF-1 expressing osteoblasts and endothelial and reticular cells.9–11 Although stem cell retention is not fully understood, emerging evidence indicates that SCF-mediated adhesion is of utmost importance in creating conditions that are “pro-retention.”18,21 Our data revealed increased SCF expression in osteoblasts and endothelial cells derived from hpa-Tg mice as well as enhanced SCF-mediated adhesion to osteoblasts of primitive Sca-1+/c-Kit+/Lin− cells from hpa-Tg mice. This indicates that heparanase overexpression modifies both hematopoietic stem and progenitor cells as well as the BM microenvironment in a manner that leads to increased retention of undifferentiated cells in the BM. Our results are in line with recently published data showing that heparanase is highly abundant in granules associated with preosteoblasts, osteoblasts, and osteocytes; some osteoblasts also presented heparanase-positive cell membranes,50 pointing to the possible importance of heparanase regulation, specifically in the endosteal osteoblastic region and bone mass turnover.
Rare populations of stem cells and their progeny with a more restricted potential are detectable at low levels in the peripheral blood, spleen, and liver. This indicates continuous processes of release and homing of primitive cells, which is part of steady-state homeostasis conditions.51 These processes are enhanced under conditions of stress, such as G-CSF-induced mobilization.52 In these situations, dynamic changes in the microenvironment lead the HSC into a “pro-egress pathway.” The molecular mechanism underlying this phenomenon involves proteases such as neutrophil elastase, as well as osteoclast recruitment, activation, and secretion of the major bone-resorbing proteinase cathepsin K that degrades SCF.13,40,53 In addition, up-regulation of SDF-1 leading to MMP-9 activation and consequent shedding of membrane-bound SCF is also involved in cell egress.22 Our data showing decreased activity of elastase and cathepsin K as well as down-regulation of the SDF-1/MMP-9 axis in the BM of hpa-Tg mice via direct affect on MMP-9 secretion as well as indirect track of TIMP-1 up regulation. These data imply that increased retention of hematopoietic stem and progenitor cells results not only from up-regulation of “pro-retention” signals but also from down-regulation of these “pro-egress” players.
Heparanase down-regulation of “pro-egress” pathways is reversed during hematopoietic cell mobilization, such as following G-CSF stimulation, in which heparanase levels are reduced (Figure 3E). This claim is further strengthened by recently published data showing that heparanase was not detected in immunohistochemical staining of osteoclasts, an important player in stem cell mobilization.13 Furthermore, although heparanase expression was observed in BM derived monocytic cells, only very low levels of heparanase mRNA were detected in their osteoclast descendents, suggesting down-regulation of heparanase expression during osteoclastogenesis.50 In all, heparanase expression and its consequent effect on SCF, SDF, and proteolytic enzymes tilt the balance between pro-egress and pro-retention situations. As part of heparanse up-regulation of “pro-retention” signals, intense rate of SDF-1 turnover and consequent higher degradation of the chemokine were documented. Decreased expression of MMP-9, elastase, and cathepsin K, on one hand, and inhibition of SDF-1 degradation using a broad-range protease inhibitor, on the other, are pointing to an unrevealed protease overexpressed in the BM of hpa-Tg mice that is responsible for this phenomena and needs to be further investigated in future studies.
Taken together, our results revealed that heparanase affects basic features of hematopoietic stem and progenitor cells and the BM microenvironment. These features include regulation of progenitor cell development, proliferation, and retention. Our data point to an important crosstalk between SDF-1, proteolytic enzymes, SCF, and heparanase in regulation of progenitor function during homeostasis and alarm situations, as part of host defense and repair mechanisms.
Acknowledgments
The authors thank Mrs. Loya Abel for expert assistance and Prof. Amiela Globerson and Prof. Sonia Berrih-Aknin for critical remarks and fruitful discussions.
This work was supported in part by the Israel Science Foundation and the Legacy Heritage Fund (T.L.), and by US Public Health Service grant R07-CA106456 from the National Cancer Institute (I.V.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.S. designed and conducted experiments, analyzed data, and wrote the manuscript; E.Z., Y.V., and T.I. conducted experiments and analyzed data; A.K., A.D., O.K., N.N., K.G., and I.S. performed experiments; A.N. provided human blood cells; N.I. and I.V. contributed to the design and supervision of the study; T.L. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tsvee Lapidot, Department of Immunology, Weizmann Institute of Science, P.O. Box 26, Rehovot, 76100, Israel; e-mail: Tsvee.Lapidot@weizmann.ac.il.
References
- 1.Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–1585. doi: 10.1182/blood-2003-05-1595. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26:426–433. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 4.Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 5.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 6.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 9.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 13.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 14.Toksoz D, Zsebo KM, Smith KA, et al. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci U S A. 1992;89:7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 16.Ponomaryov T, Peled A, Petit I, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams DA, Majumdar MK. Analysis of steel factor (stem cell factor) isoforms in the hematopoietic microenvironment. Stem Cells. 1994;12(Suppl 1):67–74. discussion 75–77. [PubMed] [Google Scholar]
- 19.Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell. 1992;3:349–362. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood. 1998;91:2231–2239. [PubMed] [Google Scholar]
- 21.Driessen RL, Johnston HM, Nilsson SK. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp Hematol. 2003;31:1284–1291. doi: 10.1016/j.exphem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires mmp-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 24.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai K, Kobayashi M, Wang J, et al. Selective secretion of chemoattractants for haemopoietic progenitor cells by bone marrow endothelial cells: a possible role in homing of haemopoietic progenitor cells to bone marrow. Br J Haematol. 1999;106:905–911. doi: 10.1046/j.1365-2141.1999.01644.x. [DOI] [PubMed] [Google Scholar]
- 26.Kollet O, Spiegel A, Peled A, et al. Rapid and efficient homing of human CD34(+)CD38-(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 27.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349–355. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 30.Bernfield M, Gotte M, Park PW, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 31.Hulett MD, Freeman C, Hamdorf BJ, Baker RT, Harris MJ, Parish CR. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nat Med. 1999;5:803–809. doi: 10.1038/10525. [DOI] [PubMed] [Google Scholar]
- 32.Vlodavsky I, Friedmann Y, Elkin M, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 33.McKenzie EA. Heparanase: a target for drug discovery in cancer and inflammation. Br J Pharmacol. 2007;151:1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471:M99–M108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 35.Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11:427–452. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkin M, Ilan N, Ishai-Michaeli R, et al. Heparanase as mediator of angiogenesis: mode of action. FASEB J. 2001;15:1661–1663. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- 37.Vlodavsky I, Goldshmidt O, Zcharia E, et al. Mammalian heparanase: involvement in cancer metastasis, angiogenesis and normal development. Semin Cancer Biol. 2002;12:121–129. doi: 10.1006/scbi.2001.0420. [DOI] [PubMed] [Google Scholar]
- 38.Zcharia E, Metzger S, Chajek-Shaul T, et al. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 2004;18:252–263. doi: 10.1096/fj.03-0572com. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel A, Kollet O, Peled A, et al. Unique SDF-1-induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood. 2004;103:2900–2907. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- 40.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 41.Nardella C, Lahm A, Pallaoro M, Brunetti M, Vannini A, Steinkuhler C. Mechanism of activation of human heparanase investigated by protein engineering. Biochemistry. 2004;43:1862–1873. doi: 10.1021/bi030203a. [DOI] [PubMed] [Google Scholar]
- 42.Shafat I, Zcharia E, Nisman B, et al. An ELISA method for the detection and quantification of human heparanase. Biochem Biophys Res Commun. 2006;341:958–963. doi: 10.1016/j.bbrc.2006.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy-Adam F, Miao HQ, Heinrikson RL, Vlodavsky I, Ilan N. Heterodimer formation is essential for heparanase enzymatic activity. Biochem Biophys Res Commun. 2003;308:885–891. doi: 10.1016/s0006-291x(03)01478-5. [DOI] [PubMed] [Google Scholar]
- 44.Janowska-Wieczorek A, Matsuzaki A, Marquez LA. The hematopoietic microenvironment: matrix metalloproteinases in the hematopoietic microenvironment. Hematology. 2000;4:515–527. [PubMed] [Google Scholar]
- 45.Janowska-Wieczorek A, Marquez LA, Nabholtz JM, et al. Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34(+) cells and their transmigration through reconstituted basement membrane. Blood. 1999;93:3379–3390. [PubMed] [Google Scholar]
- 46.Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Adams GB, Chabner KT, Alley IR, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 48.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 49.Wilson A, Murphy MJ, Oskarsson T, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kram V, Zcharia E, Yacoby-Zeevi O, et al. Heparanase is expressed in osteoblastic cells and stimulates bone formation and bone mass. J Cell Physiol. 2006;207:784–792. doi: 10.1002/jcp.20625. [DOI] [PubMed] [Google Scholar]
- 51.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 52.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 53.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]