Abstract
Background
Acute encephalitis is an important and severe disease in children in Vietnam. However, little is known about the etiology while such knowledge is essential for optimal prevention and treatment. To identify viral causes of encephalitis, in 2004 we conducted a one-year descriptive study at Children's Hospital Number One, a referral hospital for children in southern Vietnam including Ho Chi Minh City.
Methodology/Principal Findings
Children less than 16 years of age presenting with acute encephalitis of presumed viral etiology were enrolled. Diagnostic efforts included viral culture, serology and real time (RT)-PCRs. A confirmed or probable viral causative agent was established in 41% of 194 enrolled patients. The most commonly diagnosed causative agent was Japanese encephalitis virus (n = 50, 26%), followed by enteroviruses (n = 18, 9.3%), dengue virus (n = 9, 4.6%), herpes simplex virus (n = 1), cytomegalovirus (n = 1) and influenza A virus (n = 1). Fifty-seven (29%) children died acutely. Fatal outcome was independently associated with patient age and Glasgow Coma Scale (GCS) on admission.
Conclusions/Significance
Acute encephalitis in children in southern Vietnam is associated with high mortality. Although the etiology remains unknown in a majority of the patients, the result from the present study may be useful for future design of treatment and prevention strategies of the disease. The recognition of GCS and age as predictive factors may be helpful for clinicians in managing the patient.
Author Summary
Viral encephalitis is associated with high morbidity and mortality in Vietnam. However little is known about the causes of the disease due to a lack of diagnostic facilities in this relatively resource-poor setting. Knowledge about the etiologies and clinical outcome of viral encephalitis is necessary for future design of intervention studies targeted at improvement of clinical management, treatment and prevention of the disease. We report the viral agents, clinical outcome and prognostic factors of mortality of encephalitis in children admitted to a referral hospital for children in southern Vietnam. We show that about one third of the enrolled patients die acutely, and that mortality is independently associated with patient age and Glasgow Coma Scale on admission. Japanese encephalitis, dengue virus and enterovirus (including enterovirus 71) are the major viruses detected in our patients. However, more than half of the patients remain undiagnosed, while mortality in this group is as high as in the diagnosed group. This study will benefit clinicians and public health in terms of clinical management and prevention of childhood encephalitis in Vietnam.
Introduction
Acute encephalitis is associated with high morbidity and mortality and affects both children and adults. There have been few population-based studies, reporting incidences ranging between 3.5 and 7.4 cases per 100.000 patient-years [1]. Viruses are regarded as the most important etiological agents of encephalitis worldwide. However, in the majority of cases a specific infectious etiology cannot be found. Furthermore, the specific causes of the illness show considerable geographic and age dependent variation. In a population-based study in the United Kingdom, herpes simplex virus (HSV) was the most common virus diagnosed, and the proportion of cases with an identified etiology was significantly lower in children (33%) than in adults (45%) [2]. In the California Encephalitis Project, a confirmed or probable infectious etiology was found in only 16% of cases, with HSV-1 most commonly found in adult patients and enteroviruses in children [3].
In Southeast Asian countries like Cambodia and Vietnam, Japanese encephalitis virus (JEV) has been the leading reported cause of acute encephalitis in children, accounting for 31% to 45% of cases [4], [5]. However, the causes of the disease in the remaining cases were not extensively studied. Improved insight into the specific viral etiology and pathogen-specific clinical outcome of acute encephalitis in this region is essential to guide strategies for prevention and clinical management. We conducted a one-year prospective descriptive study of children with acute encephalitis who were admitted to a pediatric referral hospital in Ho Chi Minh City, Vietnam.
Methods
Study site
The Childrens's Hospital Number One (CH1) in Ho Chi Minh City is an 850-bed pediatric referral hospital for the southern provinces of Vietnam. Each year, the hospital admits between 400–800 patients less than 16 years of age with acute symptoms of fever and altered consciousness (including seizures). Of these patients, approximately 40% are considered to have acute encephalitis of viral origin based on clinical judgment of pediatricians at CH1.
Patients
Children admitted between January 1 and 31 December 2004 with suspected acute encephalitis of viral origin, based on the clinical judgment of admitting physicians, and with no preexisting neurological conditions or evidence of bacterial meningitis by microscopy or culture of cerebrospinal fluid (CSF) samples, and no febrile convulsion (defined by a single convulsion lasting less than 15 minutes with regaining of consciousness within 60 minutes in a child between 6 months and 6 years of age) were eligible for inclusion in the study after provision of written informed consent by the patient's parents or legal guardians. Tests of human immunodeficiency virus and tuberculosis infections were not performed when recruiting the patients.
Clinical data and specimen collection
Detailed demographic and clinical data, including routine blood and CSF hematology and chemistry laboratory investigations, were collected on case record forms at enrollment and during follow-up. Clinical outcomes were defined as death, full recovery, and severe, moderate or minor neurological sequelae, defined based on neurological examination, degree of independent functioning and controllability of seizures. For etiological investigations, acute and convalescent CSF, plasma and serum samples were obtained at enrollment and around 2 weeks later or at discharge. In addition, throat and rectal swabs were collected in viral transport medium (VTM) at enrollment.
Etiological laboratory investigations
Nucleic acid extraction
Total nucleic acid was isolated from 180µL of clinical specimens (CSF, plasma, VTM) using a silica-guanidinium thiocyanate-based procedure [6], [7], recovered in 100µL of TE (10 mM Tris-HCl, 1 mM EDTA, pH 8.0, Sigma-Aldrich Pte. Ltd., Singapore) and stored at −80°C until used. For detection of human parechoviruses, enterovirus in blood, and bacterial pathogens, nucleic acids were isolated with the automated easyMAG system (BioMérieux, Marcy l'Étoile, France), following the manufacturer's instructions.
Bacterial investigations
Available CSF samples of enrolled patients were retrospectively analyzed for bacterial infections using internally controlled real-time polymerase chain reaction (PCR) methods for detection of 4 bacterial pathogens that are most frequently associated with meningitis in Vietnam: Streptococcus pneumoniae, Haemophilus influenzae type b (Hib), Neisseria meningitidis, and Streptococcus suis [8], [9]. Primer sequences and probes are listed in table 1. All oligonucleotides were ordered from Sigma-Proligo (Singapore)
Table 1. Oligonucleotide sequences of primers and probes used in this study.
| Pathogen | Gene target | Sample tested | Oligo sequence (5′ – 3′) | Number of PCR samples | |||||||
| Forward | Reverse | probe | Reference | Internal control | CSF | Throat swab | Rectal swab | plasma | |||
| HSV € | gB | a | CCG TCA GCA CCT TCA TCG A | CGC TGG ACC TCC GTG TAG TC | 6-FAM-CCA CGA GAT CAA GGA CAG CGG CC-TAMRA | [36] | Y | 192 | ND | ND | ND |
| Enteroviruses ¥ | 5′UTR | a, b, c, d± | CCC TGA ATG CGG CTA AT | ATT GTC ACC ATA AGC AGC C | 6-FAM-CGG AAC CGA CTA CTT TGG GT-TAMRA | [37] | Y | 192 | 177 | 178 | 9 |
| Enterovirus 71 | VP1/VP3 | a, b, c | ATAATAGCAYTRGCGGCAGCCCA | AGAGGGAGRTCTATCTCYCC | − | [38] | ND | 3 | 23 | 37 | ND |
| Parechoviruses | 5′UTR | a | CTGGGGCCAAAAGCCA | GGTACCTTCTGGGCATCCTTC | 6-FAM-AAACACTAGTTGTA(A/T)GGCCC-NFQ | [31], [39] | ND | 162 | ND | ND | ND |
| DENV-1 | 3′UTR | a, d, e | ATCCATGCCCATCAYCAATG | GATCARTGGTGTGGATCCCTG | 6-FAM-TCAGTGTGGAATAGGGTTTGGATAGAGGAA-BHQ-1 | [26] | Y | 16 | ND | ND | 16 |
| DENV-2 | 3′URT | a, d, e | ACAAGTCGAACAACCTGGTCCAT | GAGAAGACCAATGGTGCGGC | 6-FAM-GTT+T+Tg+T+CT+TC+CA+TCCA-BHQ-1 | Y | 16 | ND | ND | 16 | |
| DENV-3 | 3′UTR | a, d, e | TTTCTGCTCCCACCACTTTCAT | AGACTRGCATCCAACGCCA | 6-FAM-AAGAAAGTTGGTAGTTCCCTGCAGACCCCA-BHQ-1 | Y | 16 | ND | ND | 16 | |
| DENV-4 | 3′UTR | a, d, e | GYGTGGTGAAGCCYCTRGAT | ATGAAGGATGGCCGYTCACT | 6-FAM-ACTTCCCTCCTCTTYTTGAACGACATGGGA-BHQ-1 | Y | 16 | ND | ND | 16 | |
| Influenzavirus A | M | a | GACAAGACCAATCCTGTCACYTCTG | AAGCGTCTACGCTGCAGTC | 6-FAM-TTCACGCTCACCGTGCCCAGTGAGC-TAMRA | [12] | ND | 191 | 178 | ND | ND |
| EAV | NSP | NA | CATCTCTTGCTTTGCTCCTTA | AGCCGCACCTTCACATTG | Cy5-GCGCTCGCTGTCAGAACAACATTATTGCCCACAGCGCG-BHQ-3 | [26] | NA | NA | NA | NA | NA |
| PhHV | gB polymerase | NA | GGG CGA ATC ACA GAT TGA ATC | GCG GTT CCA AAC GTA CCA A | Cy5-TTT TTA TGT GTC CGC CAC CAT CTG GAT C′-BHQ3 | [8] | NA | NA | NA | NA | NA |
| MTV/SFV_1 * | E | a | TATTGGCTAAAGGAAAAAGGGACAG | CATAGCGACACCACAAAAGAAGG | − | ND | 192 | ND | ND | ND | |
| MTV/SFV_2 * | E | a | TACTGGCTAAAAGAAAAAGGAACTG | CACAGGGACACCACGAAGGATGG | − | ND | ND | ND | |||
| Flavivirus | NS5 | a | AACATGATGGGiAARAGAGARAA | GTGTCCCAiCCiGCiGTRTCATCiGC | − | [40] | ND | 192 | ND | ND | ND |
| CMV | IE | a | CCAAGCGGCCTCTGATAACCAA | GGTCATCCACACTAGGAGAGCAGAC | − | [41] | ND | 192 | ND | ND | ND |
| N. meningitides | ctrAe | a | GCTGCGGTAGGTGGTTCAA | TTGTCGCGGATTTGCAACTA | 6-FAM-CATTGCCACGTGTCAGCTGCACAT-TAMRA | [9] | Y | 162 | ND | ND | ND |
| H. influenzae type B | bexA | a | GGCGAAATGGTGCTGGTAA | GGCCAAGAGATACTCATAGAACGTT | 6-FAM-CACCACTCATCAAACGAATGAGCGTGG-TAMRA | Y | 162 | ND | ND | ND | |
| S. pneumoniae | ply | a | TGCAGAGCGTCCTTTGGTCTAT | CTCTTACTCGTGGTTTCCAACTTGA | 6-FAM-TGGCGCCCATAAGCAACACTCGAA-TAMRA | Y | 162 | ND | ND | ND | |
| S. suis | cps2J | a | GGTTACTTGCTACTTTTGATGGAAATT | CGCACCTCTTTTATCTCTTCCAA | 6-FAM-TCAAGAATCTGAGCTGCAAAAGTGTCAAATTGA-TAMRA | [8] | Y | 162 | ND | ND | ND |
Notes:
*in house, a: CSF, b: throat swab, c: rectal swab, d: plasma, e: culture supernatant, ND: not done, NA: non applicable, EAV: equine arteritis virus, PhHV: phocid herpesvirus, NFQ: non-fluorescence quencher.
Due to the availability of the assay, a commercial SYBR Green PCR master mix (Bio-Rad, USA) was used for the detection of HSV DNA when the study was started and was then replaced by a HSV Taqman real-time PCR.
±: only if detected by RT-PCR in the other sample types.
¥: internal control was not applied for detection of enterovirus in CSF samples.
CSF serology and virus culture of CSF and sera were done in all 194 enrollees.
PCR and RT-PCR analyses for viral infections
Conventional or real-time reverse transcription (RT) PCRs were used to detect HSV-1 and 2, cytomegalovirus, enteroviruses (generic), enterovirus 71, influenza A virus, Me Tri virus/Semliki Forest virus [10], human parechoviruses, flaviviruses (generic), and dengue viruses (DENV) types 1 to 4 if serology and/or virus culture results were suggestive of dengue. Primer and probe sequences are listed in Table 1 and were all adapted from previous publications except for those targeted at Me Tri/Semliki Forest virus which were newly designed [10].
Serology
A capture IgM ELISA assay (Venture Technologies, Sarawak, Malaysia) that utilizes inactivated antigens from JEV and DENV1 – DENV4 was used to detect and distinguish between JEV-specific and DENV-specific IgM antibodies in CSF specimens, and was performed as previously described [11]
Virus isolation
Acute CSF and serum specimens were inoculated onto C6/36 mosquito cells, RD (human rhabdomyosarcoma cells), BHK-21 (baby hamster kidney cells) and African green monkey kidney (Vero) cells (ECACC, Wiltshire, UK). Cells were observed daily for cytopathic effect and viruses were identified using standard procedures of direct- and indirect immunofluorescence staining assays, and virus-specific (RT) PCRs.
Diagnostic interpretation
Depending on clinical sample types in which evident of viral infetion was identified, the encephalitis etiology was considered as definitive, probable or possible cause. Details on the diagnostic interpretation are presented in table 2.
Table 2. Diagnostic interpretation.
| Category | Criteria | Virus |
| Definitive | Viral detection by PCR or culture in CSF, or detection of viral specific IgM by serologic test in CSF | JEV, DENV, EV, influenza A, CMV and HSV |
| Viral detection in both rectal and throat swabs as well as in blood by PCR, ANDAbsence of other viruses detected in CSF | EV | |
| Probable | Viral detection in both rectal and throat swabs by PCR, ANDAbsence of other viruses detected in CSF | EV |
| Possible | Viral detection in either rectal or throat swab by PCR, ANDAbsence of other viruses detected in CSF | EV and influenza A |
| Viral-specific IgM detected in blood by serologic test or viral RNA detected in plasma by PCR, ANDAbsence of other viruses detected in CSF | DENV |
Statistical analysis
Chi-square test, Fisher exact test, independent samples t test and Wilcoxon rank-sum test were used to compare data between groups of patients when appropriate by using either SPSS for Windows version 14 (SPSS Inc, Chicago, USA) or statistical software R version 2.9.0 (http://www.r-project.org). In addition, to analyze potential prognostic factors for fatal outcome, univariate analyses (including linear regression) and logistic regression were applied.
Ethical approval
This study was approved by the Institutional Review Board of CH1 and the OXford TRopical Ethics Committee (OXTREC), University of Oxford, UK. Written informed consent was obtained from a parent or guardian of each enrolled patients.
Results
Patients
A total of 194 patients with a clinical syndrome of acute encephalitis of presumed viral etiology were enrolled between January 1 and December 31, 2004.
Details of clinical features and outcome of enrolled patients are presented in Table 3. Overall mortality was observed in 57/194 (29%) of the patient, while 48/194 (25%) had neurological sequelae at discharge, including: severe sequelae in 10%, moderate in 10% and mild in 5% (Table 3).
Table 3. Characteristics of enrollees and comparisons between patient groups.
| Total enrollees | Bacterial PCR results | Fit the predefined case definition | ||||||||
| Negative (n = 151) | Positive (n = 12) | P value | OR (95% CI) | Yes (n = 132) | No (n = 62) | P value | OR (95%CI) | |||
| Demographics | Male | 124(64) | 94 (62) | 7 (58) | 0.7 | ND | 80 (61) | 44 (71) | 0.16 | ND |
| Age (median; IQR) | 3 (1–7) | 3 (1–7) | 1 (0–6.5) | 0.16 | ND | 3 (1–7) | 2 (1–6) | 0.13 | NA | |
| Non-HCMC resident | 139 (72) | 107 (71) | 10 (83) | 0.5 | ND | 102 (77) | 37 (60) | 0.011 | 2.3 (1.2–4.4) | |
| Referral# | 133 (69) | 100 (62) | 11 (92) | 0.1 | ND | 95 (72) | 38 (61) | 0.133 | ND | |
| Hospitalization ≠ | 9 (6–14) | 8 (6–13) | 9.5 (6–27) | 0.3 | ND | 9 (6–13) | 8.5 (6–18) | 0.63 | NA | |
| Clinical symptoms | History of fever | 181 (93) | 139 (96) | 11 (92) | 0.4 | ND | 128 (100) | 53 (90) | 0.001 | 3.4 (2.7–4.28) |
| Fever* | 142 (73) | 105 (70) | 10 (83) | 0.5 | ND | 99 (76) | 43 (69) | 0.36 | ND | |
| History of convulsion | 144 (74) | 110 (73) | 11 (92) | 0.2 | ND | 100 (76) | 44 (71) | 0.47 | ND | |
| Convulsions | 31 (16) | 20 (13.6) | 4 (33) | 0.09 | ND | 24 (19) | 7 (12) | 0.19 | ND | |
| Limb weakness | 60 (31) | 49 (33) | 3 (25) | 1.0 | ND | 50 (39) | 10 (16) | 0.002 | 3.27 (1.5–7.0) | |
| Neck stiffness | 44 (23) | 35 (23) | 3 (27) | 0.7 | ND | 38 (29) | 6 (10) | 0.003 | 3.75 (1.5–9.4) | |
| Skin Rash | 13 (7) | 8 (5.3) | 0 | 1.0 | ND | 7 (5) | 6 (10) | 0.35 | ND | |
| GCS≤9 | 123 (63) | 94 (62) | 8 (67) | 1.0 | ND | 91 (69) | 32 (52) | 0.019 | 2.08(1.1–3.9) | |
| JEV vaccination | Total | 36 (19) | 27 (19) | 2 (18) | 0.8 | ND | 24 (19) | 12 (20) | 0.89 | ND |
| Complete | 24 (12) | 19 (13) | 1 (9) | ND | NA | 16 (13) | 8(13) | ND | NA | |
| Incomplete | 12 (6) | 8 (6) | 1 (9) | ND | NA | 8 (6) | 4 (7) | ND | NA | |
| Outcome | Death | 57 (29) | 43 (29) | 4 (33) | 0.6 | NA | 40 (30) | 17 (27) | 0.015 | NA |
| Severe sequelae | 20 (10) | 14 (9) | 2 (17) | 11 (8) | 9 (15) | |||||
| Moderate sequelae | 19 (10) | 16 (11) | 0 | 18 (14) | 1 (1.6) | |||||
| Mild sequelae | 9 (5) | 7 (5) | 0 | 8 (6) | 1 (1.6) | |||||
| Full recovery | 89 (46) | 71 (47) | 6 (50) | 55 (41) | 34 (55) | |||||
| CSF laboratory | CSF WBC count$ | 2 (1–51) | 2 (1–43) | 98 (17–741) | 0.001 | ND | 8 (1–63) | 2 (1–3) | <0.001 | NA |
| CSF pleocytosis | 83 (43) | 60 (40) | 9 (82) | 0.01 | 0.15 (0.03–0.7) | 71 (54) | 12 (20) | <0.001 | 4.73 (2.30–9.7) | |
NOTE: Data are no. (%) of patients; denominators may vary slightly; IQR = interquartile range,
median, cells/mm3, IQR,
*Fever at admission (≥37.5°C),
referred from other hospitals,
≠: day, median, IQR; GCS, Glasgow Coma Scale.
Pathogens
PCR detection of bacterial pathogens
In total, CSFs from 163 patients were available for bacterial PCR investigation. Evidence of bacterial infection was found in 12 (6%) patients, including Haemophilus influenzae type b in 6 patients and Streptococcus pneumoniae in another 6 patients. Evidence of viral co-infection was found in CSF samples of 3 patients (Haemophilus influenzae with EV or JEV in 2 patients, and Streptococcus pneumoniae with JEV in 1 patient). Except for CSF cell counts, no difference in patient characteristics and outcome between patients with negative and positive bacterial PCR was observed (Table 3).
Definitive and probable viral etiologies
A definitive or probable viral etiology was identified in 80 of 194 (41%) enrolled patients (Table 4 and Table 5). The most frequently identified etiologies were definitive JEV infection (50 cases, 26%), followed by enterovirus (18 cases, 9.3%), and definitive dengue viruses (9 cases, 4.6%). Of the enteroviral infections, only 4 of 18 were considered definitive encephalitis based on viral detection in CSF or blood. HSV, CMV and influenza A virus were detected in CSF of 1 patient each. The case of influenza encephalitis was caused by highly pathogenic avian influenza A (H5N1) virus and has previously been described [12].
Table 4. Laboratory results of three most common viruses (JEV, DENV and EV) detected in this study.
| Virus | Clinical samples | Detected by | Diagnostic category | Total (%) | ||
| Serology | PCR | Culture | ||||
| JEV | CSF | 50 | 0 | 0 | Definitive | 50 (26) |
| DENV | CSF | 7 | 4 | 6 | Definitive | 9 (4.6) |
| EV | CSF | ND* | 3 | 0 | Definitive | 4 (2.1) |
| Rectal, throat and blood | ND | 1 | ND | |||
| Rectal and throat | ND | 14 | ND | Probable | 14 (7.2) | |
NOTE:
*ND: not done.
Table 5. Viral etiology of encephalitis patients and comparisons of patient characteristics.
| *JEV | *DENV | EV | *HSV | *Inf. A | *CMV | *Diag. | *Undiag. | Diag. versus Undiag. | |||||||
| EV | EV71 | Total | P value | OR (95%CI) | |||||||||||
| Definitive | probable | Definitive | probable | ||||||||||||
| Diagnosis in | 50 (26) | 9 (4.6) | 2 | 10 (5.1) | 2 | 4 (2.1) | 18 (9.3) | 1 | 1 | 1 | 80 (41) | 114 (59) | NA | NA | |
| Outcome | Death | 8 (16) | 1 (11) | 2 | 4 (40) | 0 | 3 (75) | 9 (50) | 0 | 1 | 0 | 19 (24) | 38 (33) | 0.037 | ND |
| Severe sequelae | 5 (10) | 1 (11) | 0 | 2 (20) | 0 | 0 | 2 (11) | 0 | 0 | 1 | 9 (11) | 11 (10) | |||
| Moderate sequelae | 11 (22) | 0 | 0 | 2 (20) | 1 | 0 | 3 (17) | 0 | 0 | 0 | 14 (18) | 4 (4) | |||
| Minor sequelae | 3 (6) | 0 | 0 | 0 | 1 | 0 | 1 (6) | 0 | 0 | 0 | 4 (5) | 5 (4) | |||
| Characteristics | Age | 6 (3–10) | 8 (0–13) | 1 &13 | 1.2 (0.75–3.2) | <1& 2 | 1.2 (1–2.6) | 1.3 (0.75–3.3) | 4 | 4 | 0 | 4 (1–9) | 2 (1–6) | 0.002 | ND |
| Male | 27 (54) | 8 (89) | 2 | 10 (100) | 1 | 3 (75) | 16 (89) | 1 | 1 | 1 | 54 (68) | 70(61) | 0.3 | ND | |
| Non-HCMC resident | 43 (86) | 7 (78) | 2 | 8 (80) | 1 | 3 (75) | 14 (78) | 1 | 1 | 1 | 67 (84) | 72 (63) | 0.002 | 3 (1.5–6.1) | |
| Referral | 39 (78) | 8 (89) | 1 | 7 (70) | 1 | 1 (25) | 10 (56) | NA | 1 | 1 | 60 (75) | 73 (63) | 0.1 | ND | |
| History of fever | 48 (100) | 9 (100) | 2 | 9 (90) | 2 | 4 (100) | 17 (100) | 1 | 1 | 1 | 76 (100) | 105 (92) | 0.08 | ND | |
| Fever | 37 (75.5) | 6 (67 | 2 | 8 (80) | 1 | 3 (75) | 14 (78) | 0 | 1 | 0 | 58 (73) | 84 (74) | 0.9 | ND | |
| Limb weakness | 18 (37) | 2 (22) | 1 | 3 (30) | 1 | 3 (75) | 8 (44) | 1 | 0 | 0 | 29 (37) | 31 (27) | 0.2 | NA | |
| Neck stiffness | 19 (38) | 2 (22) | 1 | 1 (10) | 0 | 0 | 2 (11) | 0 | 0 | 1 | 23 (29) | 21 (18) | 0.08 | ND | |
| History of convulsion | 36 (72) | 6 (67) | 2 | 5 (50) | 0 | 2 (50) | 9 (50) | 1 | 1 | 1 | 54 (68) | 90 (79) | 0.07 | NA | |
| Convulsion | 3 (6.3) | 1 (11) | 0 | 2 (20) | 0 | 0 | 2 (11) | 0 | 0 | 1 | 7 (9) | 24 (21) | 0.03 | 2.7(1.1–6.7) | |
| GCS (≤9) | 32 (64) | 3 (33) | 1 | 7 (70) | 1 | 3 (75) | 12 (67) | 0 | 1 | 1 | 49 (61) | 74 (65) | 0.6 | ND | |
| White cells | 51.5 (20–102) | 2 (1–2.5) | 61 & 108 | 13.5 (1.5–96) | 22&164 | 59 (11–102) | 22 (4.3–99) | 0 | 1 | NA | 41 (2–92) | 0(1–5) | <0.001 | ND | |
| CSF pleocytosis | 40 (80) | 0 | 2 | 7 (70) | 2 | 3 (75) | 14 (78) | 0 | 0 | NA | 54 (68) | 29 (25) | <0.001 | 6.9 (3.61–13.1) | |
| Hospital stay | 9 (7–15) | 8 (7–12) | 2–8 | 3.5 (2–14) | 8–28 | 1.5 (1–7) | 6 (2–14.3) | 6 | 3 | 30 | 9(6–14) | 9 (5–15) | 0.7 | ND | |
NOTE: Data are no. (%) of patients; denominators may vary.
For continuous variables: data are presented as median (IQR); NA, non- applicable; ND, not done; OR, odds ratio; CI, confident interval; undiag., undiagnosed group; Diag., diagnosed group.
Among the patients with definitive etiological diagnoses, infections with additional viruses in single non-CNS samples (rectal or throat swab) were detected in 14 patients including EV (n = 8) or influenza A virus (n = 2) in patients with definitive JE, EV (n = 3) in patients with definitive DENV encephalitis, and influenza A virus in a patient with HSV encephalitis.
Characteristics and outcomes of patients with definitive or probable viral etiologies are shown in Table 5. The case fatality proportion was significantly higher in patients with enteroviral infection than in those with JEV infection: 9/18 (50%) versus 8/50 (16%) respectively (OR, 5.3; 95% CI, 1.6–17.3; P = 0.009). Neurological sequelae were common in both groups (Table 5). Patients with enteroviral encephalitis were significantly younger than Japanese encephalitis patients (median; IQR: 1.3; 0.75–3.3 years versus 6; 3–10 years respectively; P<0.001).
Possible- and unknown etiologies
A possible viral etiology was detected in 27/194 (14%) of patients, including enteroviruses in 11/194 patients (5.7%), dengue in 7/194 patients (3.6%), influenza A virus in 6/194 patients (3%), combined EV and dengue in 1 patient, and combined EV and influenza A virus in 2 patients. In 87/194 (45%) patients no pathogen could be found or implicated in any specimen.
As comparison found not statistical difference in clinical outcomes and patient characteristics between patients with possible viral diagnoses and those with no diagnoses (data not shown), clinical data of these two groups (undiagnosed group) were combined and compared against patient group of definitive or probable viral diagnosis (diagnosed group). A higher mortality was observed in the undiagnosed group (24% vs 33%, P = 0.037, Table 5). Patients with undiagnosed illness were younger, more likely to live in Ho Chi Minh City, more likely to present with convulsion, less likely to have CSF pleocytosis, and had lower CSF white blood cell counts (Table 5).
Temporal distribution of etiologies
Patients were enrolled throughout the year. JE occurred throughout the year but there was a peak in March and during the months of June and July consistent with the rainy season. No clear seasonality was observed for other etiologies but numbers were small (data not shown).
CSF PCR and serology in relation to illness day at admission
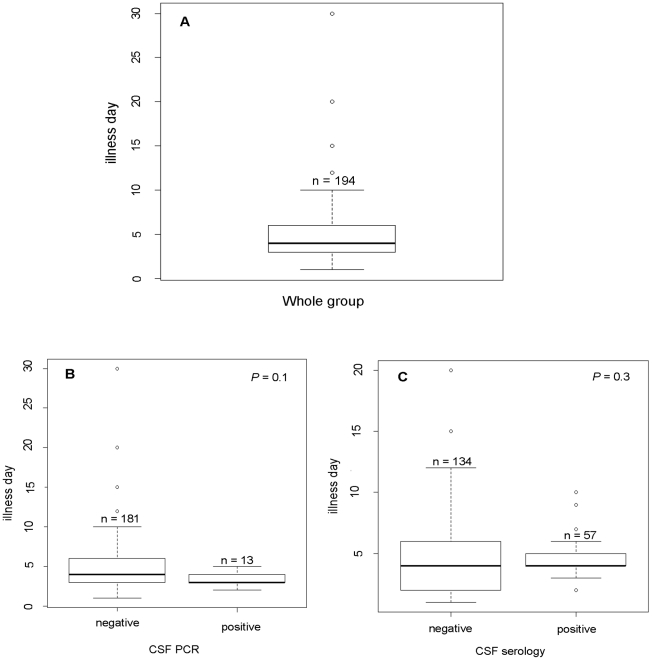
Since detection of virus or antibodies may differentially be affected by the timing of specimen collection, we analysed diagnostic yields in CSF specimens by PCR and serology in relation to illness day at the time of sampling (Figure 1). Overall, the median illness day at admission was 4 days (IQR: 3–6). No significant differences in illness days at specimen collection were observed between patients with detectable virus or IgM in CSF and those with negative diagnostic results. However, there was a trend towards a shorter illness duration at time of sampling in PCR positive patients (median, IQR: 3.5, 3–4 versus 4.8, 3–6, P = 0.1) (Figure 1).
Figure 1. Illness day on admission Vs CSF PCR and serology.
A, whole group; B, comparison of illness day on admission of viral PCR negative and viral PCR negative group; C, CSF serology negative and CSF serology positive groups.
Prognostic factors for fatal outcome
Of the 57 fatal cases, 28 patients (49%) died within 7 days after onset of illness (Table 6). Twenty-nine (51%) of fatal cases were between 0 and 1 years of age compared to 43 of 137 (31%) surviving patients. Univariate analyses were used to screen for potential factors associated with fatal outcome (Table 7), and demonstrated that the occurrence of convulsions on admission, the presence of limb weakness, GCS, and age were all significantly associated with fatal outcome, whereas illness day on admission, history of convulsion, and gender were not (Table 7). However, only GCS and age remained independently associated risk factors for fatal outcome in logistical regression analysis (Table 7).
Table 6. Time (week) to death since onset of illness.
| Time (week) | ||||
| 1 | 2 | 3 | Total | |
| Total | 28 (49) | 16 (28) | 13 (23) | 57 |
| JEV | 2 (3.5) | 3 (5) | 3 (5) | 8 (14) |
| EV | 6 (10.5) | 2 (3.5) | 1 (2) | 9 (16) |
| DENV | 1 (1.8) | 0 | 0 | 1 (2) |
| Influenza A | 0 | 1 (2) | 0 | 1 (2) |
| Undiagnosed | 19 (33) | 10 (17.5) | 9 (16) | 38 (67) |
NOTE: data are no. (% of total) fatal patients.
Table 7. Univariate and adjusted logistic regression to determine prognostic factors of fatal outcome.
| *Factors | Univariate | Adjusted# | ||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age (by +1 year) | 0.91 | 0.84–0.99 | 0.047 | 0.89 | 0.80–0.99 | 0.038 |
| GCS (by +1) | 0.62 | 0.52–0.72 | <0.001 | 0.60 | 0.51–0.71 | <0.001 |
| Convulsion | 2.26 | 0.98–5.21 | 0.053 | Excluded* | ||
| Neck stiffness | 0.43 | 0.17–1.04 | 0.063 | Excluded | ||
| Limb weakness | 2.94 | 1.49–5.78 | 0.001 | Excluded | ||
| Illness day (by +1 day later) | 1.03 | 0.94–1.13 | 0.48 | Not included± | ||
| History of convulsion | 0.75 | 0.36–1.54 | 0.44 | Not included | ||
| Rural origin | 0.86 | 0.42–1.74 | 0.68 | Not included | ||
| Gender (female) | 1.23 | 0.63–2.40 | 0.53 | Not included | ||
NOTE:
Results based on logistic regression;
*variables excluded after backwards variable selection;
±: variables not included in backwards variable selection.
Discussion
Acute encephalitis is an inflammation of the brain parenchyma, most commonly caused by viruses and associated with substantial morbidity and mortality. Worldwide, reported mortality ranges between 0–11% [3], [4], [13]–[17], but was substantially higher in our study: 30% of children died during hospitalization, with about half of deaths occurring within 3–7 days after the onset of illness, and more than half affecting infants. Furthermore, 25% of surviving children suffered from mild to severe neurological sequelae at discharge (including: severe sequelae in 10%, moderate in 10% and mild in 5%). Several prognostic factors for death or severe outcome of acute encephalitis have been proposed [14], [15]. Whereas, similar to other studies, univariate analyses in our study suggested associations between fatal outcome and age, convulsions at admission and limb weakness, GCS and age remained the only independent prognostic factors for fatal outcome in logistic regression analyses.
Our study illustrates the challenge of identifying the causative agents in children with acute encephalitis. A confirmed or probable etiology was identified in only 41% of enrolled patients which is within the same range as reported in other etiology studies [3], [4], [13], [14], [18]. Worldwide, the causes of encephalitis vary between geographical regions. While JEV was the most common cause of encephalitis in children in Cambodia, enteroviruses and Tick-borne encephalitis virus were the two most common viruses found in young patients in the United States and Sweden, respectively [3], [4], [19]. A study in China revealed enteroviruses, mumps virus and rubella virus as frequent causes of encephalitis in children between 7 months and 13 years of age [20]. Our study identified JEV, enteroviruses and DENV as the most common etiologies in southern Vietnamese children, accounting for 26%, 9.3%, and 4.6% of cases, respectively. The high prevalence of JEV in our patients is in accordance with a previous study in Ho Chi Minh City reporting a prevalence of 45% in children with encephalitis [5]. Similar to previous studies, JEV was associated with high mortality (16%) [5], [21], [22]. Our observations emphasize the need for JE vaccination programs in Vietnam and other regions of Southeast Asia where JEV is endemic [4]. Widespread JE vaccination in developing countries like Vietnam is complicated by high costs and the requirement of multiple vaccine doses. In our study population, only 19.5% of patients had received at least one dose of JE vaccine. There was a trend of lower vaccination rates in JE patients, but this did not reach statistical significance (data not shown).
Enteroviruses are well established causes of aseptic meningitis and encephalitis in young children. While reported case fatality rates of enteroviral central nervous system (CNS) infections are relatively low (0–7%) [3], [13], mortality of confirmed or probable enteroviral encephalitis in our patients was high with 9 of 18 (50%) of patients dying during hospitalisation. However, a definitive diagnosis of enteroviral encephalitis was only established in 4 of these 18 patients, 2 of whom died. In the remaining patients enteroviral encephalitis was not confirmed by virus detection in CSF but suspected based on virus detection in throat and rectum and a clinical syndrome consistent with the diagnosis. Hence, a definitive causative role of enteroviruses in these patients remains unclear, particularly since a convincing link has not yet to be established between detection of enteroviruses in non-CNS sites and encephalitis [23]. In addition, beside enterovirus 71 (EV71), we have not determined whether our patients were infected with enterovirus serotypes of particularly high virulence, such as coxsackievirus B4 and echovirus 11 which have been associated with high case-fatality rates among neonates [24]. Six of 18 (33%) enteroviral infections in our patient group were caused by EV71. In accordance with reported high mortality of EV71 CNS infections, 3 of these patients died [25].
CNS manifestations are rare clinical complications of dengue but are reported with an increasing frequency in endemic areas [26]–[28]. Dengue was found in 4% of 378 pediatric patients with suspected encephalitis in southern Vietnam [27]. Similarly, DENV was identified in nearly 5% of our study patients. The precise pathogenesis of dengue-associated CNS manifestations remains unclear [27], [29]. Patients in this study experienced mild clinical symptoms of dengue without shock or bleeding and all made a full recovery. However, the detection of virus and specific antibodies in CSF suggest that invasion and viral replication in the CNS may play a role in at least a proportion of patients with dengue-associated neurological symptoms.
Me Tri virus is a variant of Semliki Forest virus belonging to the genus Alphavirus isolated from mosquitoes collected in Vietnam [10], [30]. Previous serologic surveillance suggested that this virus might be a frequent cause of encephalitis in young patients in Vietnam with a prevalence of 14% [30]. However, the virus was not detected in any of our patients. Likewise, human parechoviruses are an emerging cause of meningitis and encephalitis in children and infants [31], [32], but were not detected in our study patients. As a previous report suggested that human parechovirus infections exhibit a two-yearly incidence peak [33], the absence of parechovirus infections in our patients may be due to the fact that our study period covered only one year. HSV encephalitis was found in only one patient in our study which is not unexpected considering the young age of our study group.
Bacterial pathogens, including S. pneumoniae and H. influenzae type B, were detected retrospectively by PCR in 12 children (6%), illustrating the difficulties in distinguishing acute viral encephalitis and bacterial meningitis in children based on clinical judgment only. Indeed, the clinical syndromes of these children seemed indistinguishable from the remaining group of patients, although CSF white cell counts were higher as expected (Table 3). Of note, 4 children with bacterial meningitis had evidence of concurrent viral encephalitis based on the detection of virus (EV, CMV) or specific IgM (JEV) in the CSF. These observations suggest that testing for other pathogens should still be considered when a single pathogen has been identified in the CSF. While the clinical relevance of these coinfections remain unclear, it is tempting to speculate about the interplay between viral and bacterial CNS infections, for example by facilitating entry of one or the other into the CNS compartment.
In 59% of the children, no confirmed or probable viral etiology could be identified. Several factors may contribute to this relatively low diagnostic yield.
Firstly, a proportion of children may not have suffered from viral or other infectious CNS illnesses. Other conditions that may have presented in this manner may include pretreated pyogenic meningitis, toxins, atypical bacteria, mycobacterial and other unknown or un-tested bacteria or viruses. As mentioned before, bacterial meningitis was in fact diagnosed retrospectively by PCR in 6% of enrolled children.
A reliable case definition for acute viral encephalitis would be helpful for triage and clinical management of patients, especially in the absence of diagnostic support. When retrospectively using a predefined case definition consisting of a) fever history of less than seven days, b) an alteration or reduction of consciousness (Glasgow coma scale≤14), c) at least one of the following symptoms or signs: seizures (excluding febrile convulsions, defined as a single convulsion lasting less than 15 minutes in patients between 6 months and 6 years of age), focal neurological signs, neck stiffness or cerebrospinal fluid (CSF) pleocytosis, d) no evidence of bacterial infection in CSF specimens, and e) no alternative diagnosis for the clinical syndrome during admission, the viral diagnostic yield only modestly increased from 41 to 45%, whereas definitive/probable laboratory diagnoses of viral CNS infections were established in 20 of 62 patients (32%) who did not meet the criteria of the case definition. Overall clinical outcome and patients characteristics were similar between the two groups even though there were slight differences in terms of patient origins, clinical outcome, history of fever, limb weakness, neck stiffness, percentage of patient with a GCS below 9 and CSF cell count (Table 3). The last five are more likely due biases toward the criteria of predefined case definition.
Diagnostic yield was similar when the criterion of a fever history of less than seven days and febrile convulsions were left out. Diagnostic yield was higher when CSF results were included into this case definition (White cell count <1000 10e6/L, CSF/blood glucose ratio >50%, protein <0.45 g/L, CSF lactate <4 mmol/L): 49% and 37% in patients that did or did not meet the case definition, respectively. However, two thirds of all patients did not meet the case definition. Clearly, further studies are needed to optimize case definitions for viral encephalitis.
The limited detection of viral pathogens in our patients may have been due to clearance of virus from CSF at the time of clinical presentation, as is the case for JEV [34]. This may also explain why, except for dengue virus, flaviviruses were not detected in any of our patients, including those with serologically confirmed JEV infections. The importance of early sampling for reliable diagnostics is suggested by findings in our study: most of the patients were admitted relatively late in the course of illness, possibly in part because of referral delays, and a trend was observed towards shorter illness duration at the time of sampling in patients with positive virus-specific PCRs. The association between earlier sampling time and PCR positivity was statistically significant when analysis was restricted to 132 patients fulfilling our predefined case definition of acute viral encephalitis (3 days versus 4 days respectively, P = 0.03).
As diagnostic assays of some viral pathogens are not available in our laboratory, we did not exhaustively look for all potential infectious causes of encephalitis in Vietnam. Thus various etiologies (e.g. measles virus, henipaviruses and Banna virus) may have been missed. In addition, undiagnosed encephalitis may be caused by as yet unknown human or zoonotic pathogens. As socio-economic, ecological and environmental factors in Southeast Asia may favor the emergence of novel zoonotic or vector-borne pathogens [35], the circulation of novel pathogens in Vietnam is probable. Therefore, efforts to identify novel or previously unrecognized pathogens in these undiagnosed patients are essential for future prevention and treatment strategies.
Supporting Information
Translation of the Abstract into Vietnamese by Le Van Tan
(0.03 MB DOC)
STROBE checklist
(0.09 MB DOC)
Acknowledgments
We thank Dr Hoang Thi Thanh Hang and Dr Marcel Wolbers for advice on statistic analyses.
Part of this study was presented at a meeting on Infectious Diseases of the Nervous System, Paris, 10th–13th September 2008 in a poster session (Poster Code: P67).
Footnotes
The authors have declared that no competing interests exist.
This work was supported by the Wellcome Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Granerod J, Crowcroft NS. The epidemiology of acute encephalitis. Neuropsychol Rehabil. 2007;17:406–428. doi: 10.1080/09602010600989620. [DOI] [PubMed] [Google Scholar]
- 2.Davison KL, Crowcroft NS, Ramsay ME, Brown DW, Andrews NJ. Viral encephalitis in England, 1989–1998: what did we miss? Emerg Infect Dis. 2003;9:234–240. doi: 10.3201/eid0902.020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaser CA, Honarmand S, Anderson LJ, Schnurr DP, Forghani B, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565–1577. doi: 10.1086/509330. [DOI] [PubMed] [Google Scholar]
- 4.Srey VH, Sadones H, Ong S, Mam M, Yim C, et al. Etiology of encephalitis syndrome among hospitalized children and adults in Takeo, Cambodia, 1999–2000. Am J Trop Med Hyg. 2002;66:200–207. doi: 10.4269/ajtmh.2002.66.200. [DOI] [PubMed] [Google Scholar]
- 5.Solomon T, Dung NM, Kneen R, Thao le TT, Gainsborough M, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 2002;125:1084–1093. doi: 10.1093/brain/awf116. [DOI] [PubMed] [Google Scholar]
- 6.Boom R, Sol C, Beld M, Weel J, Goudsmit J, et al. Improved silica-guanidiniumthiocyanate DNA isolation procedure based on selective binding of bovine alpha-casein to silica particles. J Clin Microbiol. 1999;37:615–619. doi: 10.1128/jcm.37.3.615-619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boom R, Sol J, Salimans MM, Jansen CL, Wertheim-van Dillen PM, et al. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mai NT, Hoa NT, Nga TV, Linh LD, Chau TT, et al. Streptococcus suis Meningitis in Adults in Vietnam. Clin Infect Dis. 2008 doi: 10.1086/527385. [DOI] [PubMed] [Google Scholar]
- 9.Corless CE, Guiver M, Borrow R, Edwards-Jones V, Fox AJ, et al. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Microbiol. 2001;39:1553–1558. doi: 10.1128/JCM.39.4.1553-1558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan le V, Ha do Q, Hien VM, van der Hoek L, Farrar J, et al. Me Tri virus: a Semliki Forest virus strain from Vietnam? J Gen Virol. 2008;89:2132–2135. doi: 10.1099/vir.0.2008/002121-0. [DOI] [PubMed] [Google Scholar]
- 11.Cardosa MJ, Wang SM, Sum MS, Tio PH. Antibodies against prM protein distinguish between previous infection with dengue and Japanese encephalitis viruses. BMC Microbiol. 2002;2:9. doi: 10.1186/1471-2180-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 13.Kolski H, Ford-Jones EL, Richardson S, Petric M, Nelson S, et al. Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994–1995. Clin Infect Dis. 1998;26:398–409. doi: 10.1086/516301. [DOI] [PubMed] [Google Scholar]
- 14.Klein SK, Hom DL, Anderson MR, Latrizza AT, Toltzis P. Predictive factors of short-term neurologic outcome in children with encephalitis. Pediatr Neurol. 1994;11:308–312. doi: 10.1016/0887-8994(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 15.Rautonen J, Koskiniemi M, Vaheri A. Prognostic factors in childhood acute encephalitis. Pediatr Infect Dis J. 1991;10:441–446. doi: 10.1097/00006454-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Wang IJ, Lee PI, Huang LM, Chen CJ, Chen CL, et al. The correlation between neurological evaluations and neurological outcome in acute encephalitis: a hospital-based study. Eur J Paediatr Neurol. 2007;11:63–69. doi: 10.1016/j.ejpn.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Rayamajhi A, Singh R, Prasad R, Khanal B, Singhi S. Study of Japanese encephalitis and other viral encephalitis in Nepali children. Pediatr Int. 2007;49:978–984. doi: 10.1111/j.1442-200X.2007.02495.x. [DOI] [PubMed] [Google Scholar]
- 18.Cizman M, Jazbec J. Etiology of acute encephalitis in childhood in Slovenia. Pediatr Infect Dis J. 1993;12:903–908. doi: 10.1097/00006454-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Fowler A, Stodberg T, Eriksson M, Wickstrom R. Childhood encephalitis in Sweden: etiology, clinical presentation and outcome. Eur J Paediatr Neurol. 2008;12:484–490. doi: 10.1016/j.ejpn.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Zhaori G, Vene S, Shen K, Zhou Y, et al. Viral etiology of acute childhood encephalitis in Beijing diagnosed by analysis of single samples. Pediatr Infect Dis J. 1996;15:1018–1024. doi: 10.1097/00006454-199611000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Ooi MH, Lewthwaite P, Lai BF, Mohan A, Clear D, et al. The epidemiology, clinical features, and long-term prognosis of Japanese encephalitis in central sarawak, malaysia, 1997–2005. Clin Infect Dis. 2008;47:458–468. doi: 10.1086/590008. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R, Tripathi P, Singh S, Bannerji G. Clinical features in children hospitalized during the 2005 epidemic of Japanese encephalitis in Uttar Pradesh, India. Clin Infect Dis. 2006;43:123–131. doi: 10.1086/505121. [DOI] [PubMed] [Google Scholar]
- 23.Fowlkes AL, Honarmand S, Glaser C, Yagi S, Schnurr D, et al. Enterovirus-associated encephalitis in the California encephalitis project, 1998–2005. J Infect Dis. 2008;198:1685–1691. doi: 10.1086/592988. [DOI] [PubMed] [Google Scholar]
- 24.Khetsuriani N, Lamonte A, Oberste MS, Pallansch M. Neonatal enterovirus infections reported to the national enterovirus surveillance system in the United States, 1983–2003. Pediatr Infect Dis J. 2006;25:889–893. doi: 10.1097/01.inf.0000237798.07462.32. [DOI] [PubMed] [Google Scholar]
- 25.Beig FK, Malik A, Rizvi M, Acharya D, Khare S. Etiology and clinico-epidemiological profile of acute viral encephalitis in children of western Uttar Pradesh, India. Int J Infect Dis. doi: 10.1016/j.ijid.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, et al. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg. 2001;65:848–851. doi: 10.4269/ajtmh.2001.65.848. [DOI] [PubMed] [Google Scholar]
- 27.Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Tripathi S, Tambe JJ, Arora V, Srivastava A, et al. Dengue encephalopathy in children in Northern India: clinical features and comparison with non dengue. J Neurol Sci. 2008;269:41–48. doi: 10.1016/j.jns.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Gould EA, Solomon T. Pathogenic flaviviruses. Lancet. 2008 Feb 9;371:500–509. doi: 10.1016/S0140-6736(08)60238-X. [DOI] [PubMed] [Google Scholar]
- 30.Ha DQ, Calisher CH, Tien PH, Karabatsos N, Gubler DJ. Isolation of a newly recognized alphavirus from mosquitoes in Vietnam and evidence for human infection and disease. Am J Trop Med Hyg. 1995;53:100–104. [PubMed] [Google Scholar]
- 31.Benschop K, Molenkamp R, van der Ham A, Wolthers K, Beld M. Rapid detection of human parechoviruses in clinical samples by real-time PCR. J Clin Virol. 2008;41:69–74. doi: 10.1016/j.jcv.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Verboon-Maciolek MA, Groenendaal F, Hahn CD, Hellmann J, van Loon AM, et al. Human parechovirus causes encephalitis with white matter injury in neonates. Ann Neurol. 2008;64:266–273. doi: 10.1002/ana.21445. [DOI] [PubMed] [Google Scholar]
- 33.Wolthers KC, Benschop KS, Schinkel J, Molenkamp R, Bergevoet RM, et al. Human parechoviruses as an important viral cause of sepsislike illness and meningitis in young children. Clin Infect Dis. 2008;47:358–363. doi: 10.1086/589752. [DOI] [PubMed] [Google Scholar]
- 34.Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351:370–378. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- 35.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beld M, Minnaar R, Weel J, Sol C, Damen M, et al. Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control. J Clin Microbiol. 2004;42:3059–3064. doi: 10.1128/JCM.42.7.3059-3064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perera D, Podin Y, Akin W, Tan CS, Cardosa MJ. Incorrect identification of recent Asian strains of Coxsackievirus A16 as human enterovirus 71: improved primers for the specific detection of human enterovirus 71 by RT PCR. BMC Infect Dis. 2004;4:11. doi: 10.1186/1471-2334-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oberste MS, Maher K, Pallansch MA. Specific detection of echoviruses 22 and 23 in cell culture supernatants by RT-PCR. J Med Virol. 1999;58:178–181. doi: 10.1002/(sici)1096-9071(199906)58:2<178::aid-jmv13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 40.Scaramozzino N, Crance JM, Jouan A, DeBriel DA, Stoll F, et al. Comparison of flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J Clin Microbiol. 2001;39:1922–1927. doi: 10.1128/JCM.39.5.1922-1927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boom R, Sol CJ, Schuurman T, Van Breda A, Weel JF, et al. Human cytomegalovirus DNA in plasma and serum specimens of renal transplant recipients is highly fragmented. J Clin Microbiol. 2002;40:4105–4113. doi: 10.1128/JCM.40.11.4105-4113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Translation of the Abstract into Vietnamese by Le Van Tan
(0.03 MB DOC)
STROBE checklist
(0.09 MB DOC)