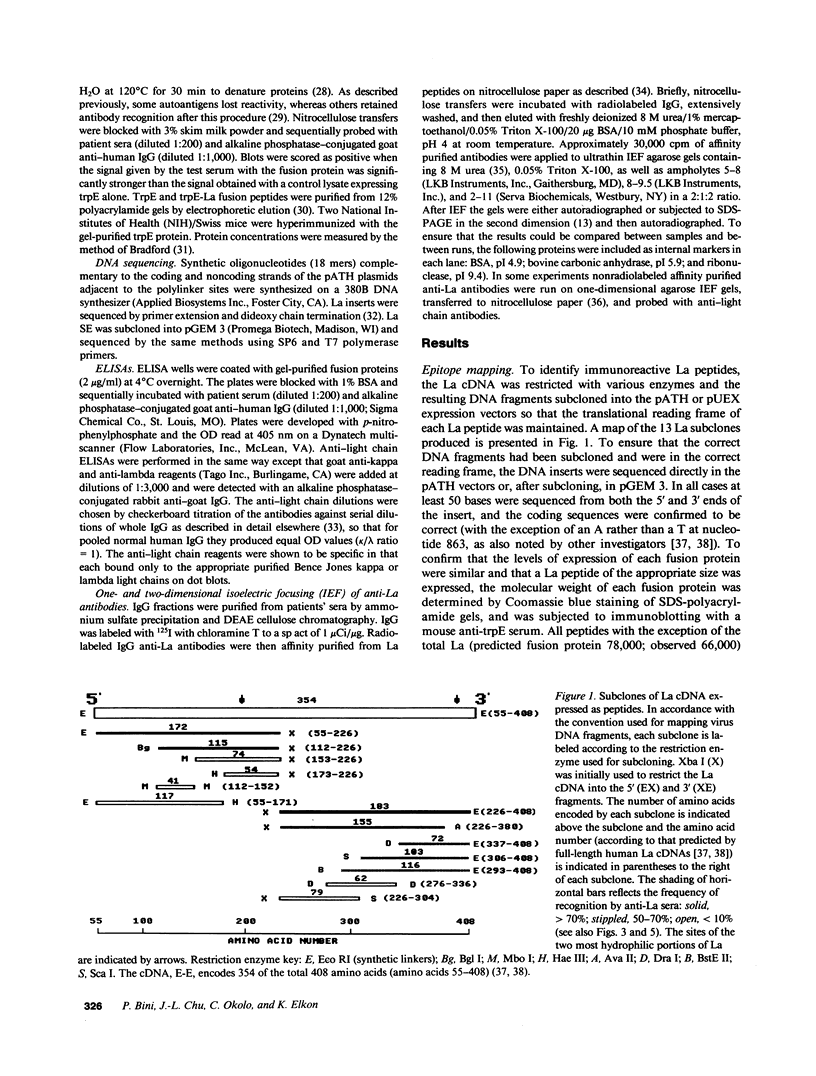
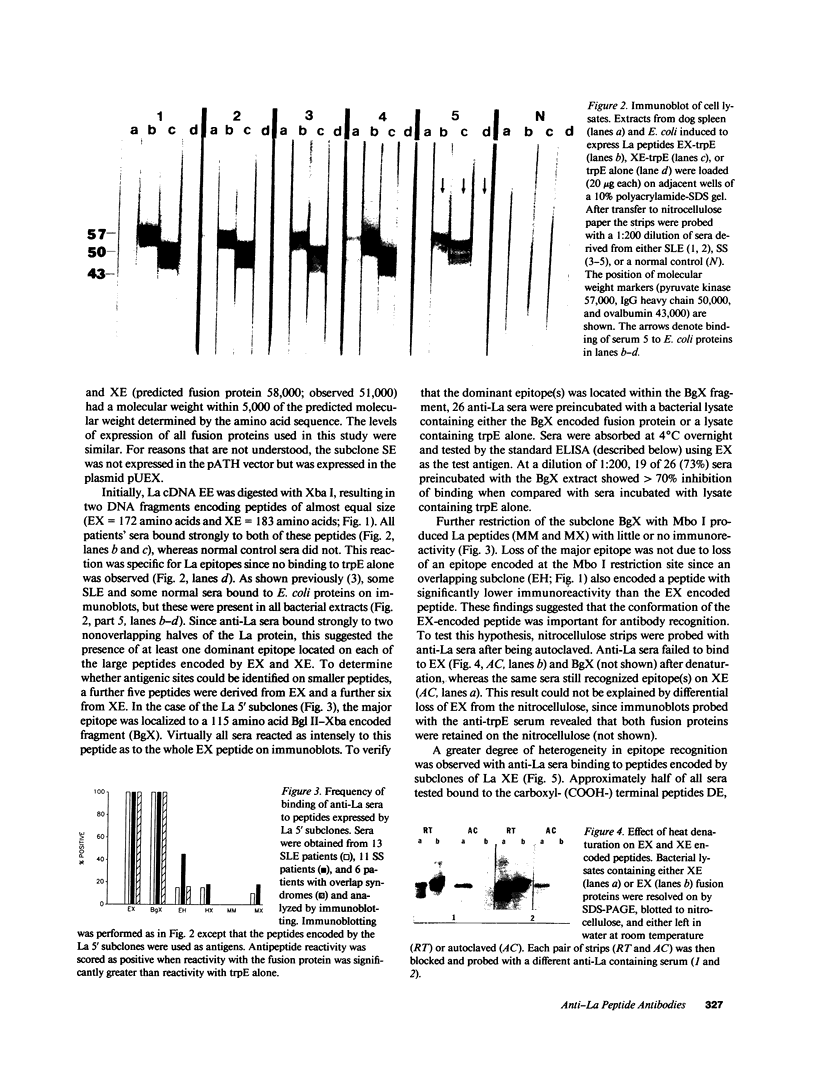
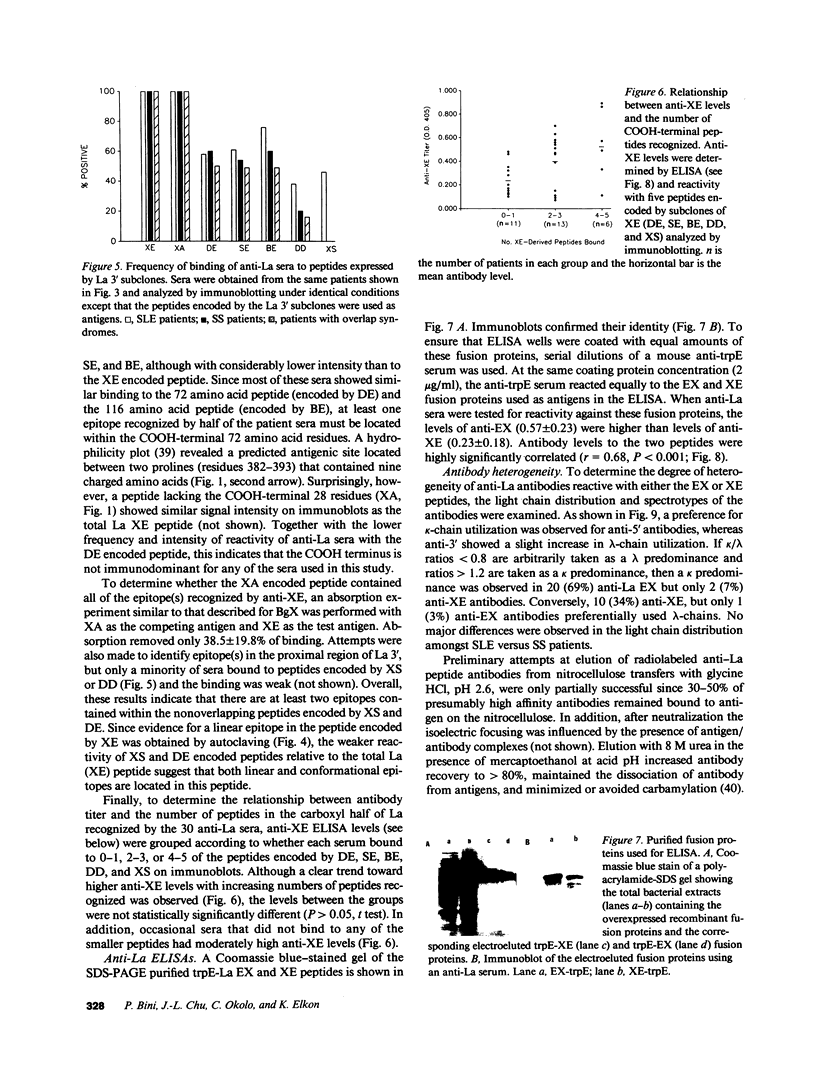
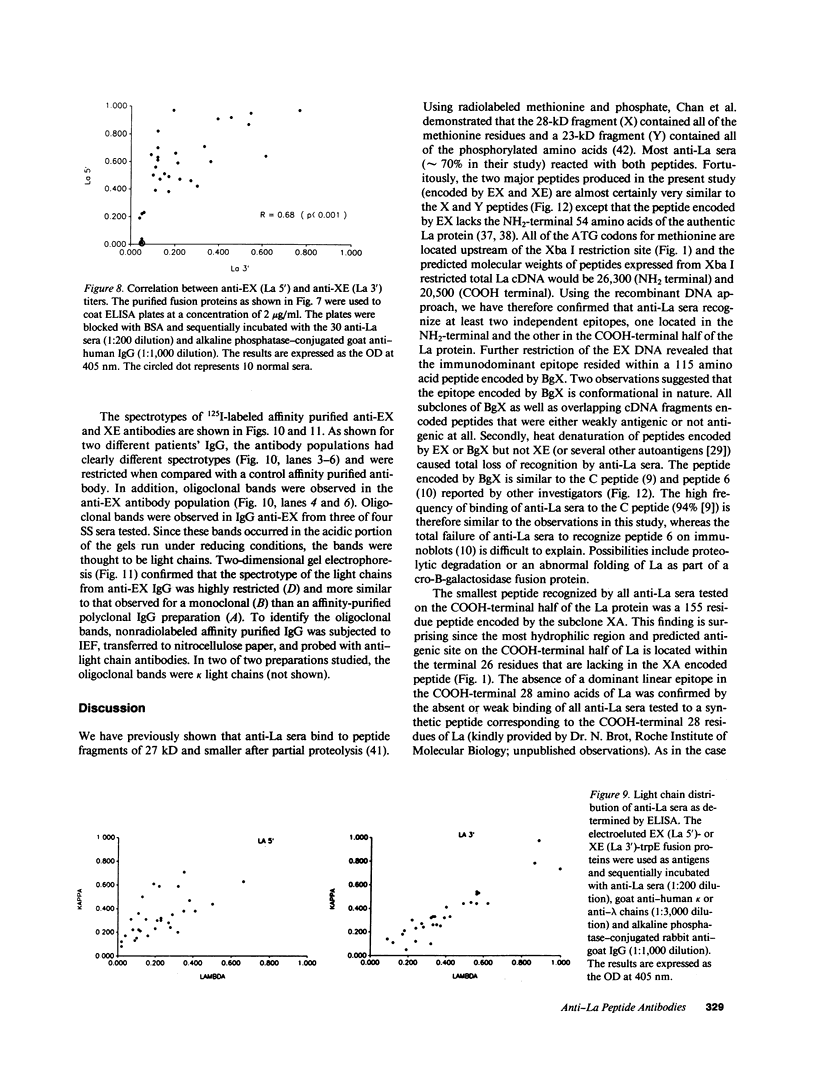
Abstract
Autoantibodies to a polymerase III transcription factor, La (SS-B), are frequently detected in the serum of patients with Sjogren's syndrome and systemic lupus erythematosus. To define the humoral immune response to this protein, we analyzed the patterns of antibody recognition toward 13 recombinant La peptides by immunoblotting and determined the heterogeneity of antibodies reactive with the immunodominant epitopes. The smallest epitopes that were strongly antigenic and recognized by greater than 70% of sera tested (immunodominant) were encoded by the subclones BgX and XA located in the 5' and 3' halves of the La cDNA, respectively. Conformation of the immunodominant La peptides played a major role in antibody recognition. Although greater diversity in antibody binding to carboxyl-terminal La peptides was observed, the overall pattern of peptide recognition by anti-La antibodies was similar in different diseases. The antibody responses to the immunodominant peptides were strongly correlated (r = 0.68, P less than 0.001). One- and two-dimensional isoelectric focusing of affinity purified IgG anti-La peptide antibodies revealed restricted heterogeneity and oligoclonal bands (kappa light chains). These observations suggest that anti-La antibodies are induced and/or maintained by the self antigen and that their diversity is constrained either by mechanisms related to tolerance or by affinity maturation of the humoral immune response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akizuki M., Boehm-Truitt M. J., Kassan S. S., Steinberg A. D., Chused T. M. Purification of an acidic nuclear protein antigen and demonstration of its antibodies in subsets of patients with sicca syndrome. J Immunol. 1977 Sep;119(3):932–938. [PubMed] [Google Scholar]
- Alspaugh M. A., Talal N., Tan E. M. Differentiation and characterization of autoantibodies and their antigens in Sjögren's syndrome. Arthritis Rheum. 1976 Mar-Apr;19(2):216–222. doi: 10.1002/art.1780190214. [DOI] [PubMed] [Google Scholar]
- Barkas T., Mauron A., Roth B., Alliod C., Tzartos S. J., Ballivet M. Mapping the main immunogenic region and toxin-binding site of the nicotinic acetylcholine receptor. Science. 1987 Jan 2;235(4784):77–80. doi: 10.1126/science.2432658. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Chu J. L., Brot N., Elkon K. B. Lupus anti-ribosomal P peptide antibodies show limited heterogeneity and are predominantly of the IgG1 and IgG2 subclasses. Clin Immunol Immunopathol. 1987 Oct;45(1):129–138. doi: 10.1016/0090-1229(87)90119-x. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Llovet R., Elkon K. Immunoblot analysis of IgG subclasses of multiple lupus autoantibodies. J Immunol. 1988 Apr 1;140(7):2231–2236. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bressan G. M., Stanley K. K. pUEX, a bacterial expression vector related to pEX with universal host specificity. Nucleic Acids Res. 1987 Dec 10;15(23):10056–10056. doi: 10.1093/nar/15.23.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. C., Kenan D., Martin B. J., Keene J. D. Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem. 1988 Dec 5;263(34):18043–18051. [PubMed] [Google Scholar]
- Chan E. K., Francoeur A. M., Tan E. M. Epitopes, structural domains, and asymmetry of amino acid residues in SS-B/La nuclear protein. J Immunol. 1986 May 15;136(10):3744–3749. [PubMed] [Google Scholar]
- Chan E. K., Sullivan K. F., Tan E. M. Ribonucleoprotein SS-B/La belongs to a protein family with consensus sequences for RNA-binding. Nucleic Acids Res. 1989 Mar 25;17(6):2233–2244. doi: 10.1093/nar/17.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian C. L., Elkon K. B. Autoantibodies to intracellular proteins. Clinical and biologic significance. Am J Med. 1986 Jan;80(1):53–61. doi: 10.1016/0002-9343(86)90048-3. [DOI] [PubMed] [Google Scholar]
- Chu J. L., Gharavi A. E., Elkon K. B. Cryoglobulinemia: analysis of isotype, idiotype and antibody activity by composite gel electrophoresis and immunoblotting. Electrophoresis. 1988 Mar;9(3):121–125. doi: 10.1002/elps.1150090303. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Machlin P. S., Bordwell B. J., Rothfield N. F., Cleveland D. W. Analysis of anticentromere autoantibodies using cloned autoantigen CENP-B. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4979–4983. doi: 10.1073/pnas.84.14.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Culhane L. Partial immunochemical characterization of the Ro and La proteins using antibodies from patients with the sicca syndrome and lupus erythematosus. J Immunol. 1984 May;132(5):2350–2356. [PubMed] [Google Scholar]
- Elkon K. B., Gharavi A. E., Hughes G. R., Moutsoupoulos H. M. Autoantibodies in the sicca syndrome (primary Sjögren's syndrome). Ann Rheum Dis. 1984 Apr;43(2):243–245. doi: 10.1136/ard.43.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Gharavi A. E., Patel B. M., Hughes G. R., Frankel A. IgA and IgM rheumatoid factors in serum, saliva and other secretions: relationship to immunoglobulin ratios in systemic sicca syndrome and rheumatoid arthritis. Clin Exp Immunol. 1983 Apr;52(1):75–84. [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B. Isoelectric focusing of human IgA and secretory proteins using thin layer agarose gels and nitrocellulose capillary blotting. J Immunol Methods. 1984 Feb 10;66(2):313–321. doi: 10.1016/0022-1759(84)90343-0. [DOI] [PubMed] [Google Scholar]
- Elkon K. B., Jankowski P. W. Fine specificities of autoantibodies directed against the Ro, La, Sm, RNP, and Jo-1 proteins defined by two-dimensional gel electrophoresis and immunoblotting. J Immunol. 1985 Jun;134(6):3819–3824. [PubMed] [Google Scholar]
- Elkon K. B., Parnassa A. P., Foster C. L. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985 Aug 1;162(2):459–471. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K., Bonfa E., Llovet R., Danho W., Weissbach H., Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5186–5189. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K., Skelly S., Parnassa A., Moller W., Danho W., Weissbach H., Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7419–7423. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharavi A. E., Chu J. L., Elkon K. B. Autoantibodies to intracellular proteins in human systemic lupus erythematosus are not due to random polyclonal B cell activation. Arthritis Rheum. 1988 Nov;31(11):1337–1345. doi: 10.1002/art.1780311101. [DOI] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldner H. H., Netter H. J., Szostecki C., Lakomek H. J., Will H. Epitope mapping with a recombinant human 68-kDa (U1) ribonucleoprotein antigen reveals heterogeneous autoantibody profiles in human autoimmune sera. J Immunol. 1988 Jul 15;141(2):469–475. [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattioli M., Reichlin M. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum. 1974 Jul-Aug;17(4):421–429. doi: 10.1002/art.1780170413. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Costello R., Drosos A. A., Mavridis A. K., Papadopoulos N. M. Demonstration and identification of monoclonal proteins in the urine of patients with Sjögren's syndrome. Ann Rheum Dis. 1985 Feb;44(2):109–112. doi: 10.1136/ard.44.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nell L. J., Hulbert C., Thomas J. W. Human insulin autoantibody fine specificity and H and L chain use. J Immunol. 1989 May 1;142(9):3063–3069. [PubMed] [Google Scholar]
- Pearce D. C., Yount W. J., Eisenberg R. A. Subclass restriction of anti-SS-B (La) autoantibodies. Clin Immunol Immunopathol. 1986 Jan;38(1):111–119. doi: 10.1016/0090-1229(86)90128-5. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Rauh A. J., Hornig H., Lührmann R. At least three distinct B cell epitopes reside in the C-terminal half of La protein, as determined by a recombinant DNA approach. Eur J Immunol. 1988 Dec;18(12):2049–2057. doi: 10.1002/eji.1830181227. [DOI] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D., Parrott D. M., Stott D. I. Quantitation of monoclonal immunoglobulins by immuno-isoelectric focusing and its application for monitoring secretory B cell neoplasia. J Immunol Methods. 1986 Jun 24;90(2):247–255. doi: 10.1016/0022-1759(86)90082-7. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. Identification of proteolytically resistant domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5673–5677. doi: 10.1073/pnas.77.10.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair E. W., Pisetsky D. S., Reich C. F., Keene J. D. Analysis of autoantibody binding to different regions of the human La antigen expressed in recombinant fusion proteins. J Immunol. 1988 Dec 15;141(12):4173–4180. [PubMed] [Google Scholar]
- Stephens R. E. High-resolution preparative SDS-polyacrylamide gel electrophoresis: fluorescent visualization and electrophoretic elution-concentration of protein bands. Anal Biochem. 1975 May 12;65(1-2):369–379. doi: 10.1016/0003-2697(75)90521-7. [DOI] [PubMed] [Google Scholar]
- Sturgess A. D., Peterson M. G., McNeilage L. J., Whittingham S., Coppel R. L. Characteristics and epitope mapping of a cloned human autoantigen La. J Immunol. 1988 May 1;140(9):3212–3218. [PubMed] [Google Scholar]
- Sugai S., Shimizu S., Hirose Y., Takiguchi T., Konda S., Yamano H. Monoclonal gammopathies in Japanese patients with Sjögren's syndrome. J Clin Immunol. 1985 Mar;5(2):90–101. doi: 10.1007/BF00915006. [DOI] [PubMed] [Google Scholar]
- Swerdlow P. S., Finley D., Varshavsky A. Enhancement of immunoblot sensitivity by heating of hydrated filters. Anal Biochem. 1986 Jul;156(1):147–153. doi: 10.1016/0003-2697(86)90166-1. [DOI] [PubMed] [Google Scholar]
- TALAL N., BUNIM J. J. THE DEVELOPMENT OF MALIGNANT LYMPHOMA IN THE COURSE OF SJOEGREN'S SYNDROME. Am J Med. 1964 Apr;36:529–540. doi: 10.1016/0002-9343(64)90101-9. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. M., Lane A. T., Barnett N. K., Bias W. B., Arnett F. C., Provost T. T. Neonatal lupus erythematosus. A clinical, serological and immunogenetic study with review of the literature. Medicine (Baltimore) 1984 Nov;63(6):362–378. [PubMed] [Google Scholar]
- Wysocki L., Manser T., Gefter M. L. Somatic evolution of variable region structures during an immune response. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1847–1851. doi: 10.1073/pnas.83.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D., Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. Monoclonal antibodies as probes of domain structure of the spectrin alpha subunit. J Biol Chem. 1982 Aug 10;257(15):9102–9107. [PubMed] [Google Scholar]