Abstract
Unloading skeletal muscle results in atrophy and weakness. Inhibition of calpain activity during unloading reduced atrophy, but the impact on force generation has not been determined. Our hypothesis was that inhibition of calpain, through muscle-specific overexpression of calpastatin, would prevent the disruption of sarcomere structure and decreased specific force (kN/m2) observed during unloading. Calpastatin-overexpressing (cp) and wild-type (wt) mice were subjected to 3, 9, or 14 days of hindlimb suspension (HS). Compared with soleus muscles of non-suspended control mice, soleus muscles of wt mice showed a 25% decline in mass after 14 days of HS while maximum isometric force (Po) decreased by 40%, resulting in a specific Po that was 35% lower than control values. Over the same time period, muscles of cp mice demonstrated 25% declines in both mass and Po but no change in specific Po. Consistent with the preservation of specific force during HS, soleus muscles of cp mice also maintained a high degree of order in sarcomere structure, in contrast to wt muscles that demonstrated misalignment of z-lines and decreased uniformity of thick filament lengths. Susceptibility to lengthening contraction-induced injury increased with the duration of HS and was not different for muscles of cp and wt mice. We conclude that inhibition of calpain activity during unloading preserves sarcomere structure such that the isometric force-generating capability is not diminished, while the effects of unloading on lengthening contraction-induced injury likely occur through calpain-independent mechanisms.
Keywords: calpastatin, isometric force, atrophy
skeletal muscle atrophy and weakness are prominent features of diseases, including cancer (30), sepsis (47), and diabetes (26), as well as during bed rest (46) and during the muscle unloading associated with travel to space (15, 45). The atrophy has been attributed to an imbalance created by factors that control protein synthesis and those that control protein degradation (36). Several proteolytic systems contribute to the turnover of muscle proteins, including the lysosomal system, the caspase system, the ubiquitin-proteasome system, and the calpain system (16). An important role for the calpain system in skeletal muscle atrophy has been implicated by several investigators (11, 33, 35), but clear conclusions have not emerged. While some reports indicated increases in calpain mRNA in skeletal muscle during unloading by hindlimb suspension (HS) (35) or spinal cord transaction (19), others showed no change in calpain mRNA with unloading by HS (34) or spaceflight (21). Regardless, mRNA levels are not indicative of protein concentrations or activity (9). Changes in calpain activity have been explored by isolating calpain from muscles following exercise or injury and incubating the protein with known substrates (1, 28), but this approach ignores both endogenous calpain inhibitors present in the muscle in vivo and the intracellular calcium concentrations critical for calpain activation. Some of the aforementioned pitfalls were circumvented in studies using transgenic mice that overexpressed calpastatin, an endogenous inhibitor of calpain (38). After 10 days of HS, the calpastatin-overexpressing mice showed reduced muscle fiber atrophy and prevention the shift to faster myosin heavy chain isoforms typical of inactivity (38).
In addition to inducing muscle atrophy, unloading also disrupts force-generating capability, which decreases muscle performance and increases susceptibility to contraction-induced injury (15, 40, 45). Decreased muscle force-generating capacity may provide an explanation for the lack of success of exercise countermeasures during muscle unloading in rats and humans (18, 40). Our preliminary studies showed weakness of muscles following a period of HS (data not shown), defined as a decrease in maximum isometric force in excess of what can be explained by atrophy, i.e., a decreased specific force (kN/m2). To elucidate the contribution of the calpain system to the development of both atrophy and weakness during unloading induced by HS, we analyzed the time course of the effects of in vivo inhibition of calpain by utilizing a line of transgenic mice with muscle-specific overexpression of calpastatin (25). Our working hypothesis is that the decreased specific force observed following the removal of weight bearing is due to the disruption of the underlying sarcomere structure by the calcium-dependent calpain system. Specifically, we hypothesized that following various periods of HS, atrophy, weakness, and susceptibility to lengthening contraction-induced injury would be less severe for muscles of transgenic calpastatin-overexpressing (cp) mice than for muscles of wild type (wt) littermates. We further hypothesized that underlying sarcomere structure would be maintained during HS in cp but not wt mice.
METHODS
All experiments were performed on 4- to 5-mo-old specific pathogen-free (SPF) male C57BL/6 mice from a colony of transgenic cp mice and wt littermates bred in house in the University of Michigan Unit for Laboratory Animal Medicine. All procedures were approved by the University of Michigan Committee for the Use and Care of Animals. A total of 26 cp and 29 wt mice were randomly assigned to one of four groups: experimental mice exposed to HS for periods of 3, 9, or 14 days and nonsuspended (control) mice that maintained normal levels of activity. During all operative procedures, mice were anesthetized with intraperitoneal injections of Avertin at a dose of 400 mg/kg tribromoethanol, with supplemental doses provided to maintain an adequate level of anesthesia to prevent response to tactile stimuli.
cp Mice
Breeding pairs of cp mice were obtained as a kind gift from Dr. Kenneth Polonsky at Washington University School of Medicine, St. Louis, MO. From these founder mice, we established an internal colony for this study. The transgene DNA was targeted to skeletal muscle using the murine muscle-specific creatine kinase promoter followed by a bovine growth hormone polyadenylation signal sequence (25).
Hindlimb Suspension
HS is a widely used method for unloading rodent hindlimbs. Our method of HS was modified from that originally described by Morey (24). Briefly, surgical tape (3M, Two Harbors, MN) was used to wrap the tail of each animal against a rigid metal wire piece with a manually bent upper hook connected to a rotating pulley. The tape was lightly soaked with liquid suture VetBond (3M) to create an instant cast. Care was taken to prevent any VetBond from dripping onto the animal's tail. During the casting process, mice were briefly restrained in a small terrycloth wrap with the tail exposed. Since the entire process of preparing the tail was completed within 2 min, no anesthetic was required during the procedure. After the tape and metal piece were attached, mice were released into a cage until the glue was dry. Once the tape hardened, the hindlegs of the mice were lifted slightly off the floor of the cage by connecting the pulley to a metal rod inside the suspension cage. The rotating pulley system enabled mice to move from one end of the cage to the other with a full 360° range of motion and obtain food and water freely. Mice were observed daily for changes in appearance and activity.
In Vitro Muscle Contractile Properties
Under deep anesthesia, soleus and extensor digitorum longus (EDL) muscles of one limb were isolated from control mice and from mice exposed to 3, 9, or 14 days of HS. Silk suture (5-0) ties were secured around the distal and proximal tendons, and the muscles were carefully removed and placed in a horizontal bath containing buffered mammalian Ringer solution (composition in mM: 137 NaCl, 24 NaHCO3, 11 glucose, 5 KCl, 2 CaCl2, 1 MgSO4, 1 NaH2PO4, and 0.025 turbocurarine chloride) maintained at 25°C and bubbled with 95% O2-5% CO2 to stabilize pH at 7.4. One tendon of the muscle was tied securely to a force transducer (model BG-50, Kulite Semiconductor Products, Leonia, NJ) and the other tendon to the lever arm of a servomotor (model 305B, Aurora Scientific, Richmond Hill, ON, Canada). The contralateral soleus and EDL muscles were removed and prepared for future analyses of protein levels and activities or sarcomere structure. After removal of the muscles, mice were euthanized with an overdose of anesthetic and administration of a bilateral pneumothorax.
For measurement of isometric contractile properties, muscles were stimulated between two stainless steel plate electrodes. The voltage of single 0.2-ms square stimulation pulses and, subsequently, muscle length (Lo) were adjusted to obtain maximal twitch force. Muscle length was measured with calipers. With the muscle held at Lo, the force developed during trains of stimulation pulses was recorded, and stimulation frequency was increased until the maximum isometric tetanic force (Po) was achieved. For EDL muscles, 300-ms trains of pulses were used, and 900-ms trains were used for soleus muscles. A stimulus frequency of 140 Hz was typically needed to achieve Po for both EDL and soleus muscles of cp mice, whereas frequencies of 120 and 130 Hz elicited Po for EDL and soleus muscles, respectively, of wt animals. For each muscle, optimum fiber length (Lf) was calculated by multiplying Lo by previously determined Lf/Lo ratios for EDL and soleus muscles of 0.45 and 0.71, respectively (6).
Following assessment of the maximum isometric tetanic forces, EDL and soleus muscles were exposed to a single constant-velocity stretch without activation (passive stretch) of 30% strain relative to Lf to obtain a measurement of the passive extension properties of the muscles. The velocity of the stretch was 2 Lf/s. The peak force achieved during the stretch was recorded. Each passive stretch was followed by a single maximum isometric contraction to verify that the stretch did not induce any injury. Following the passive stretch, each muscle was exposed to two additional 30% stretches also at 2 Lf/s initiated from the plateau of an isometric contraction at the stimulation frequency that elicited Po. Before the stretch, EDL and soleus muscles were held isometric for the first 100 or 300 ms, respectively, of each contraction to allow near-maximal activation, and stimulation was terminated at the end of the lengthening ramp. One minute of rest was allowed before the second lengthening contraction was initiated and Po was measured once again 1 min after the second lengthening contraction. The force deficit induced by the two lengthening contractions was calculated as the difference between the isometric forces measured before and after the stretches, expressed as a percentage of the force before the stretch.
After the force measurements, muscles were removed from the bath, the tendons were trimmed, and the muscle was blotted and weighed. Muscles were quick frozen in isopentane cooled with liquid nitrogen and stored at −80°C for subsequent histological analyses. Total muscle fiber cross-sectional area (CSA) was calculated by dividing the muscle mass by the product of Lf and the density of mammalian skeletal muscle, 1.06 g/cm3. Specific Po (kN/m2) was calculated by dividing Po by total fiber CSA for each muscle.
Myosin ATPase Activity
Frozen cross sections of 10-μm thickness were cut from the widest portion of the belly of each muscle. Cryosections were placed on microscope slides and incubated under various pH conditions to allow identification of type 1 and type 2 fibers on the basis of myofibrillar ATPase activity, as previously described (5). Stained sections were visualized on a microscope (Leitz Laborlux, Leica; Wetzlar, Germany) and captured with a video camera (Diagnostic Instruments; Sterling Heights, MI), and the image analyzing software ImageJ was used to calculate individual fiber CSAs. For each muscle, individual fiber CSAs were evaluated from two to three fields at 20× magnification, with each field containing ∼50 fibers. Fields were chosen randomly from the central portion of the muscle avoiding fields that reached the edge of the section. Images were analyzed from sections of n = 4–6 soleus muscles from suspended and control wt and cp mice. Consequently, single fiber areas were measured for 400–600 fibers for each experimental group.
Calpastatin Western Blot
Total protein extracts from soleus muscles were prepared by homogenizing muscles in CytoBuster Protein Extraction System and protease blocking cocktails III and VIII (Calbiochem, La Jolla, CA). Protein was resolved by SDS-PAGE and transferred to nitrocellulose membranes (Calbiochem). The membranes were blocked overnight at 4°C with 5% nonfat milk in phosphate-buffered saline containing 0.1% Tween 20. The membranes were probed with polyclonal anti-CAST (Calbiochem). Horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz BioTechnology (Santa Cruz, CA) and reagents for enhanced chemiluminescence purchased from Thermo Scientific (Rockford, IL).
Calpastatin Inhibitory Activity
Total protein extracts from soleus muscles were prepared by homogenizing muscle in CytoBuster Protein Extraction System. To verify the inhibitory capacity of the increased calpastatin protein levels, muscle homogenates from cp and wt mice soleus muscles were analyzed with the InnoZyme Calpain 1 and 2 Activity Kit from Calbiochem. This assay detects calpain activity by measuring the fluorescence produced on cleavage of a calpain-specific site on a fluorescent dye, (DABCYL)-TPLK∼SPPPSPR-(EDANS). Fluorescence was visualized using the FlexStation3 plate reader at the Center for Chemical Genomics in the Life Sciences Institute at University of Michigan.
Electron Microscopy
Soleus muscles from control mice and mice that were exposed to 14 days of HS were removed, pinned at a fixed taut length, and immediately placed in 0.1 M cacodylate-buffered Karnovsky's fixative solution (3% glutaraldahyde and 3% formaldehyde). After the tissues were fixed, each muscle was divided into three parts to allow both cross and longitudinal sections and fixed for 4 h at 40°C, pH 7.4. The muscles were then washed overnight in rinsing cacodylate buffer, postfixed in a buffered solution of 1% osmium tetroxide, and dehydrated through a graded ethanol series. Each section was propylene oxide embedded in epoxy resin and polymerized for 3 days at 450°C and 1 day at 600°C. Sections were cut on a Sorvall MT5000 ultramicrotome (DuPont, Newton, CT) and stained with 1% toluidine blue for light microscopic evaluation, and ultrathin sections were obtained by cutting the specimens with a diamond knife on the same ultramicrotome and poststaining in 1% uranyl acetate and lead citrate. These sections were examined with a Philips CM-10 transmission electron microscope (Philips Electronic Instruments, Mahwah, NJ) operating at 60 kV. Micrographs were analyzed using ImageJ to quantify the width of the A-bands through measurements of the lengths of individual thick filaments. For control mice, 20 filaments were measured from two regions of muscles from two different animals, and for mice exposed to 14 days of HS, three regions of muscles from four different mice were analyzed for a total of 640 thick filaments measured.
Statistics
All data are presented as means ± 1 SE unless indicated otherwise. The effects of genotype (wt, cp) and duration of unloading (none, 3, 9, 14 days) on muscle mass, Po, specific Po, peak force during passive stretches, force deficits following lengthening contractions, single fiber CSAs, and thick filament lengths were determined by two-factor ANOVA with a level of significance at P < 0.05. Individual differences were determined by Bonferroni post hoc analyses.
RESULTS
Phenotype of Calpastatin-Overexpressing Mice
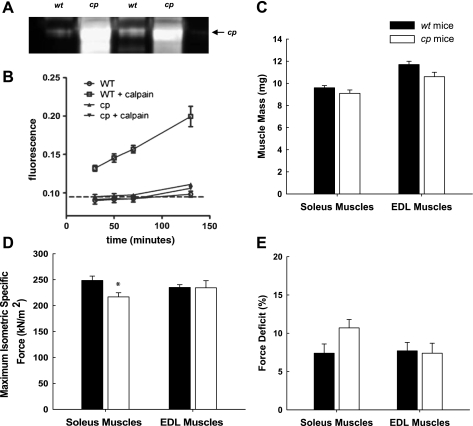
Similar to the levels of calpastatin overexpression previously reported in pooled hindlimb muscle by Polonsky's group (25), calpastatin levels were increased ∼20-fold in soleus muscles of cp compared with wt mice (Fig. 1A). The ability of this level of calpastatin overexpression in the soleus muscles to inhibit calpain activity was demonstrated using the fluorometric calpain activity assay. The sensitivity of this assay was not sufficient to detect the endogenous calpain activities in the extremely small volume of homogenates obtained from individual soleus muscles. Thus our approach was to assess the ability of endogenous calpastatin activity in the muscle homogenates to inhibit known concentrations of exogenous calpain (5 μg/ml). When exogenous calpain was added to wt muscle homogenates, cleavage of the fluorescent protein increased substantially (Fig. 1B). In contrast, the cp muscle homogenates demonstrated no calpain activity above baseline even when 5 μg/ml exogenous calpain was added to the cp muscle homogenates (Fig. 1B), indicating that the muscles of cp mice possessed substantial capacity for inhibiting calpain activity.
Fig. 1.
Characterization of calpastatin-overexpressing (cp) mice. A: confirmation by Western blot of overexpression of human calpastatin in soleus muscles of cp but not wild-type (wt) mice. B: calpain activity measurements in muscle lysates from wt (open symbols) and cp (filled symbols) mice. C: muscle mass expressed in mg. D: maximum isometric specific force expressed in kN/m2. E: deficit in isometric force measured 1 min following 2 lengthening contractions expressed as the percent decline relative to the initial force. In B, the dashed line represents the background level of fluorescence. Marked activity was detected only in wt lysates (squares) in the presence of exogenously added calpain (5 μg/ml), and this calpain activity was completely inhibited in lysates from cp mice (inverted triangles). Data are means ± SE; n = 3. All values for wt lysates with exogenous calpain were significantly (P < 0.05) above baseline as well as above the values for all other groups. Values for cp lysates were not different from baseline at any point. In C–E, bars represent means ± 1 SE for soleus and extensor digitorum longus (EDL) muscles of wt (black bars) and cp (gray bars) mice. *Significant (P < 0.05) difference between the value of cp and wt mice.
Despite the high levels of calpastatin in the muscles of cp mice, the body masses of the cp mice, 28.3 ± 0.6 g, and wt littermates, 28.5 ± 0.4 g, were not different, nor were the muscle masses for soleus or EDL muscles from control cp and wt mice (Fig. 1B). Consistent with the similarity of muscle masses between cp and wt mice, Po was not different for either soleus or EDL muscles of control cp and wt mice (data not shown). When normalized by total muscle fiber CSA, specific Po values for EDL muscles of cp and wt mice were also not different, but soleus muscles of cp mice showed a small amount of weakness as reflected in a specific Po that was just over 10% lower for muscles of cp compared with wt mice (Fig. 1C). Finally, with respect to the susceptibility to lengthening contraction-induced injury, maximally activated soleus and EDL muscles of both cp and wt mice suffered a deficit in isometric force of ∼8% following two lengthening contractions of 30% strain, with no differences between muscles or groups of mice (Fig. 1D).
Response to HS
Body mass.
All mice used in this study were weighed before HS and at the point when they were euthanized. Although most mice lost a small amount of mass during the period of HS, some mice actually showed slight gains, with no difference between the cp and wt mice. Moreover, the loss in body mass was not progressive, that is, mice suspended for 14 days did not show a greater loss of mass than those suspended for 3 or 9 days. Overall, for the 40 mice that experienced any period of HS, the average change in weight was a loss of 4.2% ± 0.8%.
Soleus muscle atrophy.
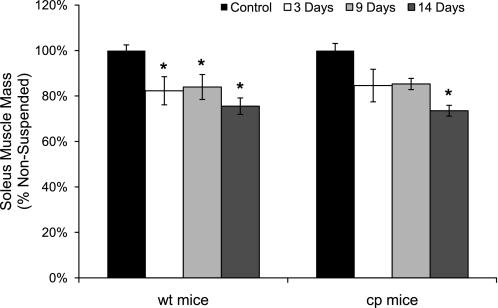
Compared with control muscles, 14 days of HS induced a decline in soleus muscle wet mass of 25% that was not different for cp and wt mice, although the decrease became statistically significant after only 3 days for muscles of wt mice compared with 14 days for cp mice (Fig. 2). Similarly, analysis of the individual single-fiber CSAs showed no overall effect of genotype on the CSA of either type 1 (P = 0.65) or type 2 (P = 0.12) fibers, either in control mice or following HS (data not shown). Analysis of the effect of HS on CSA of type 1 fibers showed a trend (P = 0.08) for a decline by day 9 and a significant decline (P = 0.02) of 30% by day 14. In contrast, while the CSAs of type 2 fibers were trending downward for soleus muscles of both cp and wt mice, the decrease in CSA compared with control muscles of ∼12% after 14 days did not reach statistical significance (P = 0.18).
Fig. 2.
Soleus muscle mass for wt and cp mice following hindlimb suspension. Data are shown for masses of soleus muscles of transgenic cp mice (right) and wt littermates (left) that were either nonsuspended (control; black bars) or exposed to 3 (white bars), 9 (light gray bars), or 14 (dark gray bars) days of hindlimb suspension. Data are expressed as a percentage of the value for muscles of the controls. Bars represent means ± 1 SE. *Significant (P < 0.05) differences from the respective values for control mice.
Soleus muscle isometric contractile properties.
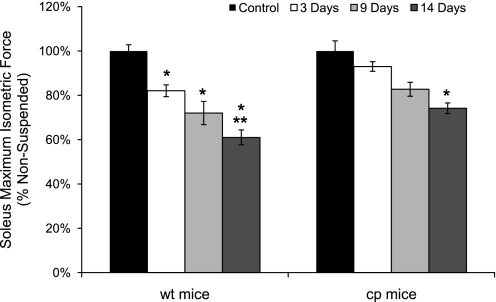
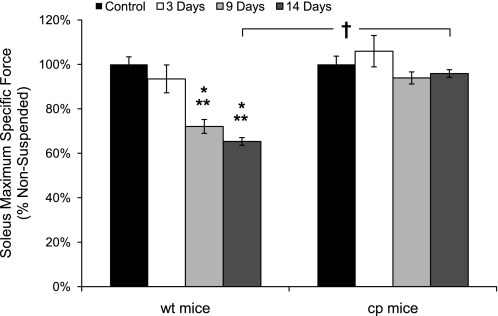
With the loss of soleus muscle mass and fiber CSA, a coincident loss in force was observed (Fig. 3). Whereas muscle mass showed only a trend for a further decrease beyond 3 days of HS for wt mice, the decrease in Po for wt mice continued to progress out to day 14, at which point Po was 40% lower than the value for soleus muscles of control wt mice. Consistent with the progressive loss of force with HS by soleus muscles of wt mice, along with no further decrease in mass after day 3, specific Po declined throughout the entire duration of HS. The 6.5% decrease in specific Po after 3 days of HS was not statistically significant, but by 9 days, specific Po was 28% lower than the value for muscles of control wt mice (Fig. 4). Specific Po continued to decline such that, after 14 days of HS, the value was decreased by 35% compared with the control value. In contrast to the profound weakness induced by HS in soleus muscles of wt mice, the specific Po for soleus muscles of cp mice exposed to HS showed no decrease compared with the value for soleus muscles of control cp mice even after 14 days of HS (Fig. 4).
Fig. 3.
Soleus muscle maximum isometric forces for wt and cp mice following hindlimb suspension. Data are shown for maximum isometric tetanic forces (Po) of soleus muscles of transgenic cp mice (right) and wt littermates (left) that were either nonsuspended (control; black bars) or exposed to 3 (white bars), 9 (light gray bars), or 14 (dark gray bars) days of hindlimb suspension. Data are expressed as a percentage of the value for muscles of the controls. Bars represent means ± 1 SE. *Significant (P < 0.05) differences from the respective values for control mice. **Significant (P < 0.05) differences from the 3-day value for mice of the same genotype.
Fig. 4.
Soleus muscle maximum isometric specific forces for wt and cp mice following hindlimb suspension. Data are shown for maximum isometric specific force (specific Po) of soleus muscles of transgenic cp mice (right) and wt littermates (left) that were either nonsuspended (control; black bars) or exposed to 3 (white bars), 9 (light gray bars), or 14 (dark gray bars) days of hindlimb suspension. Data are expressed as a percentage of the value for muscles of the controls. Bars represent means ± 1 SE. *Significant (P < 0.05) differences from the respective values for control mice. **Significant (P < 0.05) differences from the 3-day value for mice of the same genotype. †Significant (P < 0.05) difference between cp and wt mice for the same period of hindlimb suspension.
Sarcomere structure.
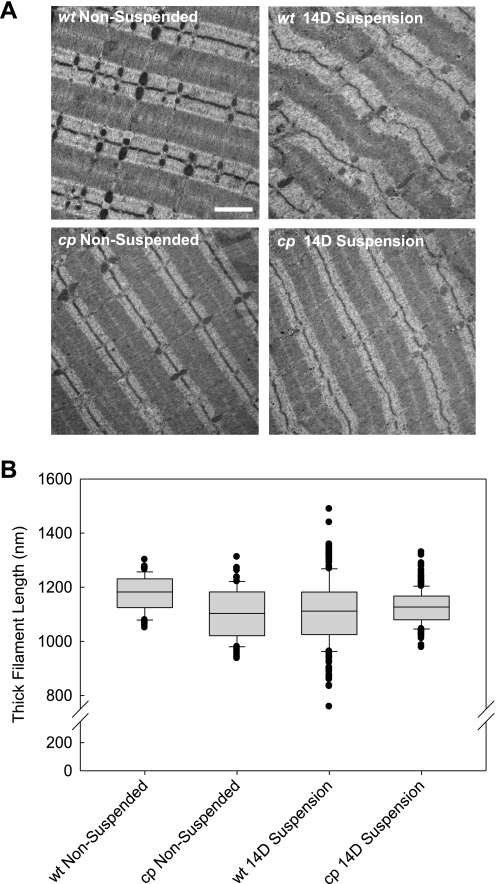
The maintenance of isometric force-generating capacity in the muscles of cp mice suggested there was preservation of the underlying myofibrillar structure in the cp mice. Therefore, sarcomere ultrastructure in muscles of cp and wt mice was examined by electron microscopy (Fig. 5A). Control muscles from both wt and cp mice demonstrated a highly ordered array of well-aligned sarcomeres with great consistency of A-band widths (Fig. 5A). Following 14 days of HS, a clear loss of the sarcomeric organization within muscles of wt mice was observed (Fig. 5A). Also clear visually was an increased variability in the width of the A-bands. In contrast, in muscles of cp mice exposed to 14 days of HS, the alignment of sarcomeres and the uniformity of the A-band widths were maintained to a much larger degree than in wt mice. These observations were supported by quantitative analysis of individual thick filament lengths (Fig. 5B). In control muscles, thick filament lengths were highly uniform and were not different for soleus muscles of wt and cp mice. Compared with the uniformity in the lengths of the thick filaments in sarcomeres of control muscles, HS resulted in a substantial increase in muscles of wt mice in the variability of the thick filament lengths (Fig. 5B). Although neither the mean nor median thick filament lengths changed for muscles of wt mice following 14 days of HS, sarcomeres contained coexisting combinations of short and long thick filaments, resulting in a doubling of the SD. In contrast, soleus muscles from the cp mice maintained roughly the same uniformity in thick filament lengths even after 14 days of HS (Fig. 5B).
Fig. 5.
Sarcomere structure for soleus muscles of nonsuspended wt and cp mice and mice exposed to 14 days of hindlimb suspension. A: representative electron micrographs (EM) from soleus muscles of wt and cp mice that were either not suspended (nonsuspended) or exposed to 14 days of hindlimb suspension (14D suspension). Scale bar = 1 μm. B: thick filament lengths measured from muscle EMs are shown in a box plot representation. The line within the boxes indicates the median, while the top and bottom edges of the box indicate 75th and 25th percentiles, respectively. The brackets above and below the boxes indicate 90th and 10th percentiles, respectively, and the additional symbols are values that fall outside the 10th to 90th percentile range. Neither means nor medians were different among the groups, but note increased variability in thick filament lengths for muscles of wt mice after hindlimb suspension.
Passive resistance to stretch.
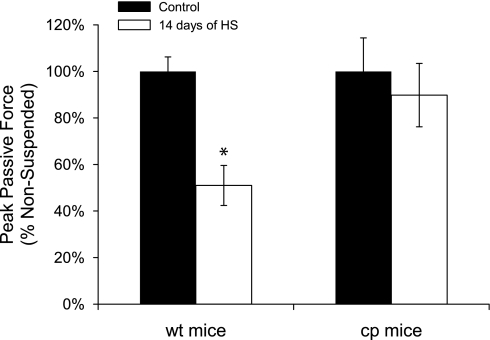
The decreased uniformity of thick filament lengths following HS for wt mice was accompanied by a substantial decrease in the resistance to passive stretch, as reflected in the lower peak force during the stretch (Fig. 6). Moreover, in cp mice, peak passive force was unaffected by HS consistent with the maintenance in these muscles of a uniformity of thick filament lengths.
Fig. 6.
Changes in passive resistance to stretch for soleus muscles of wt and cp mice following hindlimb suspension. Data are shown for the peak force during a passive stretch for soleus muscles of transgenic cp mice (right) and wt littermates (left) that were either nonsuspended (control; black bars) or exposed to 14 days of hindlimb suspension (white bars). Data are expressed as a percentage of the value for muscles of the control mice. Bars represent means ± 1 SE. *Significant (P < 0.05) difference from the control value.
Susceptibility to contraction-induced injury.
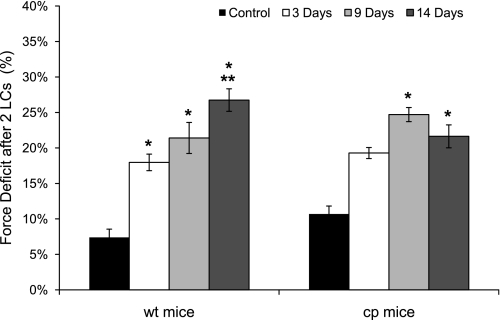
Two 30% lengthening contractions of maximally activated soleus muscles caused significant deficits in isometric force that worsened progressively after periods of HS. For wt mice, the amount of injury, as measured by the force deficit 1 min after the second lengthening contraction, increased steadily to reach a level nearly fourfold greater than that of control muscles by day 14 (Fig. 7). Soleus muscles of cp mice also demonstrated a marked increase in susceptibility to injury after HS such that after 14 days the force deficit induced by lengthening contractions for muscles of cp mice was not different from that induced in wt mice (Fig. 7).
Fig. 7.
Force deficits following lengthening contractions (LCs) of soleus muscles of wt and cp mice following hindlimb suspension. Data are shown for the deficit in isometric force induced following 2 lengthening contractions of maximally activated soleus muscles of transgenic cp mice (right) and wt littermates (left) that were either nonsuspended (control; black bars) or exposed to 3 (white bars), 9 (light gray bars), or 14 (dark gray bars) days of hindlimb suspension. Data are expressed as the difference between the initial isometric force and the force immediately following the stretches expressed as a percentage of the initial force for each group. Bars represent means ± 1 SE. *Significant (P < 0.05) differences from the value for control mice. **Significant (P < 0.05) difference from the 3-day value for mice of the same genotype.
EDL muscle mass and contractile properties.
The effects of HS on EDL muscles were minimal. In wt mice, EDL muscle mass showed a transient decrease of 20% in wt mice at day 3, but mass recovered by day 9 and remained unchanged during the remainder of the period of HS (data not shown). The small decrease in mass was not accompanied by a decrease in force generation, as neither Po nor specific Po was affected by HS. For EDL muscles of cp mice, both the mass and the contractile properties remained unchanged throughout the 14 days of HS (data not shown).
DISCUSSION
Progressive skeletal muscle atrophy and weakness are among the primary effects of skeletal muscle unloading, immobilization, and spaceflight (15, 29, 39, 45). Previous work showed that inhibition of the protease calpain, through overexpression of calpastatin in a transgenic mouse model, reduced the amount of muscle fiber atrophy in response to 10 days of HS (38), but the impact of calpain inhibition on skeletal muscle function has not previously been addressed. The major finding of the present study was that in contrast to the progressive decrease with HS in maximum isometric specific force exhibited by soleus muscles of wt mice, the development of muscle weakness was completely prevented during 14 days of HS when calpain was inhibited by increased in vivo expression of calpastatin. The protection of the muscles of cp mice from developing weakness with unloading is consistent with our hypothesis that the decreased specific force observed following the removal of weightbearing is due to the disruption by the calpain system of the underlying sarcomere structure. Further support for this hypothesis was provided by a second key finding in the present study that the maintenance of specific Po in cp mice during HS was accompanied by a striking preservation of sarcomere ultrastructure compared with the disorganization in sarcomere structure observed after 14 days of HS in wt mice. Our observation that calpain inhibition in our transgenic model did not prevent atrophy after 14 days of unloading likely reflects the longer time period of unloading in our study compared with the study of Tidball and Spencer (38) who studied mice exposed to 10 days of HS. We similarly showed no significant loss of soleus muscle mass at 9 days in cp mice as well as a 13% further loss of mass between 9 and 14 days.
Mice deficient in either of two muscle-specific ubiquitin ligases, muscle RING finger 1 (MuRF1) or muscle atrophy F-box (MAFbx, also known as atrogin-1), have also displayed an attenuation of muscle atrophy, leading to the conclusion that the ubiquitin-proteasome system is required for muscle atrophy in response to disuse (3), although those experiments employed denervation rather than HS to trigger atrophy in the muscles. The proteasome has been shown to degrade purified actin and myosin, but specific interactions between myofibrillar proteins appear to protect intact myofibrils from ubiquitin-dependent degradation, with the rate-limiting step in the degradation of myofibrillar proteins proposed to be their dissociation from the myofibril (32). One hypothesis is that cleavage of sarcomeric proteins by calpain may be necessary to provide substrate for the ubiquitin-proteasome system (22). An important role of calpain in cytoskeletal remodeling during the early phase of the muscle response to alterations in loading is supported by several investigations (11, 43), and the preservation in the present study of sarcomere structure and specific Po under circumstances when calpain was inhibited during unloading provides strong support for the conclusion that calpain targets the force-generating infrastructure during disuse. Despite substantial support for the coordinated action of calpain and the ubiquitin-proteasome system to mediate muscle atrophy in response to unloading, the present finding that inhibition of calpain during 14 days of HS did not prevent atrophy coupled with the findings that following 14 days of denervation, muscle mass decreased 30% and 20% in mice deficient in MuRF1 or MAFbx, respectively (3), suggests that the inhibition or even prevention of protein degradation may be insufficient to protect muscle completely from a loss in mass during unloading. Alternate or additional approaches targeting the enhancement of protein synthesis and muscle growth (4) rather than the inhibition of protein degradation may be necessary to fully counteract the atrophy associated with disuse.
One known calpain substrate and hypothesized in vivo target of calpain is the giant sarcomeric protein titin (16, 17, 23, 27, 42), which contains high-affinity calpain binding sites where the protein is cleaved (27). Titin is well recognized as the main determinant within muscle fibers of passive tension (14, 20) and may serve as a regulator of thick filament length during sarcomere assembly (41, 44). Recently, Udaka and colleagues (42) reported that muscle disuse, achieved through immobilization of a limb by casting, induced changes within sarcomeres that were correlated with the proteolytic cleavage of titin. Among the responses to immobilization that were associated with a preferential loss of titin were decreased mean thick filament length, decreased uniformity of thick filament length, decreased maximum isometric specific force, and decreased passive force (42). We show here strong evidence that the changes in passive force and increased variability of thick filament length can be prevented in muscles where calpain activity is inhibited, providing in vivo evidence for the role of calpain-mediated protein cleavage in these functional changes that occur during HS. Furthermore, the maintenance of passive tension and the preservation of the uniformity in thick filaments lengths for muscles of cp mice, in combination with the results by Udaka and colleagues, are consistent with the hypothesis that calpain targets titin and the inhibition of calpain may protect against loss in titin function, at least to some extent. An additional calpain substrate that may be targeted during HS is the intermediate filament protein desmin (12). Desmin functions to maintain sarcomeres within adjacent myofibrils in register, and deficiency of desmin results in muscle weakness (31). Our electron micrographs showed a loss following HS of the alignment of sarcomeres in the muscles of wt mice along with the substantial reduction in specific Po. The observation that, in cp mice, specific Po was unaffected by HS and sarcomere alignment was maintained is consistent with the hypothesis that additional proteins responsible for sarcomere integrity, such as desmin, may also be spared by the inhibition of calpain. Collectively, these findings provide evidence for a model in which muscle unloading increases in vivo calpain-mediated cleavage of sarcomere proteins, possibly titin and desmin, leading to alterations within the sarcomere that decrease force-generating capacity.
A surprising observation in the present study was that, although muscles of cp mice maintained specific force during HS, the muscles of cp mice were not protected to any great extent from the increased susceptibility to lengthening contraction-induced injury observed during HS. In many disease states such as muscular dystrophy and aging, the increased susceptibility to contraction-induced injury is tightly associated with muscle weakness (7, 8). Our results showed that the preservation of sarcomeric structure and force production afforded by the inhibition of calpain during unloading was not accompanied by maintenance of the ability to withstand the additional stress associated with lengthening contractions. These data support the conclusion that the increased susceptibility to contraction-induced injury and muscle weakness induced by HS are likely occurring through distinct cellular mechanisms and the effect of HS to increase contraction-induced injury is not mediated by calpain-dependent proteolytic activity.
Our data substantiate the common finding that the EDL muscle, which is comprised primarily of type 2 fibers, is minimally affected by unloading (10, 13, 37). Although it is generally accepted that fast muscles are less sensitive to disuse-induced atrophy than slow muscles (2), the mechanisms underlying these differences between fast and slow muscles remain an area of active investigation. Fast muscles may be less susceptible than slow muscles to HS due to differential activation of downstream cellular responses to HS in type 1 and type 2 fibers. Previous work demonstrated that slow soleus muscles exhibited increased calpain activation during atrophy within 12 h of HS, whereas a significant increase in calpain activity was not observed in fast gastrocnemius muscles until the muscles had been unloaded for more than 3 days (11). Moreover, 5 days of hindlimb immobilization resulted in increased levels of both autolyzed calpain 1 and autolyzed calpain 2 in soleus muscles but no change in either calpain 1 or 2 in fast plantaris muscles (43). Thus the sensitivity of type 1 fibers to disuse atrophy may be related to fiber-type specificity of the activation pattern of calpain following HS. Finally, the lack of atrophy of EDL muscles during HS may be a consequence of the small amount of loading of the muscles provided by flexing the ankle and lifting the hindpaws against gravity, rather than a complete insensitivity of type 2 fibers to disuse atrophy. Regardless of the precise mechanisms for the different response of slow and fast muscles, our studies demonstrate that if activation of calpain is inhibited in slow muscle, many of the effects of HS on muscle contractile function and sarcomeric structure can be prevented.
In summary, this study provides clear evidence that inhibition of the calpain system during HS preserves sarcomere structure and prevents the development of muscle weakness in soleus muscles. These findings support our working hypothesis that the decreased specific force observed following the removal of weightbearing is due to the disruption of the underlying sarcomere structure by calpains. Protection from sarcomere disruption by the inhibition of calpain, perhaps through the inhibition of cleavage of sarcomeric proteins such as titin, is also supported by our observation of the maintenance of passive tension and uniformity of thick filament lengths in soleus muscles of cp mice exposed to 14 days of HS. Finally, our findings that the loss of soleus muscle mass associated with unloading was not prevented in the cp mice and that the risk for disruption of the myofibrillar structure when challenged with lengthening contractions was substantially elevated by unloading in cp mice suggest that inhibition of the calpain system is not sufficient to completely protect muscles from the structural and functional deficits associated with disuse. Thus calpain inhibition may be an important and effective target for preventing muscle weakness during disuse, by minimizing proteolytic damage to the force-generating apparatus, especially in combination with additional pharmacological or physical therapies aimed at preventing muscle atrophy and injury.
GRANTS
Financial support for the work was provided by National Aeronautics and Space Administration Grant NNC04AA21A to S. V. Brooks and National Institutes of Health (NIH) Grant R01-HL-080388 to D. E. Michele. Fellowship support to J. J. Salazar was provided by NIH Grants T32-GM-008322 and T90-DK-070071 and the Michigan Space Grant Consortium.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Cheryl Hassett, Jonathan Gumucio, and Ajit Gogawale for help with data collection.
REFERENCES
- 1. Arthur GD, Booker TS, Belcastro AN. Exercise promotes a subcellular redistribution of calcium-stimulated protease activity in striated muscle. Can J Physiol Pharmacol 77: 42–47, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Baldwin KM, Haddad F. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil 81: S40–S51, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol 23: 369–379, 1970 [DOI] [PubMed] [Google Scholar]
- 6. Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol 497: 573–580, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Consolino CM, Brooks SV. Susceptibility to sarcomere injury induced by single stretches of maximally activated muscles of mdx mice. J Appl Physiol 96: 633–638, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev 71: 813–847, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Darr KC, Schultz E. Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J Appl Physiol 67: 1827–1834, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Enns DL, Belcastro AN. Early activation and redistribution of calpain activity in skeletal muscle during hindlimb unweighting and reweighting. Can J Physiol Pharmacol 84: 601–609, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Enns DL, Raastad T, Ugelstad I, Belcastro AN. Calpain/calpastatin activities and substrate depletion patterns during hindlimb unweighting and reweighting in skeletal muscle. Eur J Appl Physiol 100: 445–455, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Fitts RH, Riley DR, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204: 3201–3208, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Funatsu T, Higuchi H, Ishiwata S. Elastic filaments in skeletal muscle revealed by selective removal of thin filaments with plasma gelsolin. J Cell Biol 110: 53–62, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gardetto PR, Schluter JM, Fitts RH. Contractile function of single muscle fibers after hindlimb suspension. J Appl Physiol 66: 2739–2749, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci 86: E19–E35, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Goto K, Okuyama R, Honda M, Uchida H, Akema T, Ohira Y, Yoshioka T. Profiles of connectin (titin) in atrophied soleus muscle induced by unloading of rats. J Appl Physiol 94: 897–902, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Haddad F, Adams GR, Bodell PW, Baldwin KM. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J Appl Physiol 100: 433–441, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM. Atrophy responses to muscle inactivity. II. Molecular markers of protein deficits. J Appl Physiol 95: 791–802, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Horowits R, Kempner ES, Bisher ME, Podolsky RJ. A physiological role for titin and nebulin in skeletal muscle. Nature 323: 160–164, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Ikemoto M, Nikawa T, Takeda S, Watanabe C, Kitano T, Baldwin KM, Izumi R, Nonaka I, Towatari T, Teshima S, Rokutan K, Kishi K. Space shuttle flight (STS-90) enhances degradation of rat myosin heavy chain in association with activation of ubiquitin-proteasome pathway. FASEB J 15: 1279–1281, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Kandarian SC, Stevenson EJ. Molecular events in skeletal muscle during disuse atrophy. Exerc Sport Sci Rev 30: 111–116, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Lim CC, Zuppinger C, Guo X, Kuster GM, Helmes M, Eppenberger HM, Suter TM, Liao R, Sawyer DB. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem 279: 8290–8299, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Morey ER. Spaceflight and bone turnover: correlation with a new rat model of weightlessness. Bioscience 29: 168–172, 1979 [Google Scholar]
- 25. Otani K, Han DH, Ford EL, Garcia-Roves PM, Ye H, Horikawa Y, Bell GI, Holloszy JO, Polonsky KS. Calpain system regulates muscle mass and glucose transporter GLUT4 turnover. J Biol Chem 279: 20915–20920, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Price SR, Mitch WE. Mechanisms stimulating protein degradation to cause muscle atrophy. Curr Opin Clin Nutr Metab Care 1: 79–83, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Raynaud F, Fernandez E, Coulis G, Aubry L, Vignon X, Bleimling N, Gautel M, Benyamin Y, Ouali A. Calpain 1-titin interactions concentrate calpain 1 in the Z-band edges and in the N2-line region within the skeletal myofibril. FEBS J 272: 2578–2590, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Reid WD, Belcastro AN. Time course of diaphragm injury and calpain activity during resistive loading. Am J Respir Crit Care Med 162: 1801–1806, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Riley DA, Thompson JL, Krippendorf BB, Slocum GR. Review of spaceflight and hindlimb suspension unloading induced sarcomere damage and repair. Basic Appl Myol 5: 139–145, 1995 [PubMed] [Google Scholar]
- 30. Russell ST, Siren PM, Siren MJ, Tisdale MJ. Attenuation of skeletal muscle atrophy in cancer cachexia by d-myo-inositol 1,2,6-triphosphate. Cancer Chemother Pharmacol 64: 517–527, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Sam M, Shah S, Friden J, Milner DJ, Capetanaki Y, Lieber RL. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am J Physiol Cell Physiol 279: C1116–C1122, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271: 26690–26697, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Spencer MJ, Lu B, Tidball JG. Calpain II expression is increased by changes in mechanical loading of muscle in vivo. J Cell Biochem 64: 55–66, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol 551: 33–48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taillandier D, Aurousseau E, Meynial-Denis D, Bechet D, Ferrara M, Cottin P, Ducastaing A, Bigard X, Guezennec CY, Schmid HP. Coordinate activation of lysosomal, Ca2+-activated and ATP-ubiquitin-dependent proteinases in the unweighted rat soleus muscle. Biochem J 316: 65–72, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thomason DB, Biggs RB, Booth FW. Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Physiol Regul Integr Comp Physiol 257: R300–R305, 1989 [DOI] [PubMed] [Google Scholar]
- 37. Thompson LV, Shoeman JA. Contractile function of single muscle fibers after hindlimb unweighting in aged rats. J Appl Physiol 84: 229–235, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Tidball JG, Spencer MJ. Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J Physiol 545: 819–828, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tischler ME, Henriksen EJ, Munoz KA, Stump CS, Woodman CR, Kirby CR. Spaceflight on STS-48 and earth-based unweighting produce similar effects on skeletal muscle of young rats. J Appl Physiol 74: 2161–2165, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Trappe S, Costill D, Gallagher P, Creer A, Peters JR, Evans H, Riley DA, Fitts RH. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J Appl Physiol 106: 1159–1168, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Trinick J. Titin and nebulin: protein rulers in muscle? Trends Biochem Sci 19: 405–409, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Udaka J, Ohmori S, Terui T, Ohtsuki I, Ishiwata S, Kurihara S, Fukuda N. Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. J Gen Physiol 131: 33–41, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vermaelen M, Sirvent P, Raynaud F, Astier C, Mercier J, Lacampagne A, Cazorla O. Differential localization of autolyzed calpains 1 and 2 in slow and fast skeletal muscles in the early phase of atrophy. Am J Physiol Cell Physiol 292: C1723–C1731, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Whiting A, Wardale J, Trinick J. Does titin regulate the length of muscle thick filaments? J Mol Biol 205: 263–268, 1989 [DOI] [PubMed] [Google Scholar]
- 45. Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol 516: 915–930, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Widrick JJ, Romatowski JG, Bain JL, Trappe SW, Trappe TA, Thompson JL, Costill DL, Riley DA, Fitts RH. Effect of 17 days of bed rest on peak isometric force and unloaded shortening velocity of human soleus fibers. Am J Physiol Cell Physiol 273: C1690–C1699, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Williams AB, Decourten-Myers GM, Fischer JE, Luo G, Sun X, Hasselgren PO. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. FASEB J 13: 1435–1443, 1999 [DOI] [PubMed] [Google Scholar]