Abstract
Background
Increasing serum levels of N-terminal pro-hormone brain natriuretic peptide (NT-proBNP) are associated with worsening heart failure (HF) in adults. We determined whether changes in NT-proBNP level are associated with changes in symptoms and left ventricular (LV) systolic function and remodeling in children with HF secondary to dilated cardiomyopathy.
Methods
We retrospectively examined associations between serum NT-proBNP levels and NYHA/Ross functional class, LV systolic and diastolic diameter (LVSD-z and LVDD-z), LV ejection fraction (LVEF), and LV shortening fraction (LVSF-z) using generalized linear mixed models. Fluctuation in functional class of subjects was also modeled using logistic regression and receiver operating characteristic (ROC) curves.
Results
In 36 children (14 males), a 10-fold increase in NT-proBNP serum levels was associated (P<0.001) with a 9.8% decrease in LVEF, a 3.25-unit drop in LVSF-z, a 1.53-unit increase in LVDD-z, a 2.64-unit increase in LVSD-z, and an increased odds of being in functional class III/IV (OR 85.5; 95% CI, 10.9 to 671.0). An NT-proBNP level greater than 1000 pg/mL identified children constantly or intermittently in functional class III-IV with 95% sensitivity and 80% specificity. The reliability of a single NT-proBNP value was 0.61, but the means for two and three NT-proBNP values were 0.76 and 0.82, respectively.
Conclusions
In children with HF, NT-proBNP is associated with cardiac symptoms and indices of LV systolic dysfunction and remodeling. NT-proBNP >1000 pg/mL identifies highly symptomatic children. Within subject serial measurements of NT-proBNP are needed for a reliable and accurate determination of disease status and/or course.
INTRODUCTION
Brain-natriuretic peptide (BNP) is an excellent marker of heart failure (HF) in adults (1–4). Serum levels can help differentiate dyspnea caused by respiratory problems from HF (1), correlate with the severity of left ventricular (LV) dysfunction (1,2) and functional status (1), predict morbidity and mortality (3), and can guide medical treatment (4). However, the value of measuring serum levels of brain-natriuretic peptide in children with HF still needs further study before it can be recommended for clinical use. In children and adolescents, BNP level predicts outcome in those admitted to cardiac intensive care units (5), is a marker for rejection in cardiac transplant patients (6), correlates with the symptoms of HF (7), may indicate the presence of LV volume and pressure overload in the presence of a shunt (8), and may identify marked cardiac disease in acute care settings (9,10). In spite of the evidence of its diverse use, natriuretic peptide measurements are not commonly part of the routine testing performed in children with cardiac disease because relatively little is known about their function, accuracy and validity as a diagnostic test in children (10,11).
We have studied cardiac biomarkers for several years (7,12–15). In a cross-sectional study, a single NT-proBNP level was associated with clinical severity, LV systolic dysfunction, and LV dilation in children with HF (7). We now hypothesize that changes in NT-proBNP serum levels are associated with changes in echocardiographic indices of LV systolic function and LV remodeling, as well as with symptoms in children or young adults with HF related to dilated cardiomyopathy.
METHODS
Patients
We retrospectively reviewed all the NT-proBNP levels measured in our children with HF secondary to dilated cardiomyopathy between October 2003, when we began monitoring this biomarker in HF patients, and July 2008. All children were followed in the pediatric HF program at the Holtz Children’s Hospital and University of Miami’s Children’s Heart Center. Our institution is a regional referral center and is the only pediatric HF-transplant program in South Florida. The patients in this study are representative of the pediatric HF population. The study was approved by the University of Miami Institutional Review Board.
All children were followed by a single cardiologist and received the University of Miami standard of care for children with HF, which consists of angiotensin-converting enzyme inhibitors, beta-blockers, diuretics, and digoxin.
Data Collection
Patient charts were reviewed for information on age, sex, weight, cause of HF, medications, functional class, echocardiographic data, and serum NT-proBNP measurements taken during regular clinic visits. The number of NT-proBNP measurements per person varied, and was primarily a function of clinical status. NT-proBNP was measured at each clinic visit or, if the patient was hospitalized, at the time of admission and throughout the hospital stay as needed. At the time of the NT-proBNP measurements patients were at different stages of HF, including patients with acute, chronic or resolving HF. Patients may have been hospitalized or seen in the HF clinic. Functional status was indicated by the New York Heart Association (NYHA) classification for children older than 5 years and by the Ross classification (16) for younger children.
Echocardiographic data were abstracted from the echocardiogram report prepared by a cardiologist who was unaware of the serum test results. All echocardiograms were performed following standard institutional protocols. An M-mode tracing was obtained in the parasternal short-axis view at the level of the papillary muscles of the LV, and LV end-systolic and end-diastolic diameters were measured.
Regression equations relating LVSD and LVDD to body surface area and LVSF to age were derived from M-mode echocardiographic data in 580 healthy patients aged 0 to 40 years using methods described elsewhere (17,18). Using the Boyd weight-based formula for body surface area (18) or age, the estimated means and standard deviations from these regression equations were used to calculate z-scores for LVSD (LVSD-z) and LVDD (LVDD-z) relative to body surface area and z-scores for LVSF (LVSF-z) relative to age in our sample. Left ventricular ejection fraction (LVEF) was calculated using Simpson’s apical biplane method, as recommended by the American Society of Echocardiography (19).
Serum NT-proBNP was measured using the Elecsys 2010 System (Roche Diagnostics, Indianapolis, IN). Echocardiographic measurements and functional classification were obtained within 7 days of an NT-proBNP measurement.
Statistical Methods
Within-patient linear associations between log-transformed (base 10) NT-proBNP and the log odds of being in NYHA/Ross classes III or IV or echocardiographic values were estimated using generalized linear mixed models (SAS PROC MIXED/NLMIXED, Cary, NC). Random intercepts were used to account for heterogeneity between individuals and within individual correlations. This approach also accounted for unequal numbers of NT-proBNP values per patient. Predicted outcomes for a hypothetical average patient were estimated using a random intercept of zero. This mixed-model, random-intercept model primarily tests the within-patient relationship (i.e., association) between NT-proBNP and echocardiographic measures, as well as NYHA/Ross classes III or IV (versus I or II).
A between-patient analysis was also conducted to examine the more general question of the absolute measurement of NT-proBNP as a predictor of functional class. This relationship was examined by classifying patients as “in-control” (those consistently in NYHA/Ross classes I or II), “fluctuating control” (those moving back and forth between classes I or II and classes III or IV), or “poor control” (those consistently in classes III or IV) and then examining the means and distribution of the log NT-proBNP (base 10) by control status. Logistic regression was used to further quantify differences in control status. The ROC curve resulting from the logistic regression was used to estimate the sensitivity and specificity for predicting functional status and the NT-proBNP value that optimized both sensitivity and specificity.
The reliability of a single NT-proBNP measurement was estimated from children who were deemed to be “in-control”. Reliability was estimated by restricted maximum likelihood using a simple variance component model (i.e., intraclass correlation). Only patients who had a minimum of three NT-proBNP measurements were used in the between-patients reliability analysis.
The study was supported in part by grants from the National Institutes of Health (HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, CA068484, HD052102, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL095127, HD80002).
RESULTS
Within–Patient Analysis
We analyzed data from 36 children (Table I). The number of children varied between analyses, primarily as a result of missing values; the sample size for each analysis is given in the table titles and figure legends.
Table I.
Demographic and Clinical Characteristics of 36 Children with Heart Failure Secondary to Dilated Cardiomyopathy Providing Serial Measurements of NT-proBNP
| Characteristic | Value |
|---|---|
| Cause of dilated cardiomyopathy, n (%) | |
| Idiopathic | 31 (86) |
| Anthracycline-related | 3 (8) |
| Uremic | 1 (2.5) |
| Muscular dystrophy | 1 (2.5) |
| Age at diagnosis, median (min, max), years | 2.2 (0.08, 16) |
| Age at first NT-proBNP measurement, median (min, max), years | 11.65 (2.0, 24.8) |
| Male, n (%) | 14 (39) |
| Race, n (%) | |
| White | 20 (55) |
| Black | 15 (42) |
| Other | 1 (3) |
| Ethnicity | |
| Hispanic | 15 (42) |
| Outcomes, n (%) | |
| Death | 1 (3) |
| Transplant | 7 (19) |
| Length of follow-up, median (min, max), y | 1.0 (0.08, 4.1) |
| Number of NT-proBNP measurements per child, median (min, max), n | 4 (1, 33) |
| Time between serial NT-proBNP measurements, median (min, max), days | 64 (1, 763) |
NT-proBNP, N-terminal pro-hormone brain natriuretic peptide
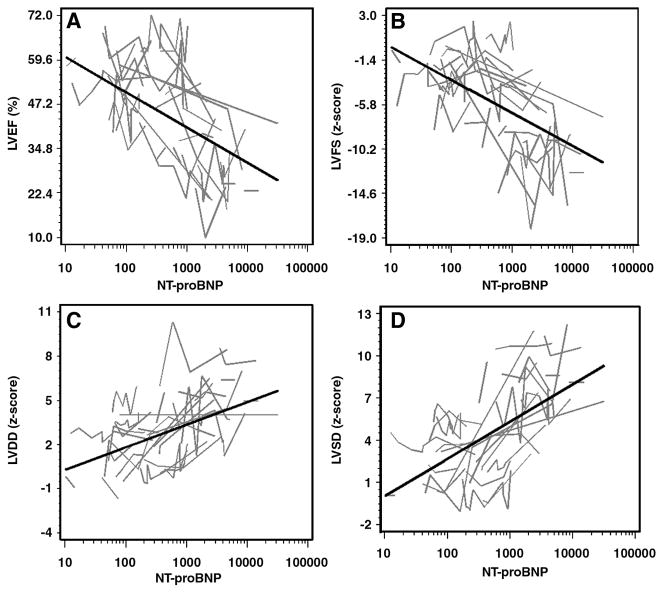
All four echocardiographic measures were associated with a corresponding within-patient change in NT-proBNP levels (Table II). The results indicate that LVEF and LVSF-z decrease and LVDD-z and LVSD-z increase with increasing NT-proBNP levels (P<0.001).
Table II.
Linear Mixed-Model Estimates of Mean Echocardiographic Measurements by Serum NT-proBNP Level in a Hypothetical Average Child with Dilated Cardiomyopathy
| Outcome | Children, n Observations, n | Estimated Outcome (95% CI)* | Δ Outcome/Δ log10 (NT- proBNP) (95% CI)† | P† |
|---|---|---|---|---|
| LVEF (%) | 34 (121) | 42.5 (38.9, 46.0) | −9.8 (−12.5, −7.1) | <0.001 |
| LVSF-z | 36 (128) | −6.04 (−7.15, −4.92) | −3.25 (−4.16, −2.34) | <0.001 |
| LVDD-z | 36 (131) | 3.07 (2.37, 3.77) | 1.53 (1.06, 2.00) | <0.001 |
| LVSD-z | 36 (128) | 4.85 (4.01, 5.71) | 2.64 (2.40, 3.25) | <0.001 |
The bivariate association between each outcome and NT-proBNP was examined using a linear mixed model with a random intercept and log10 (NT-proBNP) as a single covariate. The estimated mean outcome and its 95% CI for a hypothetical average child was estimated at a NT-proBNP value equal to the geometric mean of 660 pg/mL in the sample of 198 available measurements and at a random intercept of zero.
The within-patient change in outcome per 1-unit change in log10 (NT-proBNP) and its 95% CI were estimated using the same linear mixed model. A 1-unit change in log10 (NT-proBNP) corresponds to a 10-fold increase in NT-proBNP measured in pg/mL (e.g., from 104 pg/mL to 1040 pg/mL). The P value is for the test of the null hypothesis that the change in outcome is zero.
LVEF, left ventricular ejection fraction; LVSF-z, left ventricular shortening fraction relative to age z-score; LVDD-z, left ventricular diastolic diameter relative to body surface area z-score; LVSD-z, left ventricular systolic diameter relative to body surface area z-score; NT-proBNP, N-terminal pro-hormone brain natriuretic peptide.
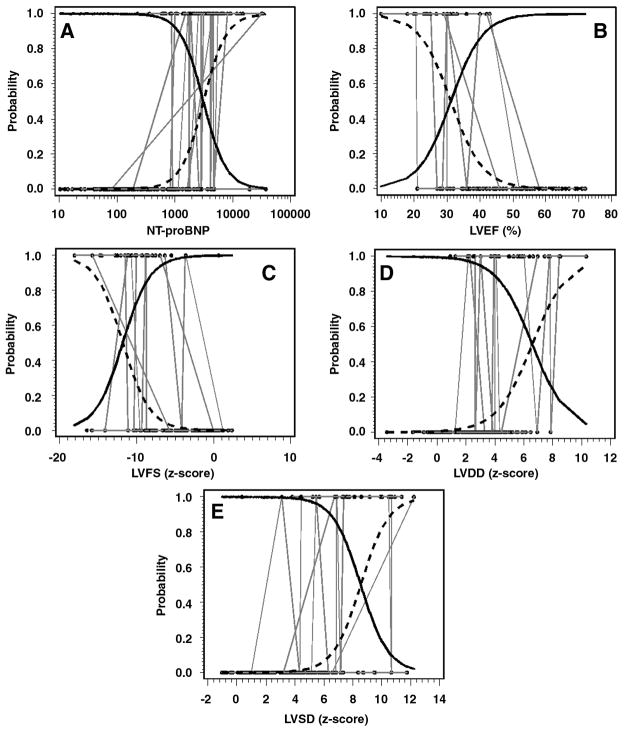
Levels of NT-proBNP and the echocardiographic measures also predicted functional status (Table III). A 10-fold increase in NT-proBNP had an odds ratio of 85.48 (P<0.001), indicating a strong within-patient relationship with NYHA or Ross classification. The echocardiographic measures also predicted NYHA/Ross class—P values were all ≤0.001—but they did not uniformly reach the performance of NT-proBNP. The bivariate plots of the relationship between the echocardiographic data and NT-proBNP are given in Figure 1. Points are grouped by individual patients (i.e., connecting lines). The bivariate relationships between the probability of being in NYHA/Ross class III/IV versus I/II and NT-proBNP and echocardiographic measures are given in Figure 2.
Table III.
Logistic-Normal Generalized Linear Mixed-Model Estimates of the Probability of Being in NYHA/Ross Class III or IV for a Hypothetical Average Child with Dilated Cardiomyopathy
| Predictor | Patients, n Observations, n | Predictor Sample Mean | P (NYHA/Ross III or IV) (95% CI)* | Odds Ratio for NYHA/Ross Class III or IV vs. Class I or II (95% CI)† | P† |
|---|---|---|---|---|---|
| log10(NT-proBNP)‡ | 34 (198) | 2.83 | 0.14 (0.03, 0.44) | 85.48 (10.89, 671.02) | <0.001 |
| LVEF (%) | 34 (121) | 44.8 | 0.08 (0.02, 0.26) | 0.83 (0.77, 0.89) | <0.001 |
| LVSF-z | 36 (128) | −5.33 | 0.09 (0.02, 0.35) | 0.58 (0.59, 0.79) | <0.001 |
| LVDD-z | 36 (131) | 3.03 | 0.06 (0.01, 0.39) | 1.75 (1.39, 2.21) | <0.001 |
| LVSD-z | 36 (128) | 4.46 | 0.03 (0.00, 0.44) | 1.67 (1.39, 2.02) | <0.001 |
The bivariate association between the probability of being in NYHA/Ross class III or IV and each predictor was examined using a logistic-normal generalized linear mixed model with a random intercept and the predictor as a single covariate. The probability of being in class III or IV and its 95% CI for a hypothetical average child was estimated at a predictor value equal to the sample mean of the predictor and at a random intercept of zero.
The within-patient change in the odds of being in NYHA/Ross class III or IV for a 1-unit change in the predictor, expressed as an odds ratio, and its 95% CI were estimated using the same logistic-normal generalized linear mixed model. A 1-unit change in log10 (NT-proBNP) corresponds to a 10-fold increase in NT-proBNP measured in pg/mL (e.g., from 104 pg/mL to 1040 pg/mL). The P value is for the test of the null hypothesis of no within-patient change in odds (or an odds ratio of 1).
The sample arithmetic mean of approximately 2.82 log10 units corresponds to a sample geometric mean of 660 pg/mL
LVEF, left ventricular ejection fraction; LVSF-z, left ventricular shortening fraction relative to age z-score; LVDD-z, left ventricular diastolic diameter relative to body surface area z-score; LVSD-z, left ventricular systolic diameter relative to body surface area z-score; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-hormone brain natriuretic peptide.
Figure 1.
(Panels A–D). Bivariate relationships between echocardiographic measurements and serum NT-proBNP measurements on a log10 scale. Black lines show the within-subject relationships in a hypothetical average subject (i.e., a subject having a random intercept of zero) estimated from these data using linear mixed models. Gray lines differentiate individuals by connecting the repeated measurements for each subject.
Figure 2.
(Panels A–E). Bivariate relationships between the probabilities of being in NYHA/Ross class I/II versus III/IV by NT-proBNP on a log10 scale and echocardiographic measurements. Solid (Probability of being in NYHA/Ross class I/II) and dashed (Probability of being in NYHA/Ross class III/IV) logistic curves show the within-subject relationships in a hypothetical average subject (i.e., a subject having a random intercept of zero) estimated from these data using logistic-normal, generalized linear mixed models. The black dots are the probability of being in NYHA/Ross class III/IV for an individual subject, which was 1 if the subject was in NYHA/Ross class III/IV at a particular observation and 0 otherwise, plotted against actual measurements of the predictor. Repetitive measurements within individual subjects are connected by gray lines. The solid and dashed logistic curves cross at the value of the predictor for which the hypothetical average subject has a 50% chance of being in NYHA/Ross class III/IV.
Between–Patient Analysis
On the distributions of mean NT-proBNP levels by control status (Figure 3), the reference line at 125 pg/mL is the upper limit of normal for adults and that at 450 pg/mL is a commonly used threshold that defines HF (20). All observation in the “fluctuating” and “poor control” groups fell above both of these thresholds. However, patients in the “in-control” group tended to straddle the thresholds. These data suggest a high negative predictive value for NT-proBNP levels, with a larger false positive rate because patients may have high NT-proBNP levels but a consistent NYHA/Ross I or II classification over time.
Figure 3.
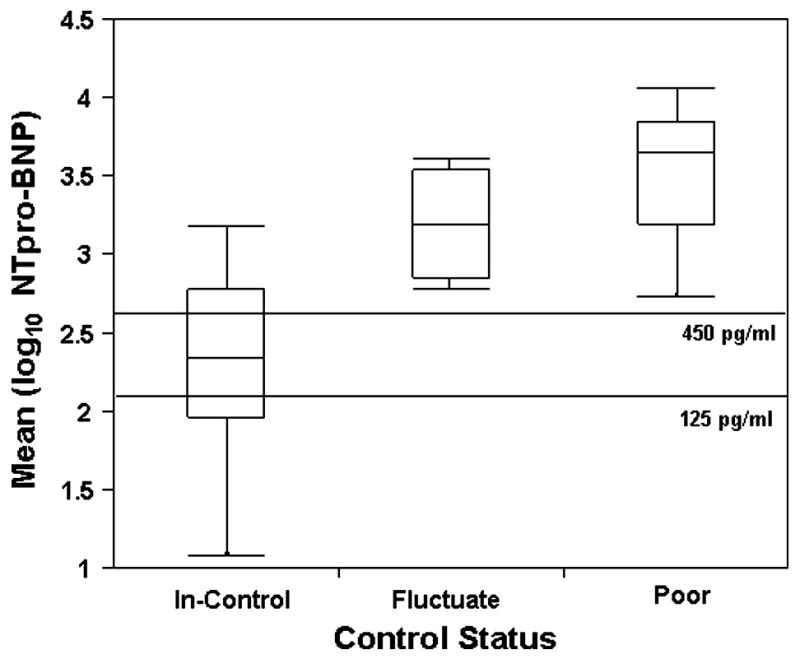
Side-by-side box plots of the average log10 of NT-proBNP by control status group (“In Control”, n = 20; “Fluctating”, n = 4; “Poor Control”, n = 6). Vertical lines indicate the range; the boxes, the 25th to the 75th percentiles; and the lines in the box, the medians. The reference lines at 125 and 450 pg/ml indicate the upper limit of normal and the diagnostic value for HF, respectively.
The log NT-proBNP value was used to predict group membership (logistic regression) between the “in-control” group and the combined “poor control/fluctuating” group. Figure 4 presents the logistic probability function along with the resulting three-dimensional ROC curve (Figure 5): True positive rate was maximized (0.95) and false positive rate minimized (0.20) when the log of NT-proBNP was approximately 3.0. This value equates to a raw NT-proBNP of 1000 pg/mL.
Figure 4.
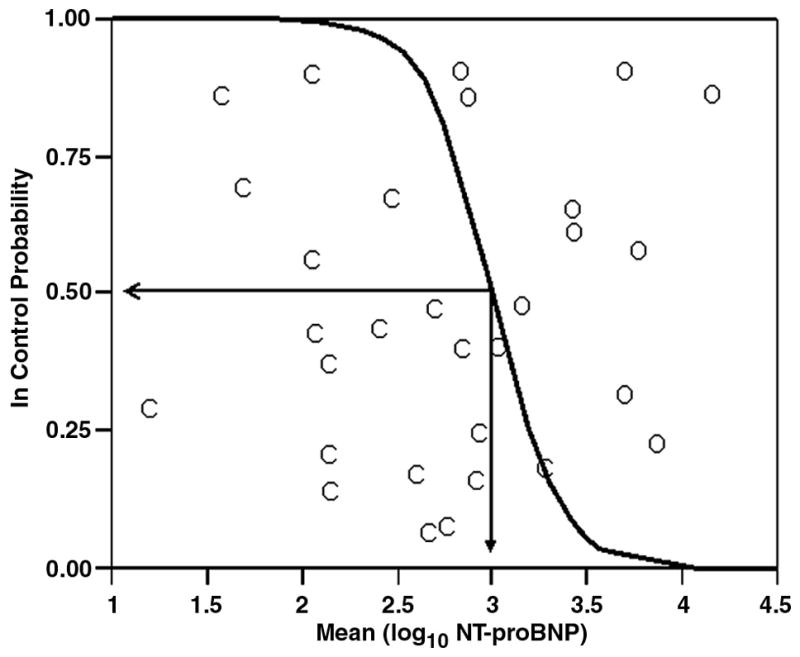
The logistic probability function (C = “In Control” subjects (n = 20), O = combined “Poor Control” and “Fluctuating” subjects (n=10)) for the probability of being “In Control” based on an averaged log10 of NT-proBNP. Plotted values are probabilities and corresponding log10 NT-proBNP for individual subjects after averaging over the repeated measurements. Log10 NT-proBNP equals 3.0 when the odds of being in or out of control are equal.
Figure 5.
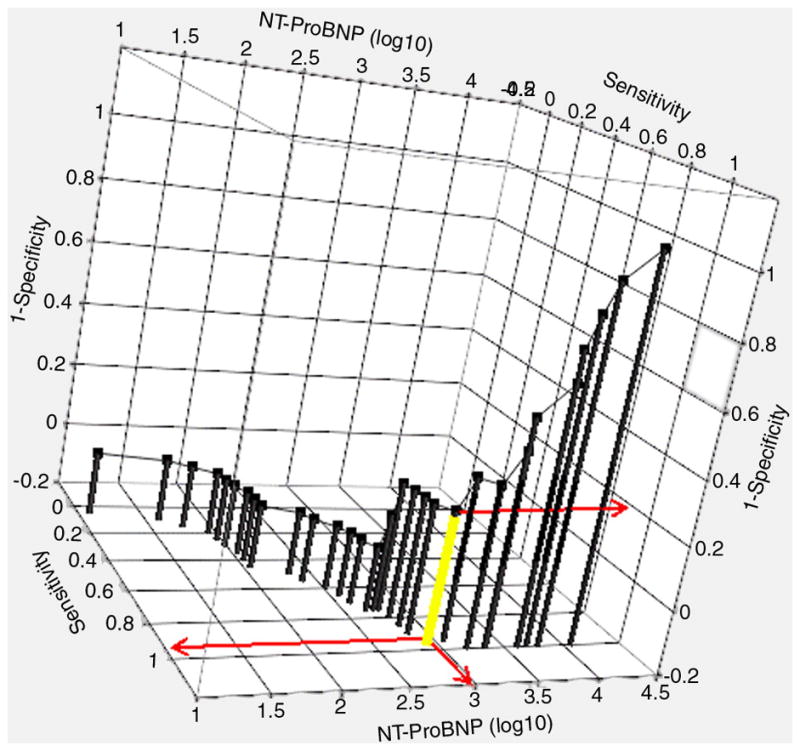
The 3-dimensional ROC curve for predicting status group (“In Control” versus combined “Fluctuating” and “Poor Control”). The optimized cut-point (log10 NT-proBNP = 3.0) is depicted by the yellow drop line and red projections (Sensitivity = 0.95, Specificity =0.80).
The crossover point obtained from the within-patient analysis (Figure 2, Panel A) is somewhat higher (i.e., 3000 pg/mL) than that obtained as the optimized cut-point from the between-patient analysis (i.e., 1000 pg/mL). This result is explained primarily by the fact that the between-patient analysis is marginal, whereas the within-patient analysis is conditional on a random intercept of zero. Also, the two analyses place subjects into functional status groups in a slightly different manner. Thus, the two crossover points are not strictly comparable. However, when averaged over the random effects, the two crossover points agree closely in order of magnitude.
The Reliability of NT-proBNP Measurements
The reliability of a single NT-proBNP measure was estimated to be 0.61. Thus, only 61% of the observed variation in a single NT-proBNP measure is attributable to a patient’s true score (i.e., the theoretical true value of a patient’s NT-proBNP). The reliabilities of the mean for two and three NT-proBNP measures were 0.76 and 0.82, respectively. These values suggest that for the type of patients in this study, multiple measurements (i.e., a minimum of 3) of NT-proBNP are required before a reliable patient value is obtained.
DISCUSSION
We evaluated the ability of serial measurements of NT-proBNP to objectively assess changes in the severity of HF in children with dilated cardiomyopathy. The results indicated that increases in NT-proBNP serum levels are associated with lower LVEF, lower LVSF-z, higher LVDD-z, higher LVSD-z, and increased odds of being in NYHA/Ross classes III or IV. We determined that an NT-proBNP value above 1,000 pg/mL identifies more symptomatic patients. In children with NT-proBNP levels between 450 and 1,000 pg/mL, serial measurements (3 at least) are required to reliably indicate functional status.
The moderate-to-strong relationship of NT-proBNP levels with several echocardiographic variables and functional status potentially makes possible a test that quickly and objectively indicates the patient’s clinical condition and corroborates the clinical assessment of functional status and echocardiographic indices of ventricular size and function. The potential clinical applications of this biomarker include tracking the progression of the disease and assessing the response to therapy. We can speculate that a patient with progressive increases in NT-proBNP may need more aggressive treatment or that one with decreasing NT-proBNP levels after an increase in medication indicates improvement in HF.
Grading the severity of HF has always been problematic in infants and children because of the difficulty in objectively assessing the degree of dyspnea, palpitation, fatigue, and chest pain, factors that are the basis of the NYHA and Ross classifications (16), in a patient going through a variety of developmental changes over relatively few years. NT-proBNP appears to be an accurate and objective measure for assessing the progression of HF without the complications associated with self-reported measures of function given by children. This marker is appealing because within the same patient, NT-proBNP has a moderate-to-strong relationship to NYHA/Ross class and echocardiographic measurements, and it appears to be superior to echocardiography alone in tracking the patient’s functional class. The test is easy to do and can be done in most clinical chemistry laboratories, allowing for frequent assessments. In addition, pediatric echocardiography is more expensive, may require sedation, and the interpretation is more subjective than a routine serum NT-proBNP (21).
Our data on the variability of NT-proBNP levels between patients also shows the importance of serial measurements of NT-proBNP, particularly in patients in NYHA/Ross classes I/II. Patients in these classes may have abnormal LV size and function, but may still be asymptomatic or with minimal symptoms. A single measurement of NT-proBNP may not be sufficient to categorize the patient as “at risk” if it is below 1000 pg/mL. As we found, NT-proBNP levels between 450 and 1000 pg/mL may not distinguish between symptomatic and asymptomatic patients (Figure 3); patients with values in this range can fluctuate between the “in-control” and the “poor control” groups. It becomes useful at this point to follow serial measurements of NT-proBNP.
The data from the current investigation do not permit a reliable analysis of how frequently NT-proBNP should be measured. The approach that we propose and have used for monitoring NT-proBNP is given in Table IV. This approach should be seen only as a suggestion for how NT-proBNP levels can be used as an adjunct tool in managing children with HF. Studies designed with fixed intervals for measuring NT-proBNP are needed to determine the optimum interval between NT-proBNP measurements.
Table IV.
Proposed Frequency of Monitoring NT-proBNP in Children with Heart Failure
|
HF, heart failure.
Most of the published papers on the importance of brain natriuretic peptide in children focus on its utility in identifying the presence of cardiac disease (9,10), but few have addressed its prognostic implications in pediatric HF. In 53 consecutive children with chronic HF, Price et al. found that a BNP level greater than 300 pg/mL was a strong indicator of mortality and morbidity, with a sensitivity of 93% and a specificity of 95% (22). In another study, Mangat et al. reviewed 98 BNP levels in 48 children with pure LV dysfunction and found that a BNP level greater than 290 pg/mL predicted poor outcome with a sensitivity of 80% and a specificity of 87% (11). Although BNP and NT-proBNP values were not comparable, the authors showed that logBNP correlates with NYHA/Ross classification (r=0.82, P<0.001), LVEDD-z (r=0.34, P<0.001) and LVSF (r=−0.66, P<0.001) (11). In our study, we confirmed that NT-proBNP, like BNP, is related to LV function and is an index of LV remodeling.
Limitations of the Study
Our study is retrospective. Blood collection for NT-proBNP measurements occurred when blood testing was required as part of standard clinical management and did not follow a predetermined time interval or sequence. Samples were measured in the hospital laboratory and constituted single samples for any particular time period. Echocardiographic data were abstracted from the echocardiographic report. Although the data were not collected with the rigor of a prospective study, the echocardiograms were performed by experienced and skilled technicians and reported by a cardiologist with expertise in echocardiography. The limited number of deaths and transplants did not allow us to determine whether NT-proBNP levels could predict mortality.
The estimates of thresholds and the subsequent suggested decision rules for NT-proBNP values as they apply to children with HF need to be validated before they are applied in clinical practice. It may not be prudent to use NT-proBNP as a stand-alone measure of functional status or as a sole indicator of disease progression. Until the predictive power of NT-proBNP is validated, values obtained in children with HF should be evaluated in light of all other measures of functional status, as well as the specific clinical history of the individual patient.
Conclusions
Levels of NT-proBNP are a good indicator of HF and LV dysfunction in children with HF secondary to dilated cardiomyopathy, and they are at least as good as echocardiography in stratifying the severity of this disease. This association is consistent across several measurements of LV systolic function and LV remodeling, as well as across functional status over a long period in the same individual, and is thus extremely important for the information it can provide in diagnosing and managing HF in children. NT-proBNP could eventually be used as relatively inexpensive tests to monitor the progression of pediatric heart failure and objectively assess the effects of treatments. NT-proBNP level above 1,000 pg/ml clearly identifies the sickest patients. NT-proBNP < 450 identifies asymptomatic patients. In patients with NT-proBNP between 450 and 1,000 serial measurement are needed to understand the direction of HF evolution.
ABBREVIATIONS AND ACRONYMS
- NT-proBNP
N-terminal pro-hormone brain natriuretic peptide
- HF
heart failure
- NYHA
New York Heart Association
- LV
left ventricular
- LVSD-z
left ventricular systolic diameter relative to body surface area z-score
- LVDD-z
left ventricular diastolic diameter relative to body surface area z-score
- LVSF-z
left ventricular shortening fraction relative to age z-score
- LVEF
left ventricular ejection fraction
- ROC
receiver operating characteristics
Footnotes
There are no relationships with industry to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maisel AS, Krishnaswamy P, Nowak RM, et al. Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 2.Richards AM, Nicholls MG, Espiner EA, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107:2786–92. doi: 10.1161/01.CIR.0000070953.76250.B9. [DOI] [PubMed] [Google Scholar]
- 3.Stanton E, Hansen M, Wijeysundera HC, et al. A direct comparison of the natriuretic peptides and their relationship to survival in chronic heart failure of a presumed non-ischaemic origin. Eur J Heart Fail. 2005;7:557–65. doi: 10.1016/j.ejheart.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733–9. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 5.Tan LH, Jefferies JL, Liang JF, et al. Concentrations of brain natriuretic peptide in the plasma predicts outcomes of treatment of children with decompensated heart failure admitted to the Intensive Care unit. Cardiol Young. 2007;17:397–406. doi: 10.1017/S1047951107000601. [DOI] [PubMed] [Google Scholar]
- 6.Rossano JW, Denfield SW, Kim JJ, et al. B-type natriuretic peptide is a sensitive screening test for acute rejection in pediatric heart transplant patients. J Heart Lung Transplant. 2008;27:649–54. doi: 10.1016/j.healun.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Ratnasamy C, Kinnamon DD, Lipshultz SE, et al. Associations between neurohormonal and inflammatory activation and heart failure in children. Am Heart J. 2008;155:527–33. doi: 10.1016/j.ahj.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Suda K, Matsumura M, Matsumot M. Clinical implications of plasma natriuretic peptides in children with ventricular septal defect. Pediatr Int. 2003;45:249–54. doi: 10.1046/j.1442-200x.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- 9.Maher KO, Reed H, Cuadrado A, et al. B-type natriuretic peptide in the emergency diagnosis of critical heart disease in children. Pediatrics. 2008;121:e1484–8. doi: 10.1542/peds.2007-1856. [DOI] [PubMed] [Google Scholar]
- 10.Law YM, Hoyer AW, Reller MD, Silberbach M. Accuracy of plasma B-type natriuretic peptide to diagnose significant cardiovascular disease in children: the Better Not Pout Children! Study. J Am Coll Cardiol. 2009;54:1467–75. doi: 10.1016/j.jacc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Mangat J, Carter C, Riley G, et al. The clinical utility of brain natriuretic peptide in paediatric left ventricular failure. Eur J Heart Fail. 2009;11:48–52. doi: 10.1093/eurjhf/hfn001. [DOI] [PubMed] [Google Scholar]
- 12.Lipshultz SE, Rifai N, Sallan SE, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation. 1997;96:2641–2648. doi: 10.1161/01.cir.96.8.2641. [DOI] [PubMed] [Google Scholar]
- 13.Herman EH, Zhang J, Rifai N, et al. The use of serum levels of cardiac troponin T to compare the protective activity of dexrazoxane against doxorubicin- and mitoxantrone-induced cardiotoxicity. Cancer Chemother Pharmacol. 2001;48:297–304. doi: 10.1007/s002800100348. [DOI] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–53. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz SE, Rusconi P, Scully RE. Assessment of cardiotoxicity during anti-cancer therapy. In: Bayes-Benis A, Januzzi JL Jr, editors. NT-proBNP as a Biomarker in Cardiovascular Diseases. Barcelona, Spain: Prous Science S.A. and Thomson Reuters; 2008. pp. 183–197. [Google Scholar]
- 16.Ross RD, Daniels SR, Schwartz DC, et al. Plasma norepinephrine levels in infants and children with congestive heart failure. Am J Cardiol. 1987;59:911–4. doi: 10.1016/0002-9149(87)91118-0. [DOI] [PubMed] [Google Scholar]
- 17.Colan SD, Parness IA, Spevak PJ, et al. Developmental modulation of myocardial mechanics: Age-and growth-related alterations in afterload and contractility. J Am Coll Cardiol. 1992;19:619–29. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 18.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 19.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 20.Januzzi JL, Jr, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Am J Cardiol. 2005;95:948–54. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Lipshultz SE, Miller TL. Establishing norms for echocardiographic measurements of cardiovascular structures and function in children. J Appl Physiol. 2005;99:386–8. doi: 10.1152/japplphysiol.00167.2005. [DOI] [PubMed] [Google Scholar]
- 22.Price JF, Thomas AK, Grenier M, et al. B-type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation. 2006;114:1063–9. doi: 10.1161/CIRCULATIONAHA.105.608869. [DOI] [PubMed] [Google Scholar]