Abstract
Improvements in the prevention or control of rejection of the kidney and liver have been largely interchangeable (1, 2) and then applicable, with very little modification, to thoracic and other organs. However, the mechanism by which anti rejection treatment permits any of these grafts to be “accepted” has been an immunological enigma (3, 4). We have proposed recently that the exchange of migratory leukocytes between the transplant and the recipient with consequent long-term cellular chimerism in both is the basis for acceptance of all whole-organ allografts and xenografts (5). Although such chimerism was demonstrated only a few months ago, the observations have increased our insight into transplantation immunology and have encouraged the development of alternative therapeutic strategies (6).
DISCOVERY OF GRAFT CHIMERISM
After Liver Transplantation
Successful transplants were long envisioned as an alien patch in a homogeneous host (Fig. 1, left). The first unequivocal evidence that whole-organ grafts in human beings become genetic composites (chimeras) was obtained in 1969 with karyotyping studies in female recipients of livers obtained from male cadaveric donors. Postoperatively, the hepatocytes and the endothelium of the major blood vessels of the grafts retained their donor sex, whereas the entire macrophage system, including the Kupffer cells, was replaced with recipient female cells (identified by their characteristic Barr bodies) within 100 days (7, 8) (Fig. 1, middle). These observations attracted considerable attention at the time, primarily because of their implication that liver-based inborn errors of metabolism could be corrected permanently by liver replacement (9, 10). This prediction has been met since then in nearly two dozen such heritable diseases (11). Each report of another liver-based metabolic disorder that was corrected by liver replacement added to the illusion that the composite (chimeric) structure of the hepatic allograft was a special feature of this organ.
Fig. 1.
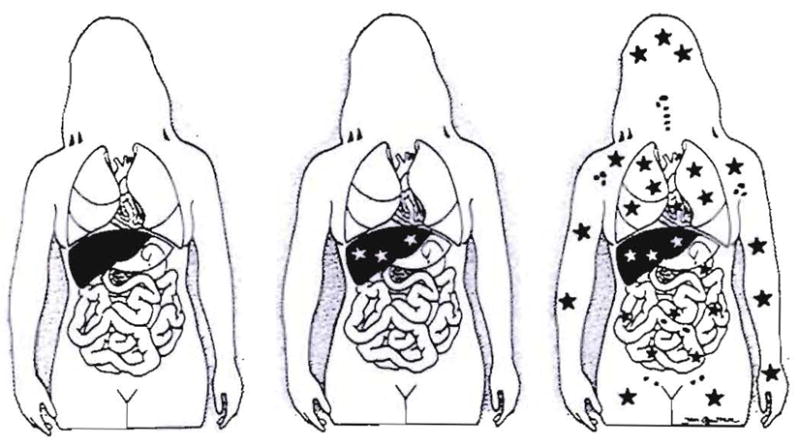
Steps in understanding liver transplantation: left– historical view; middle – realization in 1969 that the liver graft became a genetic composite (chimera); right–proof in 1992 of systemic chimerism. Stars represent cell exchange between graft and host.
After Intestinal Transplantation
The illusion of uniqueness of the hepatic graft was dispelled in 1991 with the demonstration, first in rat models (12) and then in human beings (13), that all successfully transplanted intestines also were chimeric. The epithelium of the bowel remained that of the donor, but lymphoid, dendritic and other leukocytes of recipient phenotype quickly became the dominant cells in the lamina propria, Peyer’s patches and mesenteric nodes. The transformation in experimental animals and in human beings (Fig, 2) was the same whether the bowel was transplanted alone or as a part of a multivisceral graft that also contained the liver, stomach and pancreas. As with that of the liver graft before it, the chimerism of the intestinal graft was made easier to demonstrate by the large constituency of lymphoreticular cells of the normal bowel. An additional crucial factor was the increasing sophistication of cell phenotyping techniques with which to differentiate donor from recipient cells in either experimental animals or human beings. For the first time, it was speculated in 1991 that graft chimerism might be a generic feature of all accepted grafts (13). This speculation soon was demonstrated with the kidney (14) and thoracic organs (15–17).
Fig. 2.
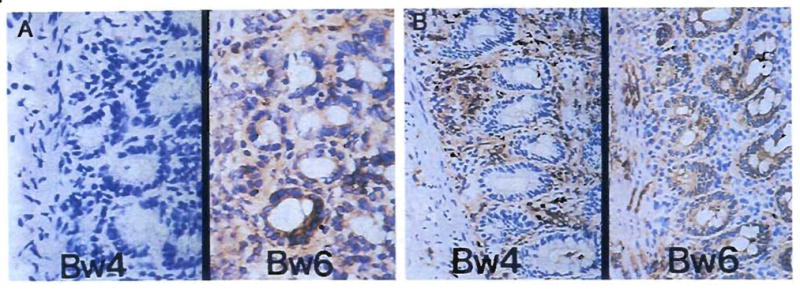
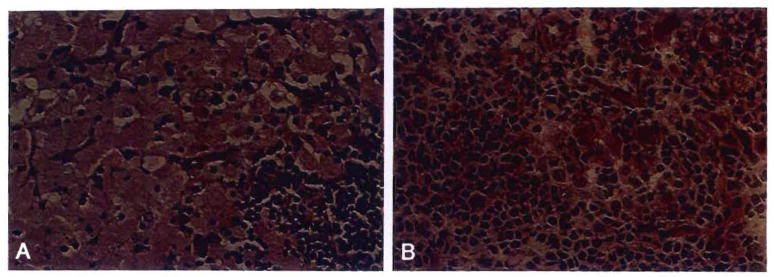
Repopulation of the lamina propria of human small intestinal grafts, demonstrated by HLA allele phenotyping. Monoclonal antibodies directed at Bw loci were used to differentiate donor (Bw6) from recipient (Bw4) cells. (A) Backtable graft biopsy specimen showed no recipient cells as expected. (Immunoperoxidase staining for Bw4 [left] and Bw6 [right]; original magnification × 250.) (B) Biopsy specimen 54 days after transplantation. The recipient cells have repopulated the lamina propria, but the epithelium and endothelium remained of donor origin. Ommunoperoxidase staining with DAB [brown] for Bw4 [left] and Bw6 [right); original magnification × 250.)
RECOGNITION OF SYSTEMIC CHIMERISM
Twenty-two years passed between the discovery of the transplanted liver’s chimerism and the discovery of that of the intestine. Throughout this time, the tacit or explicit assumption was that the cells departing the liver had been destroyed. This misapprehension would not happen again with the bowel. In a letter on February 12, 1991, accepting the article by Iwaki et al. (13) that showed the chimeric nature of the transplanted human intestine, Dr. Robin Fox, editor of the journal Lancet asked “Would you consider adding, at proof stage, a few words about the possible fate of the donor lymphocytes?” In addition to stimulating further studies of the intestine (see later), this inquiry caused a reexamination of data from much earlier investigations of kidney and liver transplant recipients. Circumstantial evidence from these cases had suggested that donor leukocytes migrated from the engrafted organs and were not promptly destroyed. However, the observations had been largely ignored or forgotten.
Kidney Transplantation
Indirect Evidence of Chimerism
Survival for at least 5 mo after clinical kidney allotransplantation was a rare achievement in patients treated through April 1962. Only eight patients survived – two in Boston (18–20) and six in Paris (21, 22). The subsequent practical exploitation of this operation hinged on the simple discovery that the rejection under azathioprine therapy of either canine (23) or human kidney allografts (24) could be reversed in the vast majority of cases with the addition or intensification of prednisone therapy. In addition, the clinical experience showed that it often became possible subsequently to taper the doses of both drugs (24). The reversibility of rejection and a change in host-graft relationship eventually were verified with all other transplanted organs, beginning with the liver (2, 25).
The mechanism of graft acceptance under such therapy could not be explained (4, 26–29). An ancient clue was the reduced allogenicity noted in thyroid tissue during a period of privileged sanctuary in the anterior chamber of the guinea pig eye before retransplantation to a subcutaneous location in the same recipient (30, 31). Such graft “adaptation” (as Woodruff called it) in combination with recipient clonal lymphocyte deletion was used in 1963 to explain the reversal of rejection in the kidney recipients and the later donor-specific diminution of recipient immunological resistance to the transplant (24, 27). The inability to explain how genetically proscribed transplant antigens could change caused the adaptation concept to lose favor.
The results of exhaustive skin test studies (tuberculin, histoplasmin, blastomycin, coccidioidin, mumps, Candida and Trichophyton) of our early Colorado kidney recipients and their donors provided an explanation for altered graft antigenicity (32), but one that was not considered plausible at the time. Seventy-seven percent of the skin reactions that were positive preoperatively in the donor but not in the patients crossed over the previously negative recipients, along with the transplanted kidney (Fig. 3). When this did not occur (the other 23%), it meant that the kidney transplant had failed. W.E.C. Kirkpatrick and Charles Wilson, the fellows who performed these studies under the supervision of the immunologist, Dr. David Talmage, speculated that the secondary acquisition of the positive skin tests was “caused by adoptive transfer of donor cellular immunity by leukocytes in the renal graft vasculature and hilar lymphoid tissue” (32).
Fig. 3.
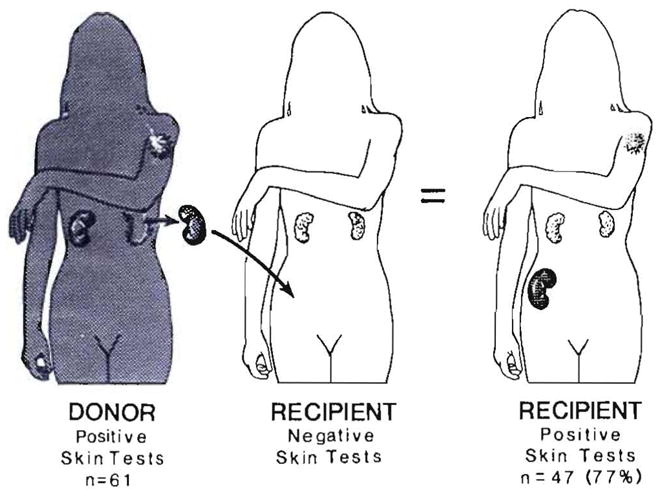
Transfer of positive skin test results from kidney donors to recipients in cases at the University of Colorado from 1962 to 1964 (32). Although inexplicable at the time, these observations reflected adoptive transfer after cell migration, repopulation and chimerism.
The implication that cells from the graft had migrated into recipient tissues was considered untenable nearly 30 yr ago because the kidney was then thought to be a leukocyte-poor organ. Consequently, the flash of potential insight faded even though the “transfer factor” of Lawrence (33) was suggested as a possible amplification device. Ironically, several of these original patients were restudied and found to have chimerism nearly three decades later (14). By then, they had become part of an elite group of forerunners bearing the longest continuously surviving kidney grafts in the world (34).
Although the empiric observations of rejection reversal and the seeming change in host-graft relationship were enigmatic in 1962 and 1963, they became the basis of the therapeutic dogma that has dominated the clinical field of whole-organ transplantation ever since. This dogma calls for daily baseline treatment (30 yr ago with azathioprine), plus intervention with the highly dose-maneuverable adrenal cortical steroids to whatever level is required to maintain stable graft function. The new drugs that have been added through the years have been increasingly effective (Table 1). However, in every case the weaning off drugs after initially heavy induction therapy requires highly individualized dose adjustments in which each change must be evaluated after the fact by the patient’s freedom from rejection or failure to maintain this state.
Table 1.
Treatment principles of organ transplantation
| Central therapeutic dogma |
| Baseline therapy with one or two drugs |
| Secondary adjustments with steroids with or without anti-lymphoid agents |
| Case to case trial (and potential error) of weaning |
| Baseline Agents |
| Azathioprine |
| Cyclophosphamide |
| Cyclosporine |
| Cyclosporine and azathioprine |
| FK 506 |
| FK 506 and azathioprine |
Technology of Chimerism Detection
The search in April through July of 1992 for chimeric cells in whole-organ recipients was made feasible by the distinctive features of two chromosomes. In female patients who had been given organs from male donors, the presence in recipient tissues (or blood) of cells with the Y chromosome was considered unequivocal evidence of systemic chimerism (14, 35). Alternatively, probes were used that detected human leukocyte antigen (HLA) alleles of chromosome 6 (5, 14, 36). For study of either the Y chromosome or chromosome 6, one or the other of two technologies, and usually both, was used (5, 14, 35, 36), One was cytostaining, which allows the location and morphological characterization of phenotypically distinct donor and recipient cells. The other was polymerase chain reaction (PCR), which distinguishes donor from recipient DNA.
The cytostaining for the Y probe was with a fluorescence method after in situ hybridization (14, 35). The immunostaining for the HLA markers was with indirect immunofluorescence, an avidin-biotin–complex immunoperoxidase or both methods, by use of monoclonal antibodies to class I and class II antigen phenotypes present in the donor but not the recipient (5, 14,36). The tissue sections were blocked with nonimmune serum from the source of the secondary antibody. Nonspecific peroxidase activity was quenched by incubation with H2O2/methanol. Staining with irrelevant HLA anti-bodies and omission of the primary antibody provided negative controls. Tissues with known HLA antigens, along with the allograft biopsy and skin sections of the recipient stained with matching HLA monoclonal antibodies, were used as positive-specificity controls.
In the PCR search for the Y chromosome, oligonucleotides specific for the satellite region of the Y chromosome centromere Y-A (37) and for the sex-determining region of the Y chromosome (38) were used as primers for determination of the presence of male DNA in the female recipient tissues. After Southern blotting, the amplified material was hybridized to radioactively labeled Y-specific probes for confirmation of the specificity of the bands visualized in an agarose gel after ethidium bromide staining. The PCR tests for donor- and recipient-specific HLA alleles of chromosome 6 were performed according to the reference protocol of the XI International Histocompatibility Workshop (39), in which preliminary generic amplification of the DRB gene is performed followed by allele-specific amplification and testing.
Direct Evidence of Chimerism
Of the five kidney recipients who were studied 27 to 29 yr after transplantation, one had stopped immunosuppression 12 yr earlier, whereas the others were still taking azathioprine with or without prednisone. These five patients were selected for the chimerism study in preference to other surviving kidney recipients from the 1963 era for three reasons. First, four of the five donors still are alive, allowing their HLA retyping and use of their lymphocytes for studies of immunological competence of the recipient; these tests showed varying degrees of donor-specific nonreactivity (tolerance) that in some cases was absolute (14). Second, known HLA incompatibilities between these four parental or aunt donors and their recipients permitted the immunocytochemical and PCR distinction of donor from recipient cells in the biopsy tissues as described in the preceding section. Finally, in the fifth case in which the donor (father to daughter) had died, chimerism in tissues could be determined by sex typing.
In each of the four kidney recipients whose donor still was alive, leukocytes with the appearance of dendritic cells that had migrated from the renal allografts were found in the recipient skin and lymph node biopsies (Table 2). Although they were few in number, these HLA-mismatched donor cells were unmistakable with the immunostaining techniques (Figure 4B). Chimerism was confirmed with PCR in the recipient tissues of all four of these patients (Fig. 5) and in the blood of two of them (Table 2).
Table 2.
Microchimerism according to HLA-allele detection with immunocytochemical (ICC) and PCR techniques in four kidney allograft recipients and according to sex in a fifth who could be studied with Y-chromosome detection (male to female donor)a
| LD no. | Donor | Date of transplantation (day/mo/yr)b | Patient age at transplantation (yr) | Donor tissue distribution |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kidney allograft |
Blood |
Lymph node |
Skin |
|||||||
| ICC | PCR | PCR | ICC | PCR | ICC | PCR | ||||
| 17 | Mother | 7/19/63 | 15 | + | + | − | + | + | − | − |
| 33 | Mother | 10/07/63 | 18 | + | + | + | + | + | + | |
| 51 | Aunt | 2/10/64 | 18 | + | +0 | − | + | + | + | + |
| 52c | Father | 2/17/64 | 16 | + | + | − | − | − | + | + |
| 90 | Mother | 7/23/65 | 18 | + | + | + | + | + | + | + |
Data from Reference 14.
Postoperative chimera studies performed in May and June of 1992.
No treatment for 12 yr except for prednisone 7.5 mg/day. Other patients still given azathioprine with or without prednisone.
Fig. 4.
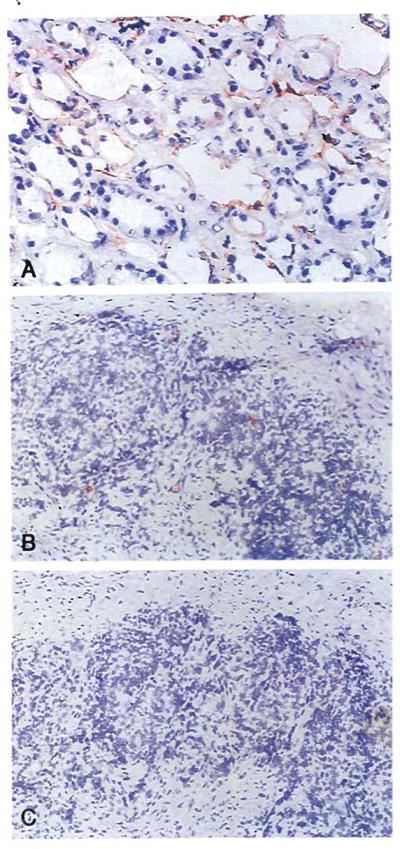
Patient LD51. Immunostained biopsy samples 29 yr after transplantation of (A) an allograft kidney (original magnification × 250) and (B) an inguinal lymph node (original magnification × 100) stained for HLA B7,40–an antigen present in the donor but not the recipient; note the sparse and evenly scattered donor cells (rust colored) in the lymph node. (C) As a control the lymph node was also stained with anti-A2,28–present in neither the donor nor the recipient (original magnification × 100).
Fig. 5.
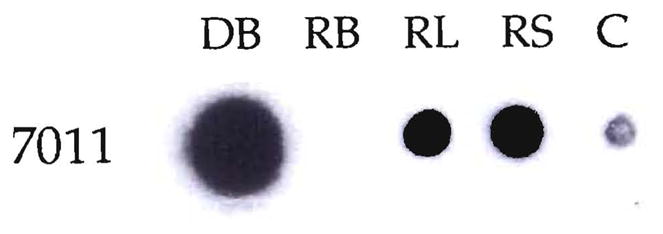
Chimerism demonstrated by HLA molecular typing in a kidney recipient, LD5l. The donor’s DR2 allele was specifically amplified by PCR from DNA extracted from the recipient’s blood (RB), lymph node (RL) and skin (RS). The amplified product was dot blotted and hybridized with 7011, a DR2-specific oligonucleotide probe. DNA from the donor’s blood (DB) was also amplified. The negative control (C) of the PCR amplification performed in the absence of DNA is also shown.
In the fifth patient, a woman who had been given her now-deceased father’s kidney 29 yr previously, cells with the Y chromosome were found in her skin with fluorescent in situ hybridization studies, which were confirmed with PCR (Fig. 6).
Fig. 6.
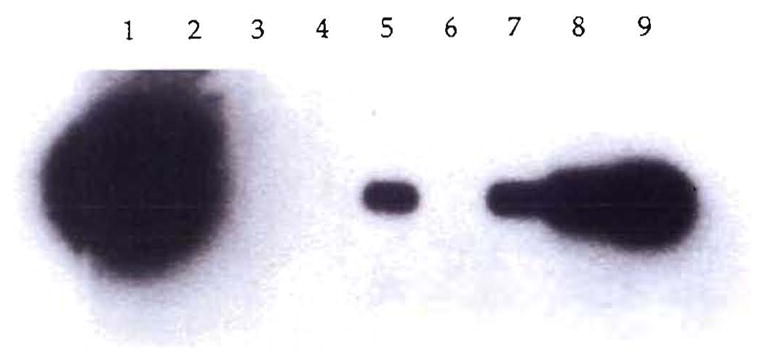
PCR demonstration of chimerism in a female recipient (LD52) of a kidney from her father. The DNA of the SRY region of the Y chromosome was PCR amplified, Southern blotted and hybridized with a homologous radioactive probe. DNA from biopsy specimens of the patient’s kidney (1), blood (3), lymph node (4) and skin (5) were tested. As controls, DNA from an unrelated female (6) and serial dilutions of male DNA in female DNA 10−4 (9), 10−5 (8) and 10−6 (7) were also analyzed. No sample was present in lane 2.
In all five cases, biopsy samples of the kidney showed that the cells departing the allograft had been replaced by similar cells from the recipient (Figure 4A). Thus, in addition to showing systemic chimerism, it was established that the kidney grafts were chimeras—composed of cells with two different genomes (14) (Fig. 7).
Fig. 7.
Systemic and graft chimerism in all five tested patients treated who had borne continuously functioning allografts for 27 to 29 yr. The stars represent the migrated cells. In these studies only the kidney, skin, lymph nodes and blood were sampled, but the more ubiquitous chimerism was inferred from the more extensive studies in recipients of livers and other organs.
The Liver and Intestine
Indirect Evidence in Liver Recipients of Systemic Chimerism
When hepatic graft chimerism was reported in 1969 (7, 8), the fate of the donor cells vacating the liver was unknown. Although it was often assumed that these cells had perished, their continued viable presence in places other than the liver was signaled by the appearance and maintenance in the recipient blood flow of new donor-specific immunoglobulin types (8, 40). As with the circumstantial evidence of chimerism in the kidney recipients of 1963, this important finding was largely ignored. However, 15 yr later emigrant cells from the graft were proposed to be the source of RBC antibodies that developed in recipients of livers from donors who had ABO nonidentity (41). Subsequently, Davies, Pollard and Calne (42) showed the appearance in liver recipients of new circulating donor-specific class I antigens, which they attributed to the graft hepatocytes. However, because these molecules are known to come from bone marrow–derived macrophages, dendritic cells or both (43–45), they presumably had the same donor origin as the additional immunoglobulin types and red cell antibodies after completion of cell migration to extrahepatic sites.
Direct Evidence of Systemic Chimerism from the Intestine
The evidence of systemic chimerism after either liver or intestinal transplantation remained circumstantial until Murase et al. (46, 47) showed with flow cytometry that the stromal leukocytes leaving the small bowel allografts in rat recipients treated with a short course of FK 506 homed through vascular routes to widely distributed host lymphoid tissues. This created a state of systemic mixed allogeneic chimerism for at least 45 days (Fig. 8), free of lethal or even detectable graft vs. host disease (GVHD) except in special strain combinations in which a poorly understood imbalance exists between the graft and recipient immune systems.
Fig. 8.
Current understanding of the graft and systemic chimerism that occurs after intestinal transplantation. The automatic production of mixed allogenic chimerism after intestinal transplantation, providing the preexisting immunological apparatus, is not iatrogenically damaged on either donor or recipient side (see text). Evolution of this concept permitted successful clinical intestinal transplantation trials (108). The stars represent the cell exchange between the graft and host.
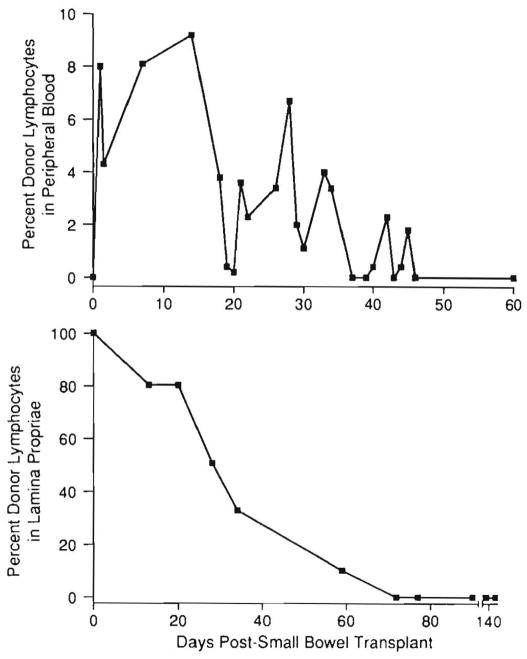
The vascular route by which cell migration occurred also was obvious in swine (48) and in human recipients of intestinal or liver-intestinal grafts (13, 49). In the patients, donor cells in the early postoperative period accounted transiently for 5% to 15% of circulating mononuclear cells, usually without evidence of GVHD. Because the circulating cells could no longer be detected with flow cytometry after 1 to 2 mo in the clinical cases (Fig. 9) and were not studied beyond this time in the rat experiments, the duration of the chimeric state remained in doubt throughout 1991.
Fig. 9.
Appearance of donor mononuclear cells in the blood of an intestinal human transplant recipient (upper) and the demonstration of progressive replacement of interstitial donor leukocytes from the graft during the same general time (lower). (Data from Iwaki et al. [13].)
Metabolic Effects of Migratory Cells from Liver Allografts
Meanwhile, an incidental and at first inexplicable observation had been made in children treated with liver transplantation for the liver failure associated with type IV glycogen storage disease (GSD IV)—pointing not only to the ubiquitous distribution of the migratory donor cells but also to their persistence and their metabolic influence. In GSD IV, a deficiency of the branching enzyme alpha-1,4-glucan:alpha-1,4-glucan 6-glycosyltransferase (50) is responsible for the widespread accumulation intracellularly and extracellularly of an insoluble and irritating amylopectin-like polysaccharide (50, 51).
The usual clinical consequence is fatal cirrhosis within 1 or 2 yr after birth However, patients who do not die from liver disease are subject to lethal cardiomyopathy or neuromuscular syndromes (52–55). Because the enzyme deficiency and consequent amylopectin deposition affect all cells, not just those in the liver, it was surprising to many experts in metabolism when absorption occurred of the amylopectin from the heart and other extrahepatic tissues of five patients with GSD IV 1.3 to 6 yr after liver replacement (Fig. 10) (56). This finding caused Howell (57) to predict in an accompanying editorial that an explanation for the benefit “will teach us a great deal about transplantation.” The prophecy was fulfilled with the further study of two of these recipients after 22 more mo of follow-up, showing that they had systemic chimeras (5, 36).
Fig. 10.
(A) An endomyocardial biopsy specimen obtained in 1989, at the time of liver transplantation, revealed diffuse amylopectin deposits within and between cells. (B) A repeat endomyocardial biopsy sample taken in 1992 revealed only traces of extracellular deposits. (Periodic acid–Schiff-D; original magnification ×100.)
The donor cells in these two patients had migrated diffusely from the transplanted liver into the heart and other tissues where they were evident 37 and 91 mo postoperatively (Table 3). There, the relocated leukocytes apparently served as enzyme carriers. Similar chimerism in a variety of host tissues including the bone marrow (Table 3) with consequential metabolic benefits (Fig. 11) also were observed 26 mo after liver transplantation in a patient whose diagnosis was type 1 Gaucher’s disease (36), a disorder caused by a deficiency of the lysosomal enzyme B glucocerebrosidase (58). The chimerism was demonstrated with the immunostaining techniques that identify individual cells with donor-specific monoclonal HLA antibodies (Fig. 12).
Table 3.
Metabolic diseasesa
| Case | Disease | Chimerism |
|---|---|---|
| 1 | GSD IV | Blood, skin, lymph node and heart |
| 2 | GSD IV | Blood, skin, lymph node and heart |
| 3 | Gaucher’s | Blood, skin, lymph node, intestine and bone marrow |
Fig. 11.
(A) Hepatic hilar lymph node removed in April 1990 at the time of liver replacement from a patient with Gaucher’s disease and (B) an inguinal lymph node obtained in June 1992. Note the marked decrease in the number of Gaucher’s cells, which were difficult to find in the latter specimen. (Periodic acid–Schiff; original magnification ×250.)
Fig. 12.
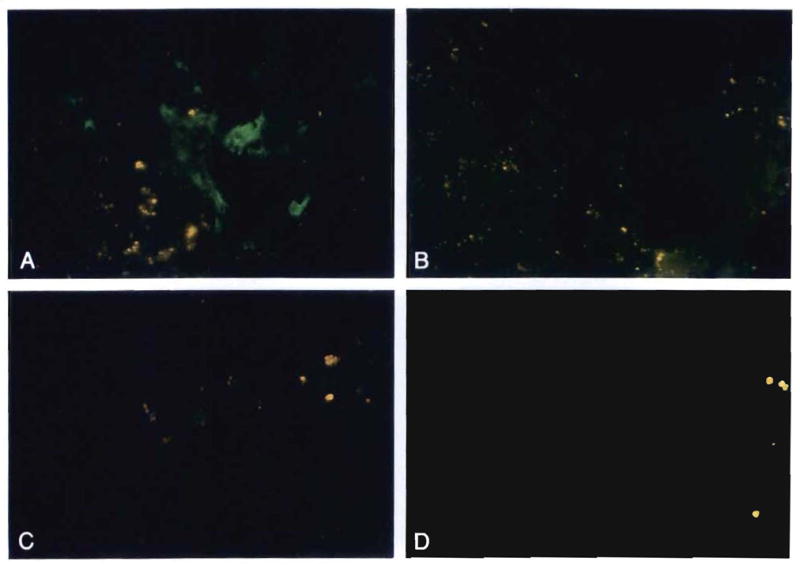
Allograft liver and inguinal lymph node biopsy specimens obtained 26 mo after the liver replacement from the recipient with Gaucher’s disease. These were stained with antibodies to DR1 (donor) and DR3 (control). (A) Note the presence of DR1-positive donor cells (green) in the portal tract of the liver (original magnification × 400). (B) Absence of staining in the control (antibodies to DR3; original magnification × 400). (C) Rare DR1-positive (donor) cells were also seen in the inguinal lymph node (original magnification × 250). (D) The lymph node control (antibodies to DR3) was negative (original magnification × 250). (All were stained with FITC indirect immunofluorescence.)
In confirmatory studies donor DNA was found with PCR in the same locations as with immunostaining (Fig. 13). Even with the limited samples available from these patients, it was evident by this time (Table 3) that the cell migration after clinical (5, 36) or experimental liver transplantation (16) was a miniversion of bone marrow transplantation (59) (Fig. 1 right).
Fig. 13.
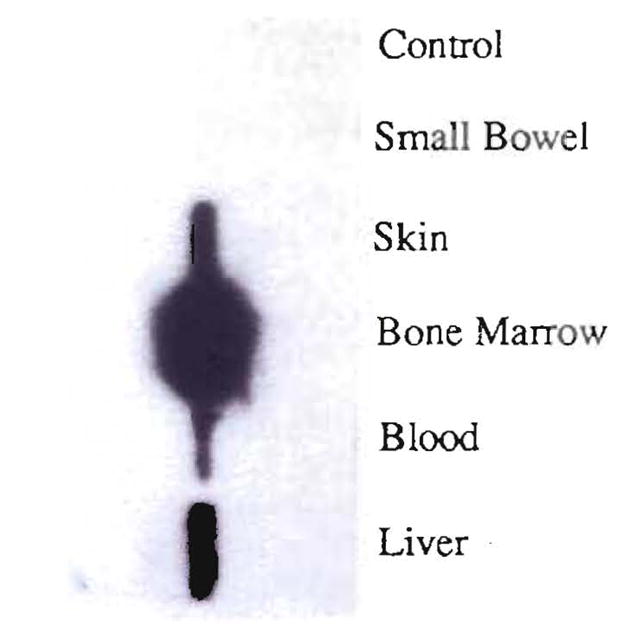
Detection of chimerism by molecular HLA class II typing in various tissues after liver transplantation in a patient with type 1 Gaucher’s disease. Southern blot analysis of DR1-specific amplification of the DNA extracted from small bowel, skin, bone marrow, blood and liver. The denaturated DNA present on the nylon membrane was hybridized to a radioactively labeled DR1 (donor) specific oligonucleotide probe (7001). In the case of the liver, only 1/100 of the amplification product was used. Note negative control of the PCR amplification.
These findings explained why patients with lysosomal storage disorders previously thought to be correctable only with bone marrow transplantation (60) had benefited from liver replacement beyond what had been predicted. These disorders included the following: Gaucher’s disease (61), Wolman disease (62), Niemann-Pick disease (63), and sea-blue histiocyte syndrome (64; Starzl et al., Unpublished follow-up). In such patients and those with GSD IV, the enzyme produced by the donor cells was postulated to have obtained efficient access to the storage substrate by a coculture effect on the contiguous recipient cells, as has been described in in vitro experiments by Neufeld (65). The number of the allogeneic cells required to achieve such a metabolic effect in vivo appeared from our clinical observations to be surprisingly low. The presumed transmission of enzymes from a small to a large cell population raised important questions about the potential cell-to-cell effect of other molecules directly involved in immunological processes including tolerance induction.
Moreover, in a strictly metabolic context, it was evident that the past use of liver transplantation to investigate certain inborn metabolic errors could have led to erroneous conclusions if it was assumed (as it commonly was) that the leukocytes replaced from the hepatic allograft were eliminated. For example, the decline in cholesterol levels after liver transplantation in patients with familial hypercholesterolemia (66) was ascribed to the new supply of low-density lipoprotein receptors of the allograft hepatocytes (67). It seems likely now that the amelioration of this disorder by liver transplantation is explained in part by low-density lipoprotein receptors of widely disseminated donor cells that are not hepatocytes. The volume of these leukocytes is large. Migratory Kupffer cells alone constitute 10% to 15% of all cells in the liver and account for one fifth of the total hepatic lysosomal volume (68).
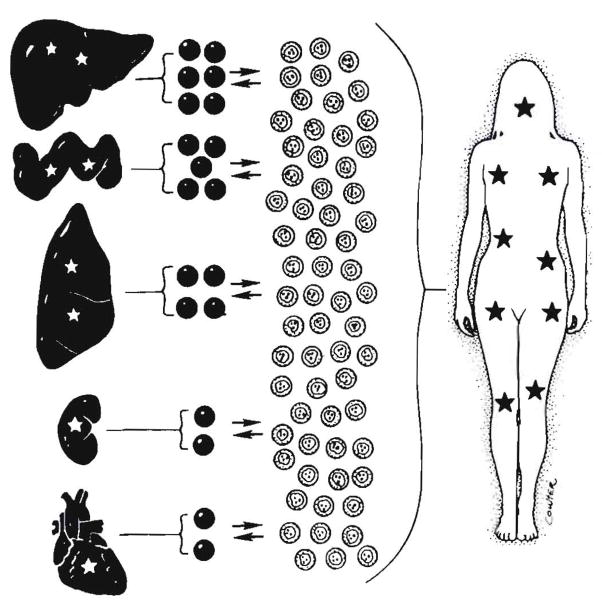
If, as we eventually concluded (5, 6, 14, 35, 36), chimerism, differing only in degree in different kinds of organs, is an integral feature of all successful engraftments, any transplantation procedure could result in a gain of metabolic function as suggested for different reasons by Groth et al. (69) two decades ago. The chimerism in our long-surviving kidney recipients was only one fifth or less of that in the liver recipients. However, there may have been enough cells migrating to the native kidneys from successfully implanted renal allografts (see Fig. 7) to explain recovery of function in the diseased kidneys, which had been shut down by the glycosphingolipid accumulations of Fabry’s storage disease (70).
The Incidence and Duration of Chimerism in Liver Recipients
The concept that long-lasting graft and systemic chimerism is a necessary condition of graft acceptance was largely speculative well into 1992. The biopsy studies of the children with GSD IV and Gaucher’s disease were not performed until April of that year, and although these gave decisive results, there were only three cases. However, in addition to these patients and those with long-surviving kidney allografts, another study was under way of liver recipients who had survived for at least 10 yr. These patients remained from a starting group of 210 treated at the University of Colorado between 1963 and 1980 (n = 184) and at the University of Pittsburgh during 1981 (n = 26) (Fig. 14). They were pioneer patients in the development of liver transplant technology (71, 72) and included the 10 longest-surviving liver recipients in the world (73).
Fig. 14.
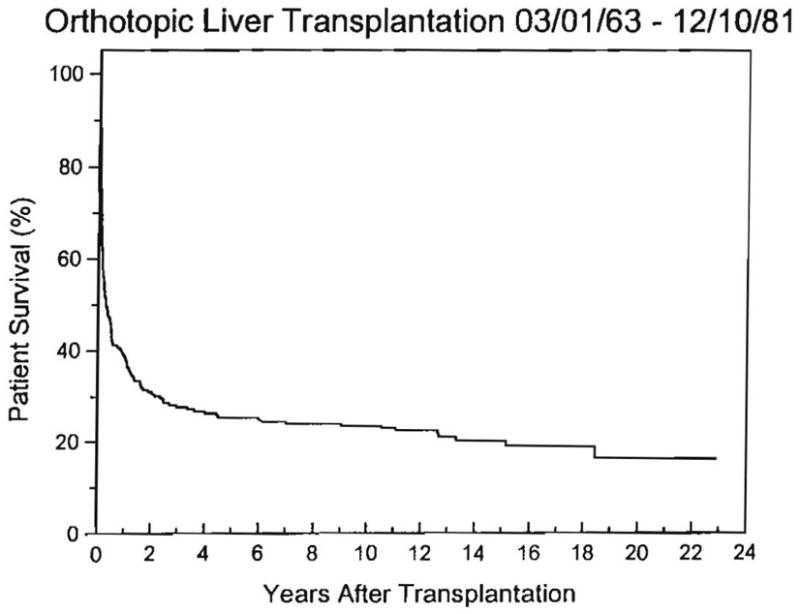
Life survival curve of the first 210 patients in our Colorado-Pittsburgh series. The survival is actual to 11 yr and actuarial thereafter. Fifty of the original 210 patients survived for more than 10 yr; 43 are still alive after 11 to 23 yr. The other 7 died after 11.1 to 18.4 yr.
From the original 210, there were 50 survivors (24%) at the end of the first decade. Of these, six died from causes shown in Table 4 beyond 10 yr but before the commencement of the study; a seventh mortality after 18.4 yr occurred in July 1992 as the result of viral CAH. Three of these seven late losses were of patients who had normal liver function; the deaths were caused by recurrent malignancy, AIDS (from a blood transfusion during an orthopedic operation) and a smoke inhalation accident during demonstration of fire-fighting equipment. Although the grafts had deteriorated in the other four cases, rejection had not been the cause of failure.
Table 4.
Liver transplantation: causes of death beyond 10 yr
| OT no. | Age at transplantation (yr) | Original diagnosis | Date of transplantation (mo/day/year) | Date of death (mo/day/year) | Survival (yr) | Liver function | Cause of death |
|---|---|---|---|---|---|---|---|
| 53 | 1.7 | A-1-A | 2/20/72 | 6/13/85 | 13.3 | Abnormal (jaundiced) | B-cell lymphoma |
| 56a | 18 | Congenital hepatic fibrosis | 5/15/72 2/2/84 |
12/29/84 | 12.6 | Abnormal (not jaundiced) | GI hemorrhage |
| 77b | 16 | Viral CAH (non-A, non-B) | 2/3/74 | 7/14/92 | 18.4 | Abnormal (jaundiced) | GI hemorrhage |
| 114 | 27 | Epithelioid hemangioendothelioma | 9/19/76 | 11/19/91 | 15.2 | Normal | Recurrent tumor |
| 135 | 34 | Alcoholic cirrhosis | 8/13/77 | 9/14/88 | 11.1 | Abnormal (jaundiced) | Recurrent alcoholic cirrhosis |
| 139 | 23 | Viral CAH (non-A, non-B) | 11/13/77 | 5/2/89 | 11.6 | Normal | AIDS |
| 155 | 11 | A-1-A | 10/6/78 | 6/9/91 | 12.7 | Normal | Inhalation accident |
The portal vein was thrombosed and unreconstructable at the time of retransplantation in February 1984.
Died during period of 1992 study.
A-1-A = α1-Antitrypsin deficiency.
The clinical profile of all 44 patients still surviving in January 1992, including the 1 who died during the study period, is shown in Table 5. All but two of the recipients were in good clinical condition, and only three had significantly perturbed liver function test results; the highest bilirubin level was 3.8 mg/dl. Immunosuppression was variable. Most of the patients treated before 1980 were given azathioprine, whereas those treated during the 1980 to 1981 period were usually still given their starting drug of cyclosporine or had been switched to FK 506. The majority of patients in both the pre-1980 and post-1980 eras were still receiving maintenance prednisone (Table 6). A particularly important group of six patients had stopped their baseline immunosuppression 1 to 11 yr after transplantation and had been free of these drugs for 5 to 13 yr (Table 7). Five of these six also had stopped prednisone treatment at the same time; the sixth recipient still took 10 mg/day.
Table 5.
Profile of 44 liver transplant survivors after 10 to 22 yra
| Parameters | Data |
|---|---|
| Original diagnosesb | |
| Biliary atresia | 11 |
| CAH | 10 |
| α1-Antitrypsin | 6 |
| PBC | 4 |
| Wilson’s disease | 3 |
| Secondary biliary cirrhosis | 3 |
| Alcoholic cirrhosis | 2 |
| Budd-Chiari syndrome | 2 |
| Hepatoma | 1 |
| Familial cholestasis | 1 |
| Neonatal hepatitis | 1 |
| Age at operation (yr)c | 20.2 ± 15.3 |
| Length of follow-up (mo)c | 174.3 ± 40.2 |
| Liver function | |
| Bilirubin (mg/dl)c | 0.8 ± 0.7 |
| Albumin (gm/dl)c | 4.0 ± 0.6 |
| AST (IU/L)c | 40.2 ± 28.9 |
Patients alive as of January 1992.
Data expressed as no. of patients.
Data expressed as mean ± S.D.
Table 6.
Immunosuppression in 44 survivors
| Group | No. of patients | Drugs | Amount administered (mg/day) |
|---|---|---|---|
| 1 | 12 | Azathioprine | 50 ± 26a |
| Prednisone | 8.7 ± 3.2a | ||
| 2 | 11 | Cyclosporine | 266 ± 132a |
| Prednisone | 6.6 ± 3.4a | ||
| 3 | 5 | Cyclosporine | 232 ± 132a |
| 4 | 3 | Azathioprine | 58 ± 14a |
| Cyclosporine | 158 ± 38a | ||
| Prednisone | 9.2 ± 1.4a | ||
| 5 | 2 | Azathioprine | 75, 50 |
| 6 | 3 | Azathioprine/cyclosporine | 25, 50/250, 100 |
| 7 | 1 | FK 506/prednisone | 6/15 |
| 8 | 1 | FK 506 | 10 |
| 9 | 1 | Prednisone | 10 |
| 10 | 5 | Nothing | – |
Data expressed as mean ± S.D.
Table 7.
Features of six liver recipients who had stopped immunosuppression
| OT no. | Year of transplant | Year drug stopped | Years drug free | Prednisone (mg/day) | Liver function |
|---|---|---|---|---|---|
| 73 | 1973 | 1982 | 10 | – | Normal |
| 92 | 1974 | 1985 | 7 | – | Normal |
| 125 | 1977 | 1987 | 5 | – | Normal |
| 150 | 1978 | 1979 | 13 | – | Normal |
| 169 | 1979 | 1981 | 11 | – | Normal |
| 182 | 1980 | 1985 | 7 | 10 | Failinga |
Recurrent CAH from HCV was the biopsy-proved diagnosis, confirmed in the graft hepatectomy specimen obtained at retransplantation.
After these preliminary inquiries were completed, 20 randomly selected patients were invited to come to Pittsburgh in May and June for biopsy studies of the liver, skin and lymph nodes. Of the 20, 15 remained on immunosuppression, whereas the other 5 had stopped all treatment 7 to 13 yr earlier. More extensive tissue collections were obtained (Tables 8 and 9) from two additional recipients. One was the male recipient (OT 77) who had died after 18.4 yr of complications of CAH caused by HBV that had been acquired after transplantation; the tissue sampling was at autopsy. The other patient was a woman (OT 182) in whom retransplantation was performed successfully on April 2, 1992 (Table 8). Her primary transplantation had been in August 1980, and although she had stopped taking cyclosporine in 1985 while continuing to take prednisone 10 mg/day, the excised allograft and several previous biopsy specimens contained evidence of recurrent chronic C virus hepatitis (HCV) but not of rejection. Multiple host tissues were taken at the reoperation, including portions of jejunum from the Roux-en-Y biliary reconstruction and an ellipse of her aorta removed for a Carrel patch arterial reconstruction.
Table 8.
Microchimerism according to Y-chromosome detection with in situ hybridization or PCR
| OT no.a | Date of transplantation (mo/day/yr)a | Age of patient at transplantation (yr) | Tissue distribution |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Liver allograft |
Blood |
Lymph node |
Skin |
||||||
| In situ hybridization | PCR | PCR | In situ hybridization | PCR | In situ hybridization | PCR | |||
| 64 | 2/18/73 | 3 | +++ | +++ | − | − | + | + | + |
| 105 | 1/21/76 | 30 | +++ | +++ | + | + | − | + | + |
| 140 | 1/04/78 | 2 | +++ | +++ | + | + | + | + | NT |
| 142 | 2/26/78 | 5 | +++ | +++ | + | + | + | + | NT |
| 166 | 9/09/79 | 35 | +++ | +++ | + | + | + | + | + |
| 171 | 3/09/80 | 29 | NT | +++ | − | NT | − | NT | + |
| 173 | 3/21/80 | 34 | +++ | +++ | + | − | − | + | + |
| 182b | 8/29/80 | 28 | +++ | +++ | NT | + | − | − | + |
| 223 | 12/28/81 | 45 | +++ | +++ | + | + | + | + | + |
In addition to Y-chromosome detection, patients underlined also were shown to have chimerism in the same tissues by immunocytochemical detection of donor HLA alleles (cases 142, 171 and 223) or by PCR molecular typing (all but 171 and 182).
NT = not tested.
All postoperative studies completed in April to June 1992.
This patient also tested positive for Y chromosome in the intestine with in situ hybridization and in multiple tissues including the aortic wall with PCR (see Fig. 16).
Table 9.
Microchimerism in 13 patients according to HLA-allele detection with immunocytochemical (ICC) phenotyping and PCRa
| OT no. | Date of transplantation (mo/day/yr)b | Age of patient at transplantation (yr) | Tissue distribution |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Liver allograft |
Blood |
Lymph node |
Skin |
||||||
| ICC | PCR | PCR | ICC | PCR | ICC | PCR | |||
| Receiving treatment | |||||||||
| 42 | 3/23/71 | 16 | NT | + | + | NT | + | NT | + |
| 46 | 7/31/71 | 3 | +++ | + | + | + | + | + | + |
| 77c | 2/03/74 | 16 | NT | + | NT | NT | + | NT | NT |
| 133 | 7/26/77 | 44 | NT | + | − | NT | + | − | + |
| 144 | 4/17/78 | 39 | +++ | + | + | + | + | − | + |
| 146 | 6/14/78 | 49 | NT | + | + | NT | + | NT | − |
| 175 | 4/13/80 | 41 | NT | + | + | NT | + | NT | + |
| 189 | 5/09/81 | 2 | +++ | + | + | + | + | + | + |
| 194 | 7/12/81 | 26 | +++ | + | − | − | + | + | + |
| Not receiving treatment | |||||||||
| 73 | 10/08/73 | 4 | +++ | + | − | + | + | − | + |
| 92 | 11/27/74 | 11 | +++ | + | − | + | + | − | + |
| 150 | 7/15/78 | 15 | NT | + | + | + | + | − | − |
| 169 | 12/03/79 | 21 | +++ | + | − | + | + | + | + |
Nine of the 22 studied were female patients who had been given livers from male donors (Table 8). In all nine the Y chromosome was demonstrated in the skin or lymph nodes with fluorescence in situ hybridization (Fig. 15) and confirmed in every case by PCR (Fig. 16). In seven of the nine cases, HLA phenotyping or HLA-DRB molecular typing also was performed on the same tissues with demonstration of chimerism in each case (Table 8).
Fig. 15.
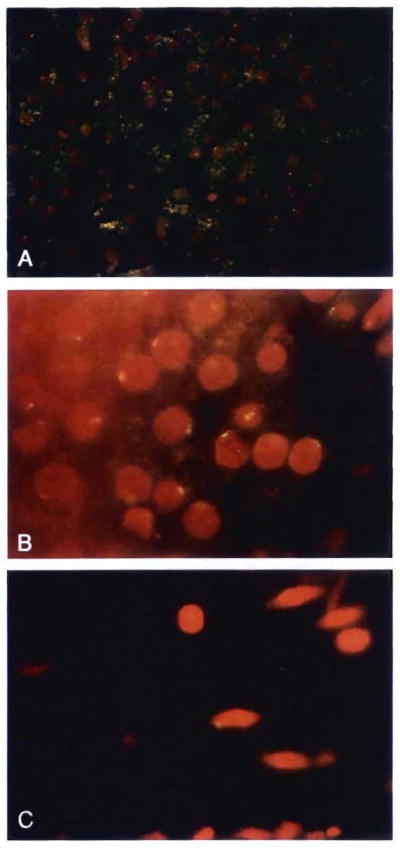
Studies of patient OT64, 19.5 yrs after her liver replacement from a male donor. Fluorescent in situ hybridization with the DYZ1 probe for the Y chromosome was used to differentiate male from female cells. (A) The allograft liver was used as a positive control. About 60% of hepatocytes and a few sinusoidal cells retained the donor genotype; the Y chromosome in the negative hepatocytes was likely excluded from the 2 μm–thick sectioning plane. The yellow cytoplasmic material is autofluorescence (original magnification × 250). (B) Oil immersion microscopy was used to illustrate the variety of signals obtained in the liver, which showed a beaded to reticular pattern, often located at the periphery of the nucleus (original magnification × 1,000). (C) Skin biopsy specimen: spindle-shaped stromal cells, with a similar signal for the Y chromosome, were sparsely distributed (original magnification × 1,000).
Fig. 16.
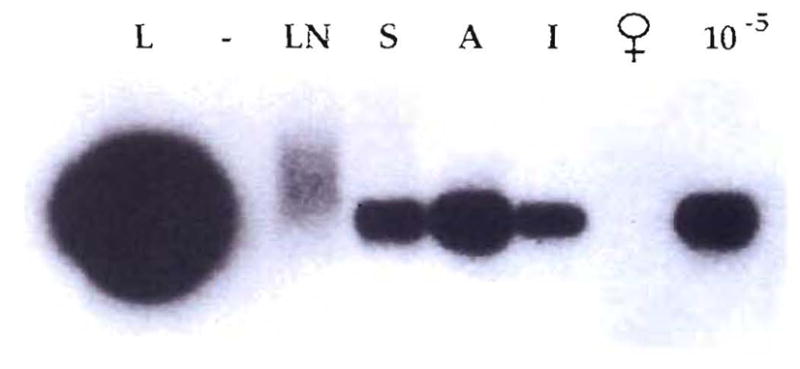
Detection of chimerism by PCR in a female recipient 12 yr after transplantation of a liver from a male donor. The samples were obtained at the time of successful retransplantation, necessitated by HCV infection of the primary graft. The DNA of the SRY region of the Y chromosome was PCR amplified, Southern blotted and hybridized with a homologous radioactive probe. Liver (L), lymph node (LN), skin (S), aorta (A) and intestine (I) of the patient were tested. DNA from an unrelated female (♀) and male DNA diluted 1: 100,000 in the female DNA (10−6) were also amplified as negative and positive controls.
Similarly, chimerism was diagnosed in the other 13 patients who were studied with only HLA detection techniques (Table 9). The availability of appropriate monoclonal antibodies made HLA phenotyping feasible in eight of these cases (Fig. 17); all eight were positive. The HLA molecular (PCR) typing showed the chimerism in all 13 cases (Fig. 18 and Table 9).
Fig. 17.
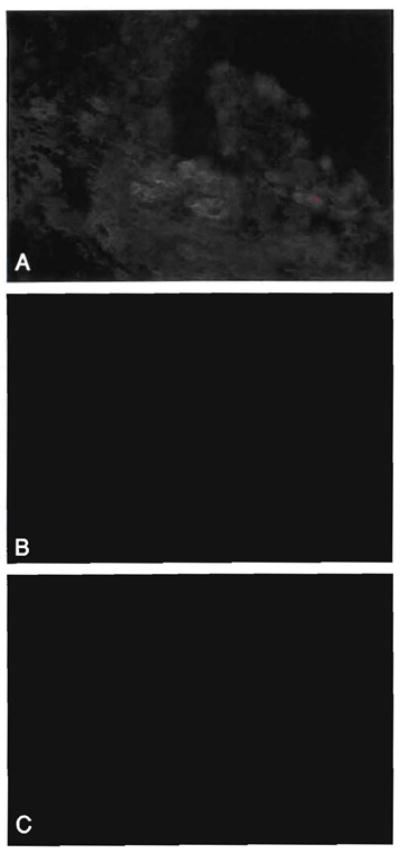
Studies of patient OT46, 21 yr after liver transplantation. An HLA-A1 (donor) antibody was used to detect donor cells in the allograft liver and in an excised inguinal lymph node. Immunofluorescence staining was used. (A) Note the staining of bile duct and endothelial cells of a mildly inflamed portal tract, whereas the inflammation is not stained. Faint sinusoidal A1 positivity can also be seen (antibodies to A1; original magnification × 400). (B) Rare A1-positive cells were also seen in the inguinal lymph node (antibodies to A1; original magnification × 400). (C) Substitution of mouse IgM for the primary antibody in the same node was negative (mouse IgM; original magnification × 400).
Fig. 18.
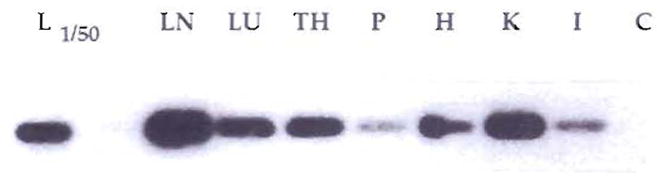
Systemic chimerism by PCR of multiple autopsy tissues of patient 77, 18.4 yr after liver transplantation; death was caused by HBV. DNA was extracted from liver (L), lymph node (LN), lung (LU), thymus (TH), pancreas (P), heart (H), kidney (K) and intestine (I) and was PCR amplified with DR4 (donor)–specific primers. A Southern blot of the amplified DNA was hybridized with a DR4 allele–specific oligonucleotide probe. In the case of liver, only 1/50 of the amplification product was used. C is the negative control of the PCR amplification performed in the absence of DNA.
Thus, all of the 22 patients had systemic tissue chimerism, which was demonstrated by one to as many as four techniques. In addition, the 65% incidence of blood chimerism with either the PCR Y probes (6 of 8; Table 8) or the HLA molecular probes (7 of 12; Table 9) was noteworthy. The ubiquitous nature of the chimerism was especially well demonstrated with the extensive tissue collections in the two patients in whose grafts recurrent viral hepatitis developed, leading to retransplantation (Table 8 and Fig. 16) or to autopsy (Table 9 and Fig. 18).
In addition to the invariable presence of systemic chimerism in these 22 patients, the studies emphasized the stability of the chimerism. Prolonged discontinuance of maintenance immunosuppression by 6 (13.6%) of the 44 patients (Tables 6, 7 and 9) had occurred. Peripheral blood lymphocytes from four of the drug-free patients and from 7 of those still on immunosuppression had normal in vitro proliferative responses to concanavalin A and phytohemagglutinin and to third-party mixed lymphocyte culture (MLR). Although donor cells were not available for MLR testing, many or most of these long survivors were thought to have stable donor-specific nonreactivity (tolerance) that was no longer drug dependent. For most of the 37 other long survivors still receiving immunosuppressive therapy, drug weaning has been started.
Thoracic Organs
In human beings (15) and rats under immunosuppression (16, 17), the thoracic viscera participate in the same cell migration process (Fig. 19). Figure 20 shows a biopsy specimen obtained on September 28, 1992, of the sigmoid colon of a female patient who had received both lungs from a male donor 3 mo previously. The male cells in the colon reflected the evolution of chimerism rather than being diagnostic of GVHD.
Fig. 19.
Lung and heart graft acceptance is also by the mechanism of cell migration and repopulation (see text). Stars represent chimeric donor cells.
Fig. 20.
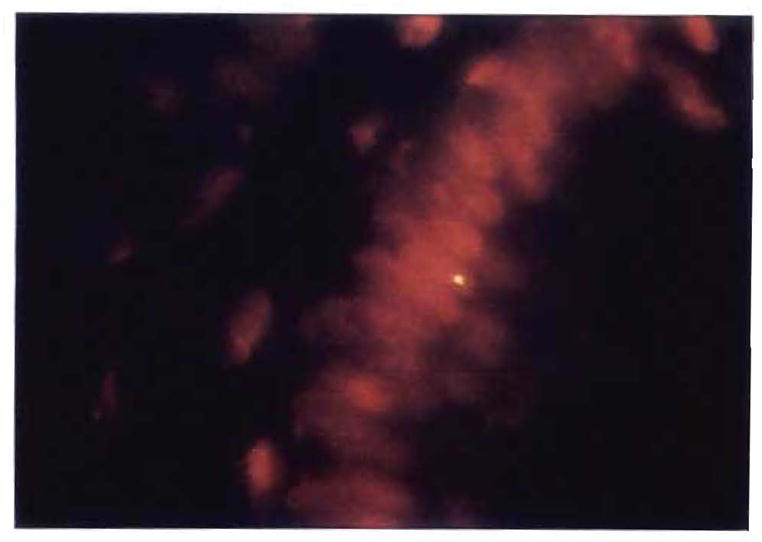
Fluorescent in situ hybridization for Y chromosome in a colon biopsy specimen of a woman who was given a lung allograft from a male donor (original magnification × 1,000). PCR analysis of the same specimen was also positive for male DNA.
Even though the responsible cell migration is common to all organs, quantitative differences have been found between the heart and lung. In untreated rats Prop and coworkers (74, 75) have shown that the leukocyte-poor heart is less vigorously rejected than the lung that contains rich bronchus-associated lymphoid tissue. However, this order of susceptibility to rejection was reversed with one or two postoperative doses of cyclosporine that often induced permanent acceptance of the lung but never of the heart. The authors explained the paradox by the greater volume of the lung’s lymphoid and dendritic cell migration in much the same context as our hypothesis of graft acceptance (5). Permanent graft acceptance after a brief induction course of FK 506 also has been shown in rats to be more difficult to achieve with the leukocyte-poor heart than with the liver (3, 4), a difference that was reflected in the disappearance histopathologically of the seeded peripheral donor cells after heart but not liver engraftment (16).
CELL TRAFFIC AND IMMUNOSUPPRESSION
Thus, cell migration is a striking phenomenon after the transplantation of all whole organs, as was first shown in detail by Häyry’s research on the kinetics of rejecting experimental kidney allografts (76). However, whether this leads to rejection or graft acceptance depends on the quality of immunosuppression, the quantity and kind of immunocytes in the organs, donor-recipient histocompatibility and perhaps multiple other factors. The donor cells departing the solid-organ graft and the recipient cells entering it include the passenger leukocytes that were suggested by Snell (77) and proved with in vivo experiments by Steinmuller (78) to be the principal cause of allograft immunogenicity. Steinman and Cohn (79–82) delineated the most important of these cells as a distinct family of bone marrow–derived antigen presenting dendritic leukocytes that are distributed throughout the body including the interstitium of the kidney, heart and other organs once thought to be nearly devoid of immunologically active cells (79, 83, 84).
The evidence implicating these antigen-presenting cells in the elicitation of primary T-cell alloimmunity (85–87) has prompted efforts to eliminate them before transplantation by a variety of means (88–91). It remains to be determined if the reduction in this way of natural graft antigenicity is beneficial and, if so, under what circumstances. The fine margin between rejection and graft acceptance was illustrated by Armstrong et al. (92), who found an association between the increased rate of dendritic cell replacement and the survival of kidney allografts transplanted to rats after they had been lightly immunized by blood transfusion from the donor strain.
Contrary to the dendritic cell–depletion approach, an alternative may be to promote, not impede, two-way cell migration while using powerful medications or other means to avoid the graft destruction, GVHD or both that are the normal and inevitable consequences of the migration without such therapeutic intervention. Viewed in this way, it does not appear to matter in principle how the immune reaction is disrupted, but only that this disruption be achieved efficiently without eliminating all of the migratory cells.
The emasculated but living cells that normally cause graft immunogenicity and rejection become instead the missionaries subserving chimerism, graft acceptance and, ultimately, tolerance. The antilymphoid agents (93, 94) and a variety of monoclonal antibodies have been particularly effective for tolerance induction in large outbred experimental animals. Figure 21 shows the steps at which some of the old and new drugs disrupt the function of the lymphocyte. This disruption can occur at the level of antigen processing (for example deoxyspergualin); at an early stage in T-cell activation, as occurs with cyclosporine and FK 506; or distal to this with rapamycin, which does not inhibit the secretion of cytokines including interleukin-2 but blocks their action. The so-called antiproliferative drugs (of which azathioprine was the prototype) work even more distally.
Fig. 21.
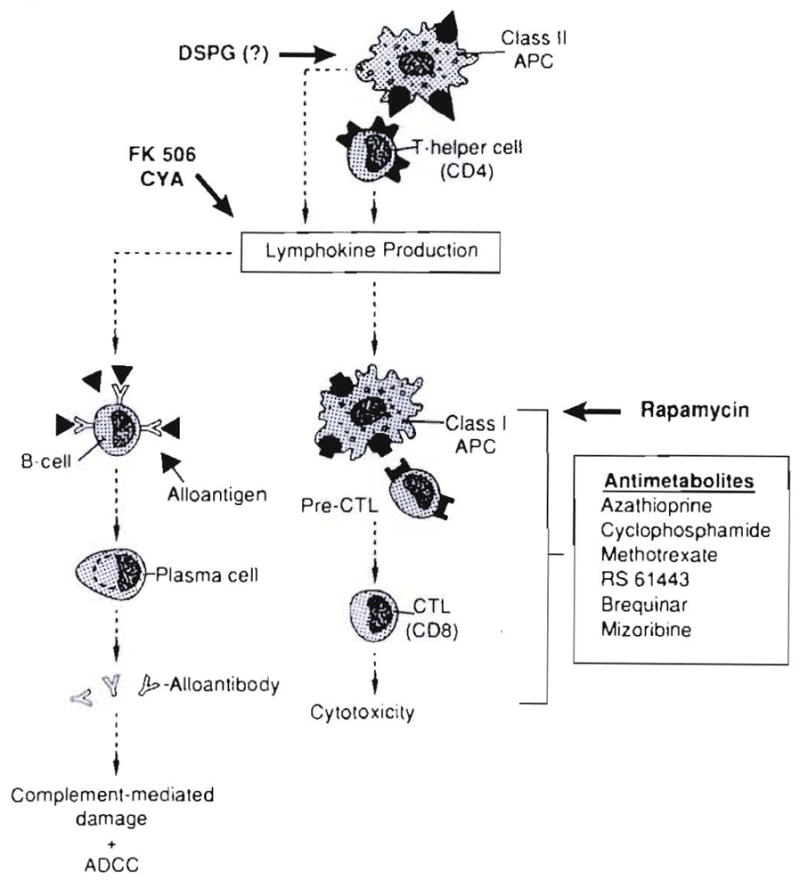
Sites of action of immunosuppressive drugs. All can induce a state of donor-specific nonreactivity if they permit cell migration and repopulation.
BACK TO THE FUTURE
The thesis that the chimerism after cell migration and repopulation is the generic explanation of graft acceptance augments the foundation of transplantation immunology that came from Medawar and his two principal associates, Billingham and Brent. If rejection was an immunological response as Medawar declared 50 yr ago (95), a logical strategy to prevent it would be to treat the recipient with agents that were known from other evidence to reduce immune reactivity. By 1951 Medawar (with Billingham and Krohn) (96) and the American Morgan (97) had taken this crucial step by showing that skin graft survival was prolonged with cortisone acetate and corticotropin. The year before, Dempster of Hammersmith Hospital, London, had demonstrated mitigation of skin graft rejection with total body irradiation (98).
The Handmaidens of Tolerance and GVHD: The In Vivo One-way MLR
Seemingly, these were small steps because the prolongation of allograft survival was limited to a few days. However, Billingham, Brent and Medawar (99) raised expectations to a new level by showing that permanent immunological tolerance to skin grafts could be acquired by inoculation of immunocompetent adult spleen cells into fetal and perinatal mice. Main and Prehn (100) were able to mimic these conditions of ontogeny in adult mice using supralethal total body irradiation followed by bone marrow alloreconstitution. When the reconstituted chimeric mice were shown in later life to be permanently tolerant to donor-strain skin, the clinical possibility of creating radiation bone marrow chimeras as a means to the end of solid-organ transplantation seemed obvious. These hopes were soon dashed when the concept was delineated by Billingham and Brent (101) that an immune-competent graft could destroy the host. This was termed GVHD or “runt disease.”
What was lost track of later was that these whole-animal models and the subsequently exploited F1 hybrid models were almost artifacts in the context of clinical whole-organ transplantation in that the interactions of the two-way cell migration and repopulation shown in Figure 22 were precluded: by the immature state of one party (the Billingham-Brent-Medawar model), by the cytoablation used by Main and Prehn (and later bone marrow transplanters) or by genetic manipulation (the parent F1 hybrid offspring model) (Fig. 23).
Fig. 22.
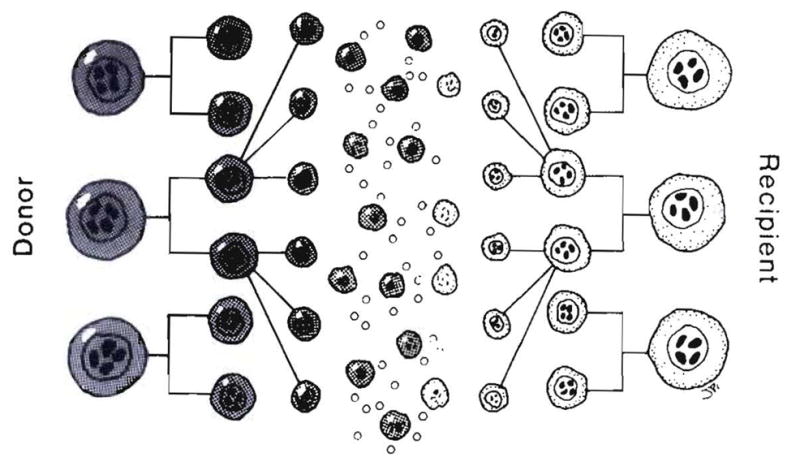
The mutual leukocyte engagement and interaction after whole-organ transplantation of cell migration. This is in essence a two-way in vivo MLR.
Fig. 23.
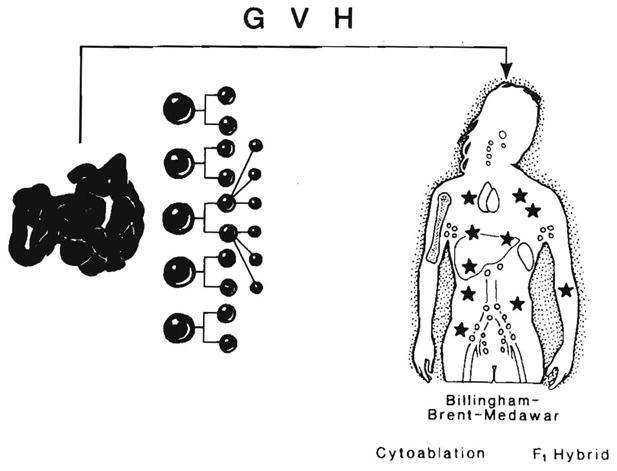
Absence of the two-way cell interaction with the removal (cytoablation) or nonreactivity (Billingham-Brent-Medawar and F1 hybrid models) of one population. These conditions are the in vivo equivalents of the one-way MLR shown in Figure 24. They are conducive to the development of GVHD. Stars represent chimeric donor cells.
These were whole-animal analogs of the one-way MLR in vitro techniques that were developed much later by Bain, Vas and Lowenstein (102) and Bach and Hirschhorn (103) (Fig. 24). The precision of the one-way in vitro systems allowed easy interpretation of results that usually showed a good correlation between histocompatibility and the immune reaction of the target lymphocytes to killed or inactivated donor lymphocytes. Because the natural phenomena of cell migration and repopulation are now known, it may be asked if the relevant in vitro (or surrogate) model of clinical transplantation for at least some purposes should be the two-way MLR in which the effect of two cell populations—each on the other—can be studied (Fig. 22).
Fig. 24.
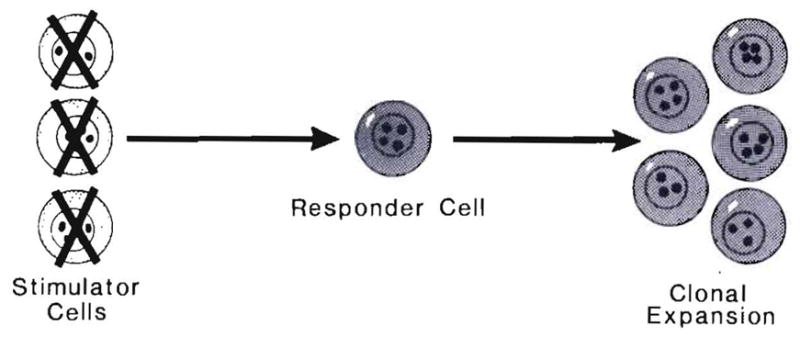
One-way MLR in which only one cell population is reactive. This is the in vitro analog of the in vivo models shown in Figure 23.
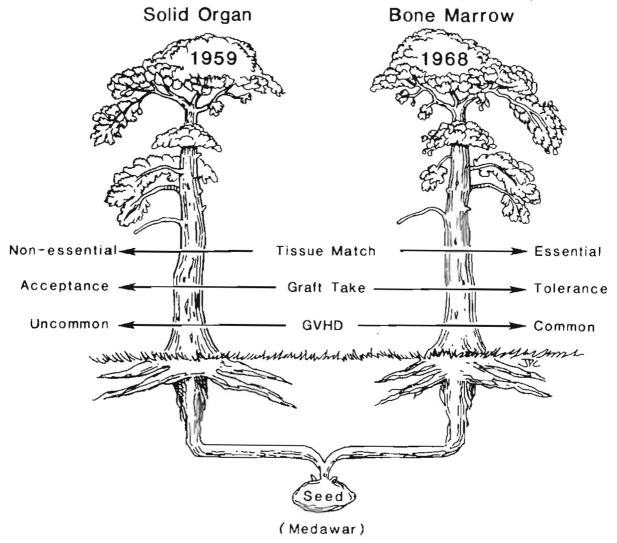
However, this is hindsight many years later. Between 1959 and 1963 and without the benefit of this information, the intellectual root that had grown from Medawar’s seed bifurcated and reached the surface looking like two separate trees (Fig. 25). The differences between what actually were branches of a common root reflected divergent therapeutic dogmas. The bone marrow branch with its precondition of cytoablation (Fig. 25, right) mimicked the Billingham-Brent-Medawar model and was the in vivo version of a one-way MLR. HLA matching was crucial. Engraftment in a drug-free state (called tolerance) was a realizable objective with perfect matching. This goal was achieved clinically in 1968 when Good’s team successfully treated a patient with severe combined immune deficiency disease (104), followed in a few months with successes by Thomas (105), Good and others for hematological disorders not characterized by immune deficiency. However, even with major histocompatibility complex compatibility, GVHD was a constant threat.
Fig. 25.
The division of transplantation into two separate disciplines by divergent therapeutic dogmas that created one-way vs. two-way in vivo MLR analogs. The policies used in bone marrow transplantation inhibited or precluded bidirectional cell migration, whereas this phenomenon was the fundamental basis for graft acceptance with the policies of whole-organ transplanters.
The reason for the virulence of the GVHD with an HLA mismatch was the complete or nearly complete removal in the cytoablated recipients of a counterweight to the transplanted immunocytes (Fig. 26). Immune modulation usually could control non–major histocompatibility complex reactions responsible for GVHD, rejection or both, and it could keep an acceptable donor-recipient immunological balance if perfect matching occurred. With significantly mismatched donors, this was not possible or required dangerous levels of immunosuppression.
Fig. 26.
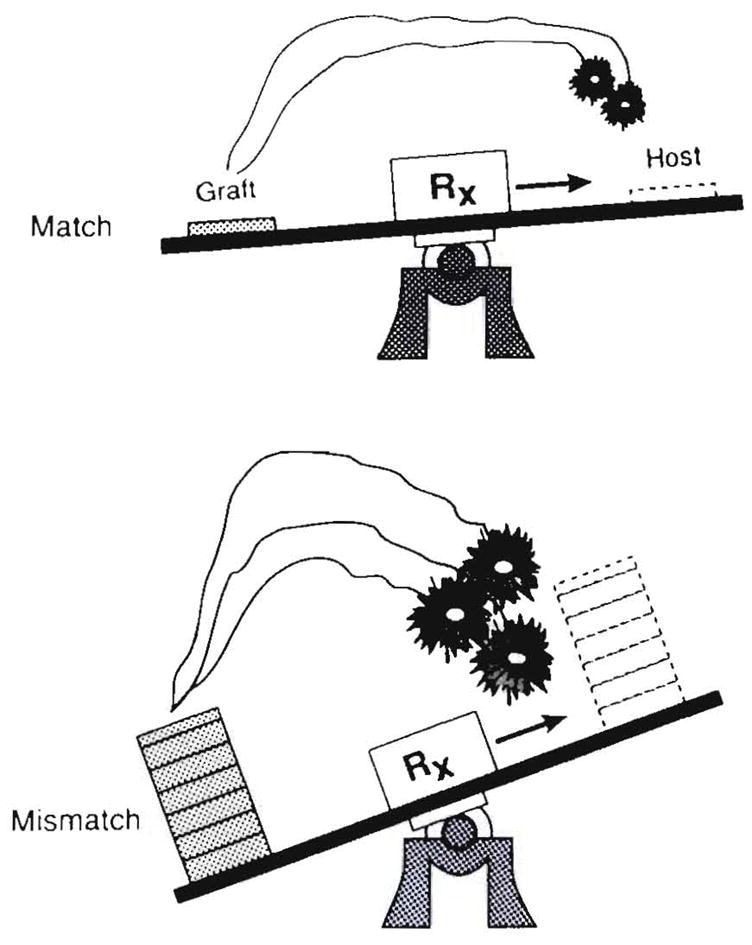
Explanation for uncontrollability of GVHD with major histocompatibility complex-mismatched donors when mutual cell engagement is prevented, thereby eliminating the element of mutual natural immunosuppression that is shown in Figure 27.
Although the concept of GVHD was developed with bone marrow and splenocyte transplantation, it was recognized early (106) that the same problem was apt to bedevil attempts to engraft lymphoid-rich whole organs such as the intestine (Fig. 23). This theory was proved by Monchik and Russell (107) in their classical intestinal transplant experiments using parent rat donors whose bowel the F1 hybrid recipients cannot recognize as alien while being subject to attack by its immunological component. The fear of GVHD generated by these results and the use of inappropriate means to prevent it discouraged efforts to transplant the human intestine for many years.
Mixed Chimerism and Whole Organs: The In Vivo Two-way MLR
By the early 1960s whole-organ transplant physicians and surgeons, who had broken ranks with their bone marrow colleagues (Fig. 25, left), empirically developed long-term immunosuppression, with which success (called graft acceptance, not tolerance) did not depend on matching and could be accomplished without GVHD—eventually extending even to the transplantation of lymphoid-rich organs such as the intestine (12, 13, 49, 108–110). The GVHD resistance now can be explained by the mutual engagement and interaction of migratory cells from the allograft with the immunocytes of the recipient (a two-way MLR) (Fig. 22) with the long survival of both (mixed chimerism). For intestinal transplantation, it finally was realized that the best strategy was not to iatrogenically unbalance the preexisting immunological apparatus of either the recipient or the graft (108, 109, 111) (Fig. 8).
The credibility of this explanation is enhanced by much earlier analogous observations in experimental bone marrow transplant models. The absence of GVHD in mixed allogeneic chimeras was observed and emphasized by Slavin et al. (112) when rat recipients of the bone marrow were conditioned with total lymphoid irradiation (TLI)–a procedure that leaves much of the host-immune system intact. GVHD resistance was demonstrated even more clearly by Ildstad and Sachs (113), who concocted various mixtures of donor and recipient bone marrow cells ex vivo and then systematically created mixed allogeneic chimeras by reconstituting cytoablated recipients with the mixtures. In both models the mixed chimeras were able to accept tissue or organ grafts from the marrow donor.
Host Vs. Graft Reactivity (Rejection) and Histocompatibility
It is unknown how the coexisting immunocyte populations in mixed chimerism come to view each other in a revised light. However, it is clear that the resulting nonreactivity accounting for GVHD resistance works the other way around, with the eventual amelioration of whole-organ rejection (host vs. graft rejection, HVG). This amelioration occurs relatively slowly under the immunosuppressive regimens in current use, even in the most successful cases. The extent and stability of the nonreactivity months or years later may not require continued immunosuppression in some recipients of the immunologically “easier” liver graft (see later) and even occasionally with the less-favorable circumstances of kidney transplantation (14, 114).
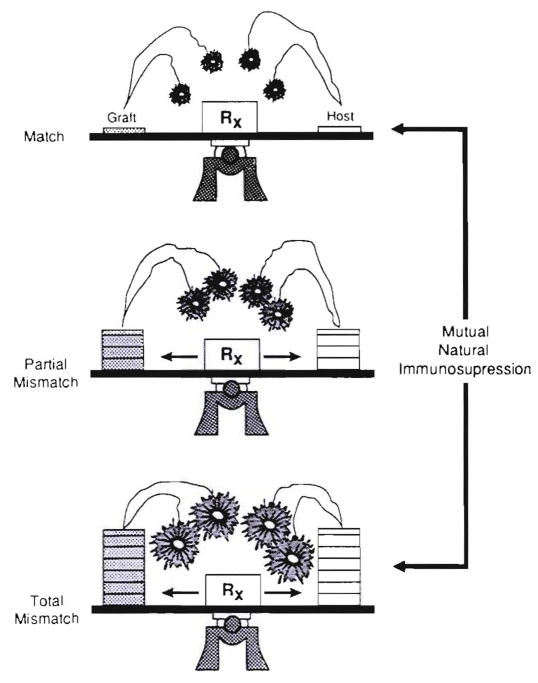
In both directions (GVH and HVG), cellular interactions that result in “mutual natural immunosuppression” are envisioned as occurring on a sliding scale in which each further level of histoincompatibility provokes countervailing, although not equal, increases in initial response (Fig. 27). If the short-term storm can be weathered long enough to allow mutual cell “accommodation,” as has been more and more possible under the protective umbrella of modern day immunosuppression, the anticipated typing effect dwindles. Such reciprocal clonal expansion followed by peripheral clonal inactivation has been called exhaustive clonal differentiation by Webb, Morris and Sprent (115). Both at the outset and in the aftermath, the number of peripheralized donor cells is small compared with the recipient leukocyte population. Figure 28 depicts a kidney with a relatively small leukocyte army of which remnants remained identifiable in recipient tissues for the lifetime of the graft (Table 2).
Fig. 27.
The reciprocal alteration of alloreactivity by coexisting immunocyte populations (mixed allogeneic chimerism). The cardinal requirement for GVHD resistance is to preserve both populations contributing to the reaction. The factor of mutual natural immunosuppression also explains the nondiscrimination of HLA matching in predicting the outcome after whole-organ transplantation (see text).
Fig. 28.
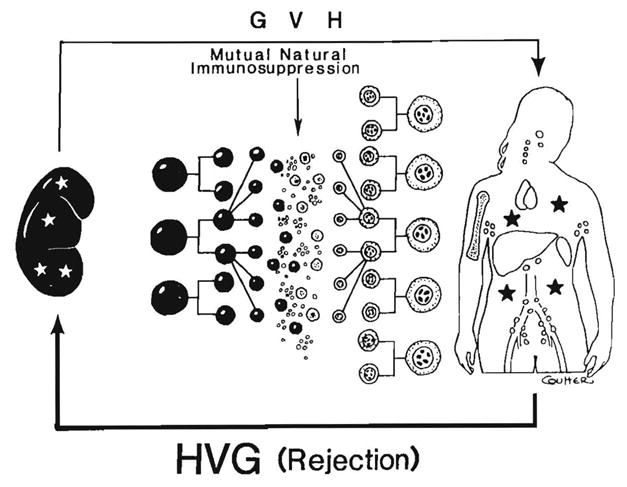
The David (kidney) and Goliath (recipient) conditions of kidney transplantation in which the graft brings a limited number of leukocytes to the mutual cell engagement. Nevertheless, the migratory donor leukocytes were demonstrated in all of the patients with continuously surviving allografts who underwent biopsies 27 to 29 yr after transplantation. Stars represent chimeric donor cells.
With this frame of reference, it is understandable why transplant surgeons have continued to report that HLA matching of the donor and recipient does not accurately predict the outcome of cadaver kidney transplantation (116–119) or of transplantation of extrarenal organs including the liver (120, 121), whereas it is universally accepted that a perfect or near-perfect match is a supreme determinant of success for bone marrow transplantation under conditions of recipient cytoablation (104, 105, 122).
Failure of Mixed Chimerism
Failure of the chimeric and recipient immunocytes to reach an immunological “truce” leads to rejection of the transplanted whole organ on one hand, to GVHD on the other or sometimes to both simultaneously. This finding has been particularly well studied after intestinal transplantation between certain rat strain combinations such as Lewis (LEW) and Brown Norway (BN) (12,46,47). In LEW rats treated daily with variable doses of FK 506 for 14 days and weekly thereafter, successful intestinal transplantation from fully allogeneic BN donors was not complicated by either rejection or by fatal GVHD (12). In contrast, with LEW to BN, rejection was difficult to control, and GVHD invariably developed when daily treatment was stopped (46, 47). Yet, the two-way lymphocyte traffic from graft-to-host lymphoid organs and vice versa was similar with either strain direction. Saat et al. (123) have described analogous findings of GVHD predisposition and rejection under cyclosporine after WAG to BN rat intestinal transplantation but not BN to WAG.
Further experiments in our laboratory have not clarified why the BN rat is an “easy” donor and “difficult” recipient (47). At a clinical level, the unresolved practical question is how to identify and avoid bad donor-recipient combinations analogous to LEW to BN, particularly when immunologically active organs such as the intestine and liver are engrafted.
With human liver transplantation, preoccupation with rejection obscured the fact until recently that the GVH reaction, which is an incipient process and a requisite for sustained engraftment in every case, can evolve to serious or fatal syndromes (124–130) in the early postoperative period. Clinically significant GVHD is observed in our liver program in approximately 5% of cases, manifesting as dermatitis. In the past, this usually was attributed to a self-limiting drug reaction or an allergic manifestation (Starzl et al., Unpublished observations, 1993).
Although most of these patients can be treated successfully with increased immunosuppression (particularly prednisone), those with extensive skin involvement, gastrointestinal symptoms and depression of the formed blood elements have a high mortality (129). The chimerism that has been documented in such patients has differed quantitatively from that seen in those after a benign convalescence. Some illustrative cases follow.
Case 1: GVHD with Lymphoproliferative Disease
On October 10, 1991, a 37-yr-old man with a neuroendocrine malignancy underwent exenteration of the native liver, pancreas, spleen, duodenum and part of the stomach, followed by liver transplantation. Control of rejection required excessive immunosuppression during the first 3 wk, including a course of OKT3. GVHD was suspected because of a skin rash after 3½ wk and was confirmed with a skin biopsy on the 34th day. Despite increases or decreases of immunosuppression, the patient deteriorated inexorably with sepsis, cardiovascular instability and multiple organ failure until his death on the 53rd day. At autopsy, donor cells were found in all recipient tissues. In addition, a widespread Epstein-Barr virus—positive polyclonal posttransplant lymphoproliferative disorder involving predominantly the liver and lymph nodes was found. The hepatic graft had an atypical portal and lobular infiltrate composed of a mixture of donor and recipient cells (60 : 40), of which most were B lymphocytes.
Case 2: GVHD Reversal with Immunosuppression
On June 4, 1992, a 34-yr-old woman with a neuroendocrine tumor had a procedure similar to that in case 1, plus pancreatic islet infusion from the same donor into the hepatic graft portal vein. She was discharged after 4½ wk but was readmitted on July 29, 1992, with bacteremia and a skin rash that on biopsy contained evidence of chimerism and GVHD including apoptosis (Fig. 29). The rash and other findings were controlled with increased immunosuppression including 1 gm prednisolone, and she was discharged 11 days later with good graft function.
Fig. 29.
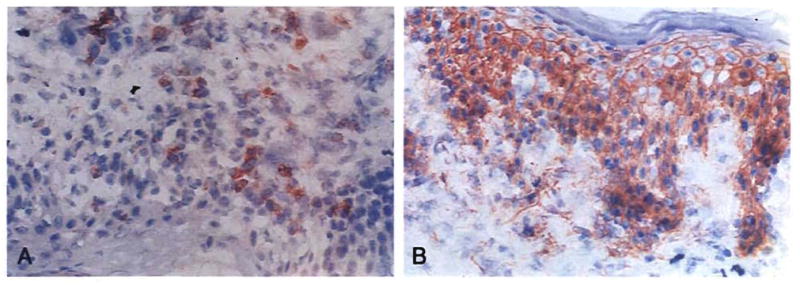
Skin biopsy specimen of a female recipient of a liver and pancreatic islets obtained from a male donor. The clinical and histopathological diagnosis was GVHD. This was easily controlled with increased immunosuppression. (A) The skin was stained for donor (Bw4) antigen, showing infiltrating cells of donor specificity. These cells also stained positive for the Y probe (not shown). (B) Stain for recipient (A3) antigen in same specimen, showing normal distribution of recipient cells (original magnification × 200).
Case 3: GVHD Reversal with Stored Autologous Bone Marrow
A 56-yr-old man with gastric leiomyosarcoma and liver metastases underwent liver transplantation on July 16, 1992, after an upper-abdominal exenteration similar to that in cases 1 and 2 but differing by the addition of a radical lymph node dissection and by preservation of the proximal pancreas and duodenum.
Just before surgery, the patient was prepared with a single dose of 5.5 Gy thoracoabdominal lymphoid irradiation followed by an intravenous dose of 19 × 109 non purged bone marrow cells. During the first postoperative week, he developed a skin rash in areas exposed to the irradiation. Although mild initially, this rash spread steadily to more than 80% of the body including the face, palms and soles with alarming desquamation and excoriation and with transient bouts of eosinophilia (2,040 mm3) and diarrhea.
The diagnosis of GVHD was made in a skin biopsy specimen on the 34th postoperative day (Fig. 30). The rash was unresponsive to either increases or decreases of immunosuppression. On August 27 and the following morning (the 42nd and 43rd postoperative days, respectively) 1.23 × 108 and 1.6 × 108 unpurged bone marrow cells per kilogram (collected and stored from before the preoperative TLI) were infused intravenously. The skin rash dramatically resolved over the next 2 wk coincident with a fall in mixed lineage donor cells in the blood from approximately 25% of total to 3% by flow cytometry. Liver function remained normal, and the patient was discharged 2 wk later.
Fig. 30.
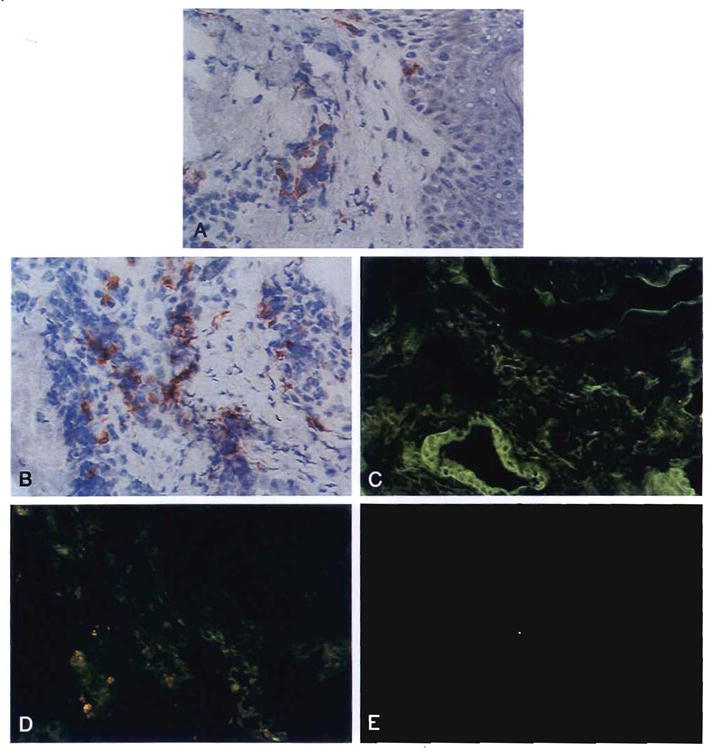
Infiltrative donor cells in the (A) epidermis and (B) adnexa in a patient with clinical GVHD 34 days after combined liver and bone marrow transplantation (antibodies to B55; original magnification × 200). (C) The GVHD was progressive and at 6 wk stored, autologous (recipient) bone marrow was infused. At 14 wk this wedge biopsy specimen of the liver showed that the biliary epithelium and endothelium were of donor origin (antibodies to B55; original magnification × 250). (D) Biliary epithelium and endothelium were negative for recipient antigens. A few positive infiltrative recipient cells were noted (antibodies to A2; original magnification × 250). (E) Scattered donor cells were detected in the lamina propria of a small bowel biopsy sample, indicating that microchimerism persisted after the GVHD was eliminated (antibodies to Bw4; original magnification × 400).
On October 13 he was readmitted with jaundice. Findings on a needle biopsy of the liver were interpreted as acute HCV. Because of an additional suspected partial biliary obstruction, the choledochojejunostomy was revised on October 22, 1992, and at reoperation another liver biopsy sample was obtained in which the histopathological diagnosis of HCV was confirmed with PCR demonstration of the viral DNA. Sparse donor cells still were present in a recipient lymph node and in a segment of bowel excised for the biliary reconstruction (Fig. 30).
After the biliary tract operation, minimum immunosuppression was given (FK 506 1 mg every other day and prednisone 5 mg/day). Interferon alfa therapy was started on October 27. The bilirubin level reached a peak of 21.9 mg/dl on October 30 but slowly declined to 2.8 mg/dl by the time the patient was discharged on November 20.
Case 4: GVHD, LPD and Recurrent HCC
On April 1, 1989, a 56-yr-old woman with micronodular cirrhosis underwent orthotopic liver transplantation from a male donor. A skin rash 5 wk later (May 6) was diagnosed initially as a drug reaction. Nine weeks after transplantation, she underwent an excisional biopsy of a cervical lymph node that showed diffuse plasmacytoid and lymphoblastic infiltration. The diagnosis was posttransplantation LPD that had both female and male cells (Fig. 31). This was treated successfully with a reduction of immunosuppression and a course of acyclovir.
Fig. 31.
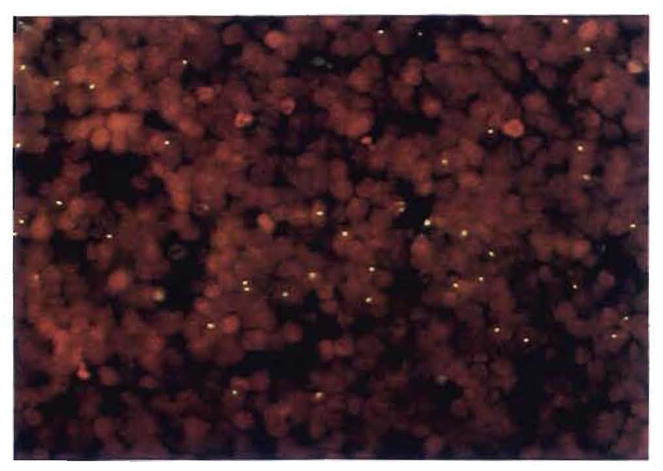
Studies in a female recipient of a male donor’s liver, who developed GVHD and a B-cell lymphoma in the first 3 postoperative mo. Note the Y chromosomes in a cervical lymph node biopsy sample containing an Epstein-Barr virus–positive posttransplant lymphoproliferative disorder (fluorescent in situ hybridization for Y chromosome; original magnification × 400). Double labeling for the Y probe and lymphocyte phenotypic markers revealed that at least one third of the donor cells were activated T cells (UCHL-1 +, not shown).
Two years later in June 1991, she was found to have an unresectable multicentric HCC in her liver allograft. The tumor did not contain cells with the Y chromosome, prompting reexamination of her cirrhotic native liver. The diagnosis was made of metastases from a previously undiagnosed HCC of the native liver, and she began chemotherapy in August 1991. The mass lesions in the liver have continued to grow, and she has developed pulmonary metastases.
Comments
In all four cases a potential donor-recipient immunological imbalance preexisted or was produced by the conditions of treatment. Although it was not measured, the loss of recipient immunological surveillance that has been associated with malignant tumors or with prior chemotherapy may have occurred. In addition, three of the four patients had lymphoid-rich upper-abdominal viscera resected, including the spleen.
In case 3, the recipient also underwent TLI and a donor bone marrow infusion. Florid multilineage blood chimerism in this patient developed during the first month, coincident with clinical GVHD. The response of the patient to treatment with autologous bone marrow was dramatic. The ability of the stored autologous marrow cells to turn off the potentially lethal GVHD may be a clue to the changes that occur during the mutual cell engagement of mixed chimerism. Although the cells used for rescue from GVHD were thought to be smaller in number than those in the circulation of the patient, they had not been exposed to donor marrow. Their therapeutic effect resembled the ability of virgin donor strain immunocytes to “break tolerance” as originally described by Billingham, Brent and Medawar (131).
Hepatic Tolerogenicity and Its Implications
That every long-surviving organ recipient in this study, whether under immunosuppression or not, was found to have chimerism is in accord with our hypothesis that cell migration and repopulation is the central mechanism of acceptance of all whole-organ grafts (5, 6). Although this is a generic process, quantitative differences exist between organs that we attribute to the variable density of the potentially migratory dendritic cells, macrophages and lymphoid collections. The heavy endowment of the liver with macrophages, Kupffer cells and dendritic cells is a particularly striking feature that invites further speculation about the role of these cells in the well-known tolerogenicity of this organ.
The immunological advantage of the liver relative to other organs includes a greater ease of inducing the acceptance of hepatic allografts or xenografts after a limited course of immunosuppression (4, 25, 132) or in swine (133–135) and some rat strain combinations (136, 137) with no treatment at all. In addition, the transplanted liver graft is relatively resistant to the preformed antigraft antibodies that cause hyperacute rejection of the kidney and heart (138–141). Another quality is its unusual ability to induce a state of unresponsiveness to other tissues and organs transplanted concomitantly or subsequently from the donor or donor strain (136, 139, 142) and even to shield these organs from the hyperacute rejection caused by preformed allospecific (143) or xenospecific (144) donor antibodies. In all of these circumstances, the liver can quickly transform the recipient environment to one more favorable for all donor tissues including itself.
These foregoing observations have been attributed to “hepatic tolerogenicity,” incorrectly we believe because the term implies that the hepatocytes are responsible. We propose that the crucial variable distinguishing the tolerogenicity of one organ graft from another under effective immunosuppression is its leukocyte (not its parenchymal) component, in a reversal of the immunogenic role described classically for these cells (77–82, 84–91). Thus, because of its dense constituency of these migratory leukocytes, the liver is high on the favorable tolerogenic list with the lung and intestine following and the kidney and heart bringing up the rear (Fig. 32). Experimental studies showing less-striking tolerogenicity of the lymphoreticular-rich spleen (145–147), intestine (12) and lung (74, 75) are compatible with this generalization.
Fig. 32.
The explanation for the variable ability under immuno-suppression to induce acceptance and ultimately tolerance of different organs. We postulate that the dendritic leukocyte is the single most important, although not the only, tolerogenic cell. The tissue content of these potentially migratory cells is liver > intestine > lung > kidney and heart. Stars represent chimeric donor cells in the recipient.
Strategies to elevate the underprivileged kidney and heart to the same level of advantage as the liver could be by the perioperative infusion of lymphoreticular cells obtained from bone marrow of the organ donor or possibly from the spleen. The strategy of concomitant infusion of bone marrow from the whole-organ donor proposed by Ildstad et al. (148) also has been advocated by Monaco et al. (149, 150) and Thomas et al. (151) for tolerance induction of kidneys and carried out clinically by Barber et al. (152). Now, the cycle is complete because this was the starting point of acquired allograft tolerance for Billingham, Brent and Medawar (99) and then Main and Prehn (100).
WHOLE-ORGAN XENOTRANSPLANTATION
When organs are transplanted from a significantly disparate species, the first immunological hurdle is that of preformed xenospecific antibodies, which quickly devascularize the graft and exclude it from recipient circulation by damaging its blood vessels (153). If this barrier can be surmounted, the process of xenograft acceptance involves the same bidirectional cell migration and consequent systemic chimerism as with allotransplantation. After hamster-to-rat xenotransplantation, the cells displaced from the xenografts can be detected in widespread rat recipient tissues with polyclonal rat-absorbed hamster leukocyte antibodies (Fig. 33) and confirmed with PCR techniques (154, 155). As with allotransplantation, the chimerism is more extensive after liver than after heart transplantation.
Fig. 33.
Hamster cells in the interstitium of the recipient heart 100 days after hamster-to-rat xenotransplantation (hamster antibody immunofluorescence; original magnification × 400). The periarterial lymphatic sheath of the spleen also contained these cells.
Chimerism was observed recently in a patient who survived for 70 days after receipt of a baboon liver (Fig. 34). Death was caused by infectious complications and by complications of biliary stasis rather than rejection or GVHD (156). This means that successful clinical xenotransplantation must be visualized along the same lines of donor-recipient cellular migration and repopulation, as with allograft acceptance.
Fig. 34.
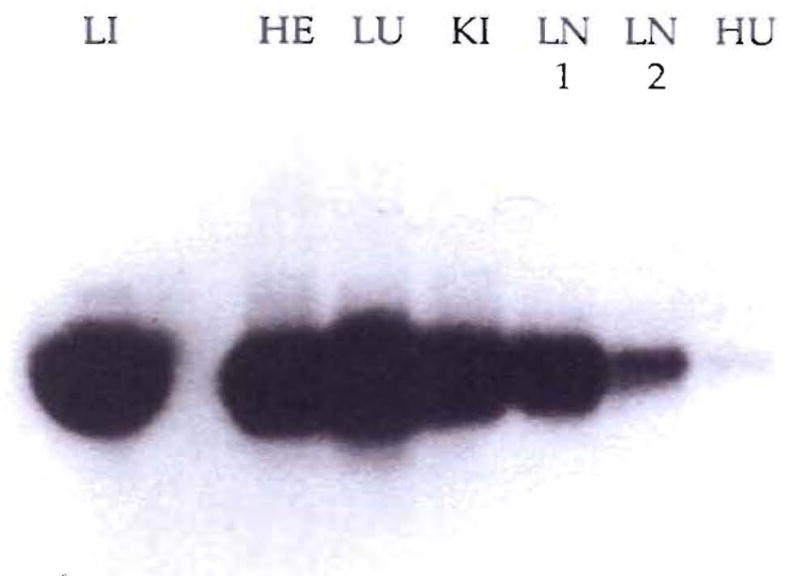
PCR amplification of a baboon-specific DNA from recipient tissues. Lane LI is the PCR product from the baboon liver and contains only 1% of the PCR reaction so that the other lanes won’t be overwhelmed (HE, heart; LU, lung; KI, kidney; LN1 and LN2, lymph nodes 1 and 2; and HU, human blood used as a negative control).
Acknowledgments
Aided by project grant no. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Starzl TE. Experience in renal transplantation. Philadelphia: WB Saunders Co; 1964. pp. 1–383. [Google Scholar]
- 2.Starzl TE, Putnam CW. Experience in hepatic transplantation. Philadelphia: WB Saunders Co; 1969. pp. 1–553. [Google Scholar]
- 3.Murase N, Kim DG, Todo S, Cramer DV, Fung JJ, Starzl TE. Suppression of allograft rejection with FK 506 I: prolonged cardiac and liver survival in rats following short course therapy. Transplantation. 1990;50:186–189. doi: 10.1097/00007890-199008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murase N, Kim DG, Todo S, Cramer DV, Fung JJ, Starzl TE. FK 506 suppression of heart and liver allograft rejection II: the induction of graft acceptance in rat. Transplantation. 1990;50:739–744. doi: 10.1097/00007890-199011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE. Cell migration and chimerism: a unifying concept in transplantation: with particular reference to HLA matching and tolerance induction. Transplant Proc. 1993;25:8–12. [PubMed] [Google Scholar]
- 7.Porter KA. Pathology of the orthotopic homograft and heterograft. In: Starzl TE, editor. Experience in hepatic transplantation. Philadelphia: WB Saunders Co; 1969. pp. 464–465. [Google Scholar]
- 8.Kashiwagi N, Porter KA, Penn I, Brettschneider L, Starzl TE. Studies of homograft sex and of gamma globulin phenotypes after orthotopic homotransplantation of the human liver. Surg Forum. 1969;20:374–376. [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE. Experience in hepatic transplantation. Philadelphia: WB Saunders Co; 1969. Candidacy; pp. 3–15. [Google Scholar]
- 10.Starzl TE. The puzzle people: memoirs of a transplant surgeon. Pittsburgh: University of Pittsburgh Press; 1992. The little drummer girls; pp. 318–333. [Google Scholar]
- 11.Starzl TE, Demetris AJ, Van Thiel DH. I. Medical progress: liver transplantation. N Engl J Med. 1989;321:1014–1022. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murase N, Demetris AJ, Matsuzaki T, Yagihasi A, Todo S, Fung J, Starzl TE. Long survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. 1991;110:87–98. [PMC free article] [PubMed] [Google Scholar]
- 13.Iwaki Y, Starzl TE, Yagihashi A, Taniwaki S, Abu-Elmagd K, Tzakis A, Fung J, et al. Replacement of donor lymphoid tissue in human small bowel transplants under FK 506 immunosuppression. Lancet. 1991;337:818–819. doi: 10.1016/0140-6736(91)92517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Demetris AJ, Trucco M, Zeevi A, Ramos H, Terasaki P, Rudert WA, et al. Chimerism and donor specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993 doi: 10.1097/00007890-199306000-00012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung JJ, Zeevi A, Kaufman C, Paradis IL, Dauber JH, Hardesty RL, Griffith B, et al. Interactions between bronchoal veolar lymphocytes and macrophages in heart-lung transplant recipients. Hum Immunol. 1985;14:287–294. doi: 10.1016/0198-8859(85)90236-8. [DOI] [PubMed] [Google Scholar]
- 16.Demetris AJ, Murase N, Starzl TE. Donor dendritic cells in grafts and host lymphoid and non-lymphoid tissues after liver and heart allotransplantation under short term immunosuppression. Lancet. 1992;339:1610. doi: 10.1016/0140-6736(92)91875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdivia LA, Demetris AJ, Langer AM, Celli S, Fung JJ, Starzl TE. Dendritic cell replacement in long-surviving liver and cardiac xenografts. Transplantation. 1993 doi: 10.1097/00007890-199308000-00048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray JE, Merrill JP, Dammin GJ, Dealy JB, Jr, Walter CW, Brooke MS, Wilson RE. Study of transplantation immunity after total body irradiation: clinical and experimental investigation. Surgery. 1960;48:272–284. [PubMed] [Google Scholar]
- 19.Murray JE, Merrill JP, Dammin GJ, Dealy JB, Jr, Alexandre GW, Harrison JH. Kidney transplantation in modified recipients. Ann Surg. 1962;156:337–355. doi: 10.1097/00000658-196209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268:1315–1323. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 21.Hamburger J, Vaysse J, Crosnier J, Auvert J, Lalanne CL, Hopper J., Jr Renal homotransplantation in man after radiation of the recipient. Am J Med. 1962;32:854–871. doi: 10.1016/0002-9343(62)90032-3. [DOI] [PubMed] [Google Scholar]
- 22.Kuss R, Legrain M, Mathe G, Nedey R, Camey H. Homologous human kidney transplantation: experience with six patients. Post grad Med J. 1962;38:528–531. doi: 10.1136/pgmj.38.443.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchioro TL, Axtell HK, LaVia MF, Waddell WR, Starzl TE. The role of adrenocortical steroids in reversing established homograft rejection. Surgery. 1964;55:412–417. [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Marchioro TL, Porter KA, Taylor PD, Faris TD, Herrmann TJ, Hlad CJ, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 26.Murray JE, Sheil AGR, Moseley R, Knight R, McGavic Dickinson J, Dammin GJ. Analysis of mechanism of immunosuppressive drugs in renal homotransplantation. Ann Surg. 1964;160:449–473. doi: 10.1097/00000658-196409000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE. Experience in renal transplantation. Philadelphia: WB Saunders Co; 1964. Host-graft adaptation; pp. 164–170. [Google Scholar]
- 28.Medawar PB. Transplantation of tissues and organs: introduction. Br Med Bull. 1965;21:97–99. [Google Scholar]
- 29.Eto M, Mayumi H, Nishimura Y, Maeda T, Yoshikai T, Nomoto K. Similarity and difference in the mechanisms of neonatally induced tolerance and cyclophosphamide-induced tolerance in mice. J Immunol. 1991;147:2439–2446. [PubMed] [Google Scholar]
- 30.Woodruff MFA, Woodruff HG. The transplantation of normal tissues: with special reference to auto- and homotransplants of thyroid and spleen in the anterior chamber of the eye, and subcutaneously, in guinea pigs. Philos Trans R Soc Lond [Biol] 1950;234:559–581. [PubMed] [Google Scholar]
- 31.Woodruff MFA. Evidence of adaptation in homografts of normal tissue. In: Medawar PB, editor. Biological problems of grafting. Oxford: Blackwell Scientific Publications; 1959. pp. 83–94. [Google Scholar]
- 32.Wilson WEC, Kirkpatrick CH. Immunologic aspects of renal homotransplantation. In: Starzl TE, editor. Experience in renal transplantation. Philadelphia: WB Saunders Co; 1964. pp. 239–261. [Google Scholar]
- 33.Lawrence HS. The transfer of hypersensitivity of the delayed type in man. In: Lawrence HS, editor. Cellular and humoral aspects of the hypersensitive states. New York: Hoeber-Harper; 1959. p. 279. [Google Scholar]
- 34.Graver B. World transplant records–1991: kidney. In: Terasaki PA, Cecka JM, editors. Clinical transplants. Los Angeles: UCLA Press; 1991. p. 431. [PubMed] [Google Scholar]
- 35.Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, Kocova M, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–77. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, Demetris AJ, Trucco M, Ricordi C, Ildstad S, Terasaki P, Murase N, et al. Chimerism after liver transplantation for type IV glycogen storage disease and type I Gaucher’s disease. N Engl J Med. 1993;328:745–749. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warburton PE, Greig GM, Haaf T, Willard HF. PCR amplification of chromosome-specific alpha satellite DNA: definition of centromeric STS markers and polymorphic analysis. Genomics. 1991;11:324–333. doi: 10.1016/0888-7543(91)90139-6. [DOI] [PubMed] [Google Scholar]
- 38.Nakagome Y, Seki S, Fukutani K, Nagafuchi S, Nakahori Y, Tamura T. PCR detection of distal Yp sequences in an XX true hermaphrodite. Am J Med Genet. 1991;41:112–114. doi: 10.1002/ajmg.1320410127. [DOI] [PubMed] [Google Scholar]
- 39.Hsia S, Tong JY, Parris GL, Nghein DD, Cottington EM, Rudert WA, Trucco M. 1992 Molecular compatibility and renal graft survival: the HLA DRB1 genotyping. Transplantation. 1993;55:395–399. doi: 10.1097/00007890-199302000-00030. [DOI] [PubMed] [Google Scholar]
- 40.Kashiwagi N. Special immunochemical studies. In: Starzl TE, editor. Experience in hepatic transplantation. Philadelphia: WB Saunders Co; 1969. pp. 394–407. [Google Scholar]
- 41.Ramsey G, Nusbacher J, Starzl TE, Lindsay GD. Isohemagglutinins of graft origin after ABO-unmatched liver transplantation. N Engl J Med. 1984;311:1167–1170. doi: 10.1056/NEJM198411013111807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies HFFS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–527. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 43.Russo C, Pellegrino MA, Ferrone S. A double-determinant immunoassay with monoclonal antibodies to the HLA-A, -B, -C complex. Transplant Proc. 1983;15:66–68. [Google Scholar]
- 44.Krangel MS. Secretion of HLA-A and -B antigens via an alternative RNA splicing pathway. J Exp Med. 1986;163:1173–1190. doi: 10.1084/jem.163.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh PB, Brown RE, Roser B. Class I transplantation antigens in solution in body fluids and in the urine. J Exp Med. 1988;168:195–211. doi: 10.1084/jem.168.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murase N, Demetris AJ, Woo J, Furuya T, Nalesnik M, Tanabe M, Todo S, et al. Lymphocyte traffic and graft-versus-host disease after fully allogeneic small bowel transplantation. Transplant Proc. 1991;23:3246–3247. [PMC free article] [PubMed] [Google Scholar]
- 47.Murase N, Demetris AJ, Woo J, Tanabe M, Furuya T, Todo S, Starzl TE. Graft versus host disease (GVHD) after BN to LEW compared to LEW to BN rat intestinal transplantation under FK 506. Transplantation. 1993;55:1–7. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnaud-Battandier F, Salmon H, Vaiman M, Revillon Y, Gallix P, Olivier M, Ricour C. Small intestinal allotransplantation in swine with cyclosporine treatment: studies of the intestinal lymphoid populations. Transplant Proc. 1985;17:1440–1441. [Google Scholar]
- 49.Grant D, Wall W, Mimeault R, Zhong R, Ghent C, Garcia B, Stiller C, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–184. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 50.Brown BI, Brown DH. Lack of an alpha-1,4-glucan:alpha-1,4-glucan 6-glycosyl transferase in a case of type IV glycogenosis. Proc Natl Acad Sci USA. 1966;56:725–729. doi: 10.1073/pnas.56.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson DH. Studies on glycogen disease with report of a case in which the glycogen was abnormal. In: Najjar VA, editor. Carbohydrate metabolism. Baltimore: Johns Hopkins Press; 1952. pp. 28–42. [Google Scholar]
- 52.Hers HG, Van Hoof F, DeBarsy T. Glycogen storage diseases. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic basis of inherited disease. 6. 1989. pp. 425–453. [Google Scholar]
- 53.Servidei S, Riepe R, Langston C, Tani LY, Bricker JT, Crisp-Lindgren N, Travers H, et al. Severe cardiopathy in branching enzyme deficiency. J Pediatr. 1987;111:51–56. doi: 10.1016/s0022-3476(87)80341-4. [DOI] [PubMed] [Google Scholar]
- 54.McMaster KR, Powers JM, Hennigar GR, Jr, Wohlbmann HJ, Farr OH., Jr Nervous system involvement in type IV glycogenosis. Arch Pathol Lab Med. 1979;103:105–111. [PubMed] [Google Scholar]
- 55.Zellweger H, Mueller S, Ionasescu V, Schochet SS, McCormick W. Glycogenosis IV: a new cause of infantile hypotonia. J Pediatr. 1972;80:842–844. doi: 10.1016/s0022-3476(72)80144-6. [DOI] [PubMed] [Google Scholar]
- 56.Selby R, Starzl TE, Yunis E, Brown BI, Kendall RS, Tzakis A. Liver transplantation for type IV glycogen storage disease. N Engl J Med. 1991;324:39–42. doi: 10.1056/NEJM199101033240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howell RR. Continuing lessons from glycogen storage diseases. N Engl J Med. 1991;324:55–56. doi: 10.1056/NEJM199101033240111. [DOI] [PubMed] [Google Scholar]
- 58.Brady RO, Kanfer JN, Bradley RM, Shapiro D. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher’s disease. J Clin Invest. 1966;45:1112–1115. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ricordi C, Ildstad ST, Demetris AJ, Abou El-Ezz AY, Murase N, Starzl TE. Donor dendritic cells repopulate ubiquitously in recipients following rat to mouse bone marrow transplantation. Lancet. 1992;339:1610–1611. doi: 10.1016/0140-6736(92)91876-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parkman R. The application of bone marrow transplantation to the treatment of genetic diseases. Science. 1986;232:1373–1378. doi: 10.1126/science.3520819. [DOI] [PubMed] [Google Scholar]
- 61.DeCerf C, Bancel B, Caillon P, Adham M, Guibaud P, Spay G, Pouyet M. Orthotopic liver transplantation for type I Gaucher’s disease. Transplantation. 1992;53:1141–1143. [PubMed] [Google Scholar]
- 62.Ferry GD, Whisennand HH, Finegold MJ, Alpert E, Globmicki A. Liver transplantation for cholesteryl ester storage disease. J Pediatr Gastroenterol Nutr. 1991;12:376–378. doi: 10.1097/00005176-199104000-00016. [DOI] [PubMed] [Google Scholar]
- 63.Daloze P, Delvin EE, Glorieux JH. Replacement therapy for inherited enzyme deficiency: liver replacement in Niemann-Pick disease type A. Am J Med Genet. 1977;1:229–239. doi: 10.1002/ajmg.1320010209. [DOI] [PubMed] [Google Scholar]
- 64.Gartner JC, Jr, Bergman I, Malatack JJ, Zitelli BJ, Jaffe R, Watkins JB, Shaw BW, Jr, et al. Progression of neurovisceral storage disease with supranuclear ophthalmoplegia following orthotopic liver transplantation. J Pediatr. 1986;77:104–106. [PMC free article] [PubMed] [Google Scholar]
- 65.Neufeld EF. Lessons from genetic disorders of lysosomes. In: Changeux JP, Hanafusa H, Deluca HF, Neufeld E, Greengard P, Samuelsson B, Tonegawa S, editors. The Harvey lectures. Series 75. New York: Academic Press; 1981. pp. 41–60. [PubMed] [Google Scholar]
- 66.Starzl TE, Bilheimer DW, Bahnson HT, Shaw BW, Jr, Hardesty RL, Griffith BP, Iwatsuki S, et al. Heart-liver transplantation in a patient with familial hypercholesterolaemia. Lancet. 1984;1:1382–1383. doi: 10.1016/s0140-6736(84)91876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bilheimer DW, Goldstein JL, Grundy SC, Starzl TE, Brown MS. Liver transplantation provides low density lipoprotein receptors and lowers plasma cholesterol in a child with homozygous familial hypercholesterolemia. N Engl J Med. 1984;311:1658–1664. doi: 10.1056/NEJM198412273112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones EA, Summerfield SA. Kupffer cells. In: Arias IM, Popper H, Schachter D, Shafritz DA, editors. The liver: biology and pathobiology. New York: Raven Press; 1982. pp. 507–523. [Google Scholar]
- 69.Groth C, Collste H, Dreborg S, Hakansson G, Lundgren G, Svennerholm L. Attempts at enzyme replacement in Gaucher disease by renal transplantation. Acta Paediatr Scand. 1979;68:475–479. [PubMed] [Google Scholar]
- 70.Desnick RJ, Simmons RL, Allen KY, Woods JE, Anderson CF, Najarian JS, Krivit W. Correction of enzymatic deficiencies by renal transplantation: Fabry’s disease. Surgery. 1972;72:203–211. [PubMed] [Google Scholar]
- 71.Starzl TE, Iwatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Starzl TE. The puzzle people: memoirs of a trans plant surgeon. Pittsburgh: University of Pittsburgh Press; 1992. pp. 1–353. [Google Scholar]
- 73.Graver B. World transplant records–1991: liver. In: Terasaki PA, Cecka JM, editors. Clinical transplants. Los Angeles: UCLA Press; 1991. p. 435. [Google Scholar]
- 74.Prop J, Kuijpers K, Petersen AH, Bartels HL, Nieuwenhuis P, Wildevuur CRH. Why are lung allografts more vigorously rejected than hearts? Heart Transplantation. 1985;4:433–436. [PubMed] [Google Scholar]
- 75.Westra AL, Prop J, Kuijpers KC, Wildevuur CRH. A paradox in heart and lung rejection. Transplantation. 1990;49:826–828. [PubMed] [Google Scholar]
- 76.Nemlander A, Soots A, Willebrand EV, Husberg B, Häyry P. Redistribution of renal allograft responding leukocytes during rejection. II. Kinetics and specificity. J Exp Med. 1982;156:1087–1100. doi: 10.1084/jem.156.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snell GD. The homograft reaction. Ann Rev Microbiol. 1957;11:439–458. doi: 10.1146/annurev.mi.11.100157.002255. [DOI] [PubMed] [Google Scholar]
- 78.Steinmuller D. Immunization with skin isografts taken from tolerant mice. Science. 1967;158:127–129. doi: 10.1126/science.158.3797.127. [DOI] [PubMed] [Google Scholar]
- 79.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139:380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell in peripheral lymphoid organs of mice. III. Functional properties in vivo. J Exp Med. 1974;139:1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 83.Hart DNJ, Fabre JW. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981;154:347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hart DNJ, McKenzie JL. Interstitial dendritic cells. Int Rev Immunol. 1990;6:128–149. doi: 10.3109/08830189009056624. [DOI] [PubMed] [Google Scholar]
- 85.Hart DNJ, Winearls CG, Fabre JW. Graft adaptation: studies on possible mechanisms in long-term surviving rat renal allografts. Transplantation. 1980;30:73–80. [PubMed] [Google Scholar]
- 86.Batchelor JR, Welsh KI, Maynard A, Burgos H. Failure of long surviving, passively enhanced allografts to provoke T-dependent alloimmunity. I. Retransplantation of (AS X AUG) F1 kidneys into secondary AS recipients. J Exp Med. 1979;150:455–464. doi: 10.1084/jem.150.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor-strain dendritic cells. J Exp Med. 1982;155:31. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Talmage DW, Dart G, Radovich J, Lafferty KJ. Activation of transplant immunity: effect of donor leukocytes on thyroid allograft rejection. Science. 1976;191:385–387. doi: 10.1126/science.1082167. [DOI] [PubMed] [Google Scholar]
- 89.Lafferty KJ, Bootes A, Dart G, Talmage DW. Effect of organ culture in the survival of thyroid allografts in mice. Transplantation. 1976;22:138–149. doi: 10.1097/00007890-197608000-00009. [DOI] [PubMed] [Google Scholar]
- 90.Faustman D, Hauptfeld V, Lacy P, Davie J. Prolongation of murine islet allograft survival by pretreatment of islets with antibody directed to Ia determinants. Proc Natl Acad Sci USA. 1981;78:5156–5159. doi: 10.1073/pnas.78.8.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Austyn JM, Steinman RM. The passenger leukocyte – a fresh look. Transplant Rev. 1988;2:139–176. [Google Scholar]
- 92.Armstrong HE, Bolton EM, McMillan I, Spencer SC, Bradley JA. Prolonged survival of actively enhanced rat renal allografts despite accelerated cellular infiltration and rapid induction of both class I and class II MHC antigens. J Exp Med. 1987;164:891–907. doi: 10.1084/jem.165.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 94.Scanfield I, Wolf JS, Wren SF, MacLean LD, Hume DM. Mechanism of permanent survival of canine renal allografts following a limited course of ALS treatment. Transplant Proc. 1973;5:533–534. [PubMed] [Google Scholar]
- 95.Medawar PB. The behavior and fate of skin autografts and skin homografts in rabbits. J Anat. 1944;78:176–199. [PMC free article] [PubMed] [Google Scholar]
- 96.Billingham RE, Krohn PL, Medawar PB. Effect of cortisone on survival of skin homografts in rabbits. Br Med J. 1951;1:1157–1163. doi: 10.1136/bmj.1.4716.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morgan JA. The influence of cortisone on the survival of homografts of skin in the rabbit. Surgery. 1951;30:506–515. [PubMed] [Google Scholar]
- 98.Dempster WJ, Lennox B, Boag JW. Prolongation of survival of skin homotransplants in the rabbit by irradiation of the host. Br J Exp Pathol. 1950;31:670–679. [PMC free article] [PubMed] [Google Scholar]
- 99.Billingham RE, Brent L, Medawar PB. “Actively acquired tolerance” of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 100.Main JM, Prehn RT. Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J Natl Cancer Inst. 1955;15:1023–1029. [PubMed] [Google Scholar]
- 101.Billingham R, Brent L. Quantitative studies on transplantation immunity. IV. Induction of tolerance in newborn mice and studies on the phenomenon of runt disease. Philos Trans R Soc Lond [Biol] 1956;242:439–477. [Google Scholar]
- 102.Bain B, Vas MR, Lowenstein L. The development of large immature mononuclear cells in mixed leukocyte cultures. Blood. 1964;23:108–116. [PubMed] [Google Scholar]
- 103.Bach F, Hirschhorn K. Lymphocyte interaction: a potential histocompatibility test in vitro. Science. 1964;143:813–814. doi: 10.1126/science.143.3608.813. [DOI] [PubMed] [Google Scholar]
- 104.Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 105.Thomas ED. Allogeneic marrow grafting: a story of man and dog. In: Terasaki PI, Cecka JM, editors. Clinical transplants. Los Angeles: UCLA Press; 1991. pp. 381–390. [Google Scholar]
- 106.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monchik GJ, Russell PS. Transplantation of the small bowel in the rat: technical and immunologic considerations. Surgery. 1971;70:693–702. [PubMed] [Google Scholar]
- 108.Starzl TE, Todo S, Tzakis A, Alessiani M, Casavilla A, Abu-Elmagd K, Fung JJ. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335–344. [PMC free article] [PubMed] [Google Scholar]
- 109.Todo S, Tzakis AG, Abu-Elmagd K, Reyes J, Nakamura K, Casavilla A, Selby R, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223–234. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goulet O, Revillon Y, Brousse N, Jan D, Canion D, Rambaud C, Cerf-Bensussan N, et al. Successful small bowel transplantation in an infant. Transplantation. 1992;53:940–943. doi: 10.1097/00007890-199204000-00046. [DOI] [PubMed] [Google Scholar]
- 111.Starzl TE, Rowe M, Todo S, Jaffe R, Tzakis A, Hoffman A, Esquivel C, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;26:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 112.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 114.Strober S, Dhillon M, Schubert M, Holm B, Englemans E, Benike C, Hoppe R, et al. Acquired immune tolerance to cadaveric renal allografts: a study of three patients treated with total lymphoid irradiation. N Eng J Med. 1989;321:28–33. doi: 10.1056/NEJM198907063210106. [DOI] [PubMed] [Google Scholar]
- 115.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 116.Matas AJ, Sutherland DER, Najarian JS. The impact of HLA matching on graft survival. Transplantation. 1992;54:568–569. doi: 10.1097/00007890-199209000-00039. [DOI] [PubMed] [Google Scholar]
- 117.Ferguson R the Transplant Information Share Group (TISG) A multicenter experience with sequential ALG/cyclosporine therapy in renal transplantation. Clin Transpl. 1988;2:285–294. [Google Scholar]
- 118.Salvatierra O., Jr Optimal use of organs for transplantation. N Engl J Med. 1988;318:1329–1331. doi: 10.1056/NEJM198805193182009. [DOI] [PubMed] [Google Scholar]
- 119.Gjertson D, Terasaki P, Takamoto S. National allocation of cadaveric kidneys by HLA matching: projected effect on outcome and costs. N Engl J Med. 1991;324:1032–1036. doi: 10.1056/NEJM199104113241505. [DOI] [PubMed] [Google Scholar]
- 120.Donaldson PT, O’Grady J, Portmann B, Davis H, Alexander GJM, Neuberger J, Thick M, et al. Evidence for an immune response to HLA class I antigens in the vanishing bile duct syndrome after liver transplantation. Lancet. 1987;1:945–948. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 121.Markus BH, Duquesnoy RJ, Gordon RD, Fung JJ, Venek M, Klintmalm G, Bryan C, et al. Histocompatibility and liver transplant outcome. Does HLA exert a dualistic effect? Transplantation. 1988;46:372–377. [PMC free article] [PubMed] [Google Scholar]
- 122.Meuwissen HJ, Gatti RA, Terasaki PI, Hong R, Good RA. Treatment of Iymphopenic hypogammaglobulinemia and bone marrow aplasia by transplantation of allogeneic marrow: crucial role of histocompatibility matching. N Engl J Med. 1969;281:691–697. doi: 10.1056/NEJM196909252811302. [DOI] [PubMed] [Google Scholar]
- 123.Saat RE, De Bruin RWF, Heineman E, Jeekel J, Marquet RL. The limited efficacy of cyclosporine in preventing rejection and graft-versus-host disease in orthotopic small bowel transplantation in rats. Transplantation. 1990;50:374–377. doi: 10.1097/00007890-199009000-00003. [DOI] [PubMed] [Google Scholar]
- 124.Burdick JF, Vogelsang GB, Smith WJ, Farmer ER, Bias WB, Kaufmann SH, Horn J, et al. Severe graft-versus-host disease in a liver transplant recipient. N Engl J Med. 1988;319:689–691. doi: 10.1056/NEJM198803173181107. [DOI] [PubMed] [Google Scholar]
- 125.Marubayashi S, Matsuzaka C. Fatal generalized acute graft versus host disease in liver transplant recipient. Transplantation. 1990;50:709–711. doi: 10.1097/00007890-199010000-00036. [DOI] [PubMed] [Google Scholar]
- 126.Bhaduri BR, Tan KC, Humphreys S, Williams R, Donaldson P, Vergani D, Mowat AP, et al. Graft-versus-host disease after orthotopic liver transplantation in a child. Transplant Proc. 1990;22:2378–2380. [PubMed] [Google Scholar]
- 127.Roberts JP, Ascher NL, Lake J, Capper J, Purohit S, Garovoy M, Lynch R, et al. Graft vs. host disease after liver transplantation in humans: a report of four cases. Hepatology. 1991;14:274–281. [PubMed] [Google Scholar]
- 128.Jamieson NV, Joysey V, Friend PJ, Marcus R, Ramsbottom S, Baglin T, Johnston PS, et al. Graft-versus-host disease in solid organ transplantation. Transpl Int. 1991;4:67–71. doi: 10.1007/BF00336399. [DOI] [PubMed] [Google Scholar]
- 129.Rosen CB, Moore SB, Batts KP, Santrach PJ, Noel P, Wiesner RH, Krom RAF. Clinical and pathological features of graft-versus-host disease after liver transplantation: a case report and review of the literature. Clin Transpl. 1993;7:52–58. [Google Scholar]
- 130.Comenzo RL, Malachowski ME, Rohrer RJ, Freeman RB, Rabson A, Berkman EM. Anomalous ABO phenotype in a child after an ABO-incompatible liver transplantation. N Engl J Med. 1992;326:867–889. doi: 10.1056/NEJM199203263261305. [DOI] [PubMed] [Google Scholar]
- 131.Billingham R, Brent L, Medawar P. Quantitative studies on tissue transplantation immunity. III. Actively acquired tolerance. Philos Trans R Soc Lond [Biol) 1956;239:357–412. doi: 10.1098/rstb.2014.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Starzl TE. Experience in hepatic transplantation. Philadelphia: WE Saunders Co; 1969. pp. 184–192.pp. 198–206. [Google Scholar]
- 133.Garnier H, Clot J, Bertrand M, Camplez P, Kumlim A, Gorim JKP, LeGoaziou F, et al. Liver transplantation in the pig: surgical approach. Cr Acad Sci Paris. 1965;260:5621–5623. [PubMed] [Google Scholar]
- 134.Peacock JH, Terblanche J. Orthotopic homotransplantation of the liver in the pig. In: Read AE, editor. The liver. London: Butterworth & Co. Ltd; 1967. pp. 333–336. [Google Scholar]
- 135.Calne RY, White HJO, Yoffa DE, Maginn RR, Binns RM, Samuel JR, Molina VP. Observations of orthotopic liver transplantation in the pig. Br Med J. 1967;2:478–480. doi: 10.1136/bmj.2.5550.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zimmerman FA, Butcher GW, Davies HS, Brons G, Kamada N, Turel O. Techniques for orthotopic liver transplantation in the rat and some studies of the immunologic responses to fully allogeneic liver grafts. Transplant Proc. 1979;11:571–577. [PubMed] [Google Scholar]
- 137.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of the multivisceral allograft in rats: a sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery. 1990;108:880–889. [PMC free article] [PubMed] [Google Scholar]
- 138.Starzl TE, Ishikawa M, Putnam CW, Porter KA, Pi cache R, Husberg BS, Halgrimson CG, et al. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129–139. [PMC free article] [PubMed] [Google Scholar]
- 139.Kamada N, Davies HFFS, Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840–842. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 140.Houssin D, Gugenheim J, Bellon B, Brunard M, Gigou M, Charra M, Crougneau S, et al. Absence of hyperacute rejection of liver allografts in hypersensitized rats. Transplant Proc. 1985;17:293–295. [Google Scholar]
- 141.Valdivia LA, Fung JJ, Demetris AJ, Starzl TE. Differential survival of hamster-to-rat liver and cardiac xenografts under FK 506 immunosuppression. Transplant Proc. 1991;23:3269–3271. [PMC free article] [PubMed] [Google Scholar]
- 142.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;233:472–474. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 143.Fung J, Makowka L, Tzakis A, Klintmalm G, Duquesnoy R, Gordon R, Todo S, et al. Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20(suppl 1):88–91. [PMC free article] [PubMed] [Google Scholar]
- 144.Valdivia L, Demetris AJ, Fung JJ, Celli S, Murase N, Starzl TE. Successful hamster to rat liver xenotransplantation under FK 506 immunosuppression induces unresponsiveness to hamster heart and skin. Transplantation. 1993;55:659–661. [PMC free article] [PubMed] [Google Scholar]
- 145.Marchioro TL, Rowlands DT, Jr, Rifkind D, Waddell WR, Starzl TE, Fudenberg H. Splenic homotransplantation. Ann NY Acad Sci. 1964;120:626–651. doi: 10.1111/j.1749-6632.1964.tb34757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wakely E, Oberholser JH, Corry RJ. Elimination of acute GVHD and prolongation of rat pancreas allograft survival with DST, cyclosporine, and spleen transplantation. Transplantation. 1990;49:241–245. doi: 10.1097/00007890-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 147.Bitter-Suermann H, Save-Soderbergh JS. The course of pancreas allografts in rats conditioned by spleen allografts. Transplantation. 1978;26:28–34. doi: 10.1097/00007890-197807010-00008. [DOI] [PubMed] [Google Scholar]
- 148.Ildstad ST, Wren SM, Oh E, Hronakes ML. Mixed allogeneic reconstitution (A + B → A) to induce donor-specific transplantation tolerance. Transplantation. 1991;51:1262–1266. doi: 10.1097/00007890-199106000-00022. [DOI] [PubMed] [Google Scholar]
- 149.Monaco AP, Wood ML. Studies on heterologous antilymphocyte serum in mice. VII. Optimal cellular antigen for induction of immunologic tolerance with ALS. Transplant Proc. 1970;2:489–496. [PubMed] [Google Scholar]
- 150.Monaco AP, Wood ML, Maki T, Gozzo JJ. Post transplantation donor-specific bone marrow transfusion in polyclonal antilymphocyte serum-treated recipients: the optimal cellular antigen for induction of unresponsiveness to organ allografts. Transplant Proc. 1988;20:1207–1212. [PubMed] [Google Scholar]
- 151.Thomas J, Carver M, Cunningham P, Park K, Gonder J. Promotion of incompatible allograft acceptance in rhesus monkeys given posttransplant antithymocyte globulin and donor bone marrow. I. In vivo parameters and immunohistologic evidence suggesting microchimerism. Transplantation. 1987;43:332–338. doi: 10.1097/00007890-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 152.Barber WH, Mankin JA, Laskow DA, Dierhoi MH, Julian BA, Curtis JJ, Diethelm AG. Long term results of a controlled prospective study with transfusion of donor-specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991;51:70–75. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 153.Starzl TE. The future of xenotransplantation. Ann Surg. 1992;216(suppl) [PMC free article] [PubMed] [Google Scholar]
- 154.Murase N, Starzl TE, Demetris AJ, Valdivia L, Tanabe M, Cramer D, Makowka L. Hamster to rat heart and liver xenotransplantation with FK506 plus antiproliferative drugs. Transplantation. 1993 doi: 10.1097/00007890-199304000-00003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Starzl TE, Murase N, Demetris AJ, Giorda R, Valdivia L, Trucco M. Drug development and testing in relation to cell migration and chimerism. Transplant Proc. 1993;25:469–472. [PMC free article] [PubMed] [Google Scholar]
- 156.Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, Doyle H, et al. Baboon to human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]