Abstract
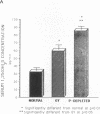
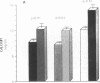
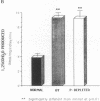
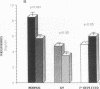
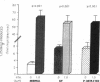
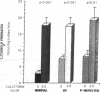
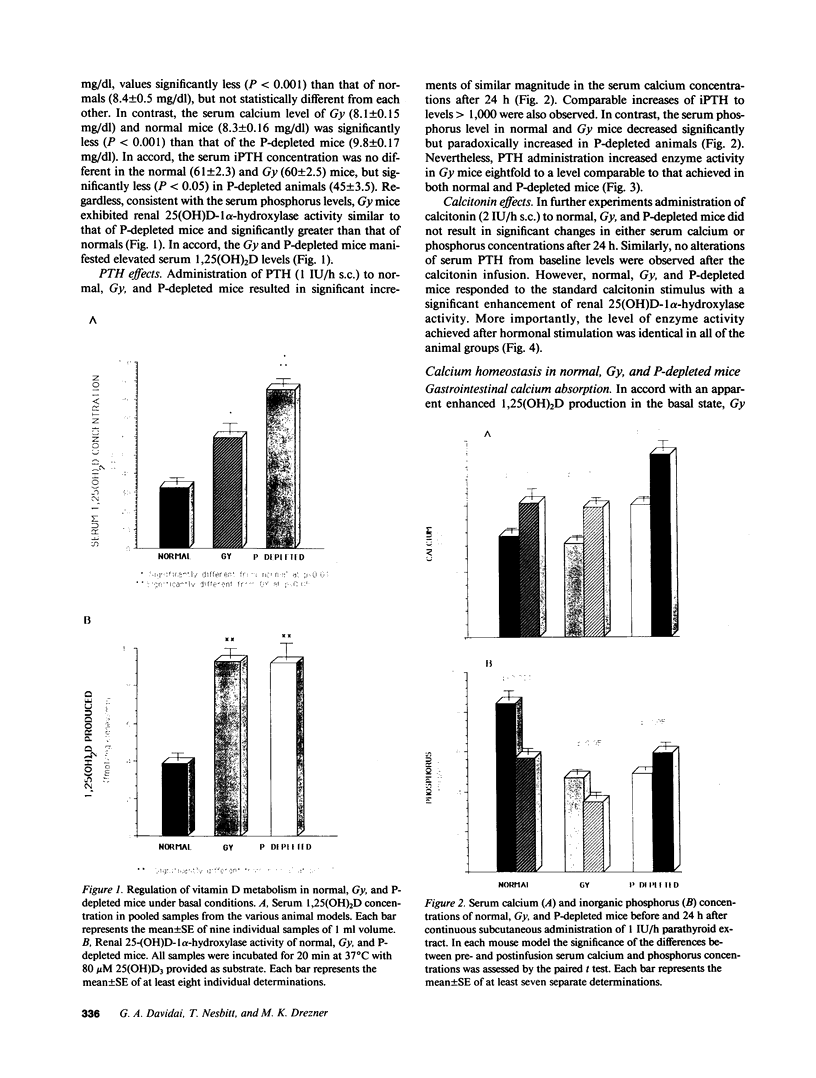
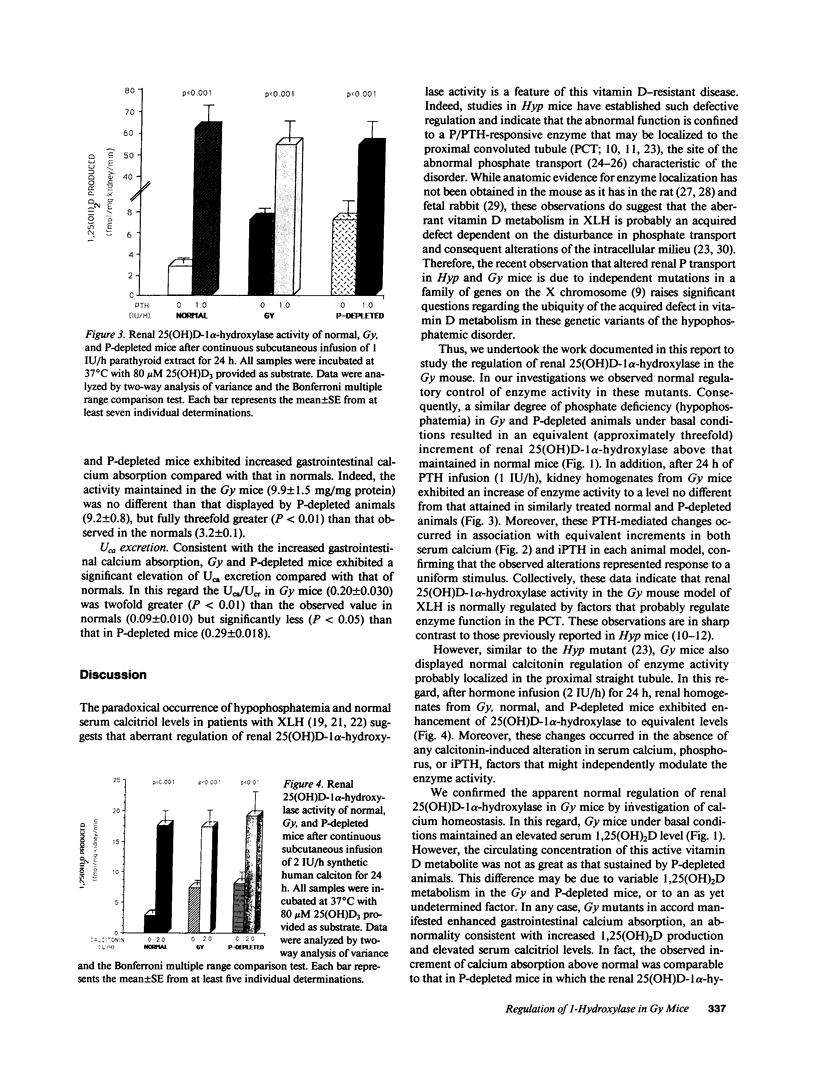
Phenotypic heterogeneity in X-linked hypophosphatemic rickets (XLH) is ascribed to variable penetrance of the genetic abnormality. However, studies of hypophosphatemic (Hyp) and gyrorotary (Gy) mice indicate that mutations at different loci along the X chromosome may underlie the genetically transmitted hypophosphatemic disorders. Thus, genetic heterogeneity may be a determinant of the phenotypic variability in XLH. To determine if such variance includes biochemical diversity, we examined whether Gy mice, similar to Hyp mice, exhibit abnormal regulation of renal 25-hydroxyvitamin D (25[OH]D)-1 alpha-hydroxylase. Serum phosphorus in Gy (4.7 +/- 0.3 mg/dl) and phosphate (P)-depleted mice (4.9 +/- 0.4) was significantly less than normal (8.4 +/- 0.5). Consistent with P depletion, the Gy mice exhibited enhanced renal 25(OH)D-1 alpha-hydroxylase activity (9.3 +/- 0.6 fmol/mg kidney per min), similar to that of P-depleted normals (9.1 +/- 1.5), but significantly greater than that of controls (3.1 +/- 0.3). Such normal enzyme responsiveness was confirmed upon PTH stimulation (1 IU/h s.c.), which revealed that Gy mice increased renal 1-hydroxylase (59 +/- 7.7) similarly to normals (65 +/- 7.7) and P-depleted animals (58.4 +/- 7.8). Calcitonin administration also enhanced enzyme function comparably in the animal models. Evidence confirming normally responsive calcitriol production in untreated Gy mice included increased serum 1,25-dihydroxyvitamin D levels, gastrointestinal calcium absorption, and urinary calcium. The normally regulated vitamin D metabolism in Gy mice indicates that biochemically diverse disease may result from mutations in the gene family regulating renal P transport and underlying X-linked hypophosphatemia. We suspect such heterogeneity is due to altered P transport at variable segments of the proximal convoluted tubule.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiba T., Endou H., Koseki C., Sakai F., Horiuchi N., Suda T. Localization of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity in the mammalian kidney. Biochem Biophys Res Commun. 1980 May 14;94(1):313–318. doi: 10.1016/s0006-291x(80)80222-1. [DOI] [PubMed] [Google Scholar]
- BURNETT C. H., DENT C. E., HARPER C., WARLAND B. J. VITAMIN D-RESISTANT RICKETS. ANALYSIS OF TWENTY-FOUR PEDIGREES WITH HEREDITARY AND SPORADIC CASES. Am J Med. 1964 Feb;36:222–232. doi: 10.1016/0002-9343(64)90085-3. [DOI] [PubMed] [Google Scholar]
- Boneh A., Reade T. M., Scriver C. R., Rishikof E. Audiometric evidence for two forms of X-linked hypophosphatemia in humans, apparent counterparts of Hyp and Gy mutations in mouse. Am J Med Genet. 1987 Aug;27(4):997–1003. doi: 10.1002/ajmg.1320270434. [DOI] [PubMed] [Google Scholar]
- Briard-Guillemot M. L., Raverdy E., Balsan S., Rey J., Frézal J. Etude critique de l'hypophosphatémie pour l'étude génetique du rachitisme vitamini-résistant hypophosphatérmique familial. Arch Fr Pediatr. 1972 Dec;29(10):1059–1068. [PubMed] [Google Scholar]
- Chesney R. W., Mazess R. B., Rose P., Hamstra A. J., DeLuca H. F. Supranormal 25-hydroxyvitamin D and subnormal 1,25-dihydroxyvitamin D: their role in X-linked hypophosphatemic rickets. Am J Dis Child. 1980 Feb;134(2):140–143. doi: 10.1001/archpedi.1980.02130140014005. [DOI] [PubMed] [Google Scholar]
- Cowgill L. D., Goldfarb S., Lau K., Slatopolsky E., Agus Z. S. Evidence for an intrinsic renal tubular defect in mice with genetic hypophosphatemic rickets. J Clin Invest. 1979 Jun;63(6):1203–1210. doi: 10.1172/JCI109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J. F., Muhtadie S. F., Hamilton D. C., Cole D. E. The comparative toxicity of vitamin D metabolites in the weanling mouse. Toxicol Appl Pharmacol. 1985 Aug;80(1):119–126. doi: 10.1016/0041-008x(85)90106-1. [DOI] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Davies M., Kane R., Valentine J. Impaired hearing in X-linked hypophosphataemic (vitamin-D-resistant) osteomalacia. Ann Intern Med. 1984 Feb;100(2):230–232. doi: 10.7326/0003-4819-100-2-230. [DOI] [PubMed] [Google Scholar]
- Dostal L. A., Toverud S. U. Effect of vitamin D3 on duodenal calcium absorption in vivo during early development. Am J Physiol. 1984 May;246(5 Pt 1):G528–G534. doi: 10.1152/ajpgi.1984.246.5.G528. [DOI] [PubMed] [Google Scholar]
- Giasson S. D., Brunette M. G., Danan G., Vigneault N., Carriere S. Micropuncture study of renal phosphorus transport in hypophosphatemic vitamin D resistant rickets mice. Pflugers Arch. 1977 Oct 19;371(1-2):33–38. doi: 10.1007/BF00580769. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Kurokawa K. Unique hormonal regulation of vitamin D metabolism in the mammalian kidney. Miner Electrolyte Metab. 1983;9(4-6):227–235. [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in proximal straight tubule of rat kidney. Nature. 1981 May 28;291(5813):327–329. doi: 10.1038/291327a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Abnormal regulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the X-linked hypophosphatemic mouse. J Clin Invest. 1983 Feb;71(2):400–403. doi: 10.1172/JCI110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Measurement of 25-hydroxyvitamin D-1 alpha-hydroxylase activity in mammalian kidney. Anal Biochem. 1983 Mar;129(2):416–424. doi: 10.1016/0003-2697(83)90571-7. [DOI] [PubMed] [Google Scholar]
- Lyles K. W., Clark A. G., Drezner M. K. Serum 1,25-dihydroxyvitamin D levels in subjects with X-linked hypophosphatemic rickets and osteomalacia. Calcif Tissue Int. 1982 Mar;34(2):125–130. doi: 10.1007/BF02411222. [DOI] [PubMed] [Google Scholar]
- Lyon M. F., Scriver C. R., Baker L. R., Tenenhouse H. S., Kronick J., Mandla S. The Gy mutation: another cause of X-linked hypophosphatemia in mouse. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4899–4903. doi: 10.1073/pnas.83.13.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie P. J., Travers R., Glorieux F. H. Bone response to phosphate and vitamin D metabolites in the hypophosphatemic male mouse. Calcif Tissue Int. 1982 Mar;34(2):158–164. doi: 10.1007/BF02411227. [DOI] [PubMed] [Google Scholar]
- Nesbitt T., Davidai G. A., Drezner M. K. Abnormal adenosine 3'.5'-monophosphate stimulation of renal 1,25-dihydroxyvitamin D production in hyp mice: evidence that 25-hydroxyvitamin D-1 alpha-hydroxylase dysfunction results from aberrant intracellular function. Endocrinology. 1989 Mar;124(3):1184–1189. doi: 10.1210/endo-124-3-1184. [DOI] [PubMed] [Google Scholar]
- Nesbitt T., Drezner M. K., Lobaugh B. Abnormal parathyroid hormone stimulation of 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the hypophosphatemic mouse. Evidence for a generalized defect of vitamin D metabolism. J Clin Invest. 1986 Jan;77(1):181–187. doi: 10.1172/JCI112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt T., Lobaugh B., Drezner M. K. Calcitonin stimulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in hypophosphatemic mice. Evidence that the regulation of calcitriol production is not universally abnormal in X-linked hypophosphatemia. J Clin Invest. 1987 Jan;79(1):15–19. doi: 10.1172/JCI112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE D. S., WALLACE W. M., HERNDON C. H. LONG-TERM TREATMENT OF VITAMIN-D RESISTANT RICKETS. J Bone Joint Surg Am. 1964 Jul;46:978–997. [PubMed] [Google Scholar]
- Sarkar B. C., Chauhan U. P. A new method for determining micro quantities of calcium in biological materials. Anal Biochem. 1967 Jul;20(1):155–166. doi: 10.1016/0003-2697(67)90273-4. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Reade T. M., DeLuca H. F., Hamstra A. J. Serum 1,25-dihydroxyvitamin D levels in normal subjects and in patients with hereditary rickets or bone disease. N Engl J Med. 1978 Nov 2;299(18):976–979. doi: 10.1056/NEJM197811022991803. [DOI] [PubMed] [Google Scholar]
- Stickler G. B., Beabout J. W., Riggs B. L. Vitamin D-resistant rickets: clinical experience with 41 typical familial hypophosphatemic patients and 2 atypical nonfamilial cases. Mayo Clin Proc. 1970 Mar;45(3):197–218. [PubMed] [Google Scholar]
- Stickler G. B. Familial hypophosphatemic vitamin D resistant rickets. The neonatal period and infancy. Acta Paediatr Scand. 1969 May;58(3):213–219. doi: 10.1111/j.1651-2227.1969.tb04709.x. [DOI] [PubMed] [Google Scholar]
- TAPIA J., STEARNS G., PONSETI I. V. VITAMIN-D RESISTANT RICKETS. A LONG-TERM CLINICAL STUDY OF 11 PATIENTS. J Bone Joint Surg Am. 1964 Jul;46:935–958. [PubMed] [Google Scholar]
- Tenenhouse H. S. Investigation of the mechanism for abnormal renal 25-hydroxyvitamin D3-1-hydroxylase activity in the X-linked Hyp mouse. Endocrinology. 1984 Aug;115(2):634–639. doi: 10.1210/endo-115-2-634. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. Effect of 1,25-dihydroxyvitamin D3 on phosphate homeostasis in the X-linked hypophosphatemic (Hyp) mouse. Endocrinology. 1981 Aug;109(2):658–660. doi: 10.1210/endo-109-2-658. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R., McInnes R. R., Glorieux F. H. Renal handling of phosphate in vivo and in vitro by the X-linked hypophosphatemic male mouse: evidence for a defect in the brush border membrane. Kidney Int. 1978 Sep;14(3):236–244. doi: 10.1038/ki.1978.115. [DOI] [PubMed] [Google Scholar]
- Tieder M., Arie R., Modai D., Samuel R., Weissgarten J., Liberman U. A. Elevated serum 1,25-dihydroxyvitamin D concentrations in siblings with primary Fanconi's syndrome. N Engl J Med. 1988 Sep 29;319(13):845–849. doi: 10.1056/NEJM198809293191307. [DOI] [PubMed] [Google Scholar]
- Tieder M., Modai D., Samuel R., Arie R., Halabe A., Bab I., Gabizon D., Liberman U. A. Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med. 1985 Mar 7;312(10):611–617. doi: 10.1056/NEJM198503073121003. [DOI] [PubMed] [Google Scholar]
- Tieder M., Modai D., Shaked U., Samuel R., Arie R., Halabe A., Maor J., Weissgarten J., Averbukh Z., Cohen N. "Idiopathic" hypercalciuria and hereditary hypophosphatemic rickets. Two phenotypical expressions of a common genetic defect. N Engl J Med. 1987 Jan 15;316(3):125–129. doi: 10.1056/NEJM198701153160302. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Seino Y., Satomura K., Tanaka Y., Yabuuchi H., Haussler M. R. Abnormal relationship between serum phosphate concentration and renal 25-hydroxycholecalciferol-1-alpha-hydroxylase activity in X-linked hypophosphatemic mice. Miner Electrolyte Metab. 1986;12(3):194–198. [PubMed] [Google Scholar]