Abstract
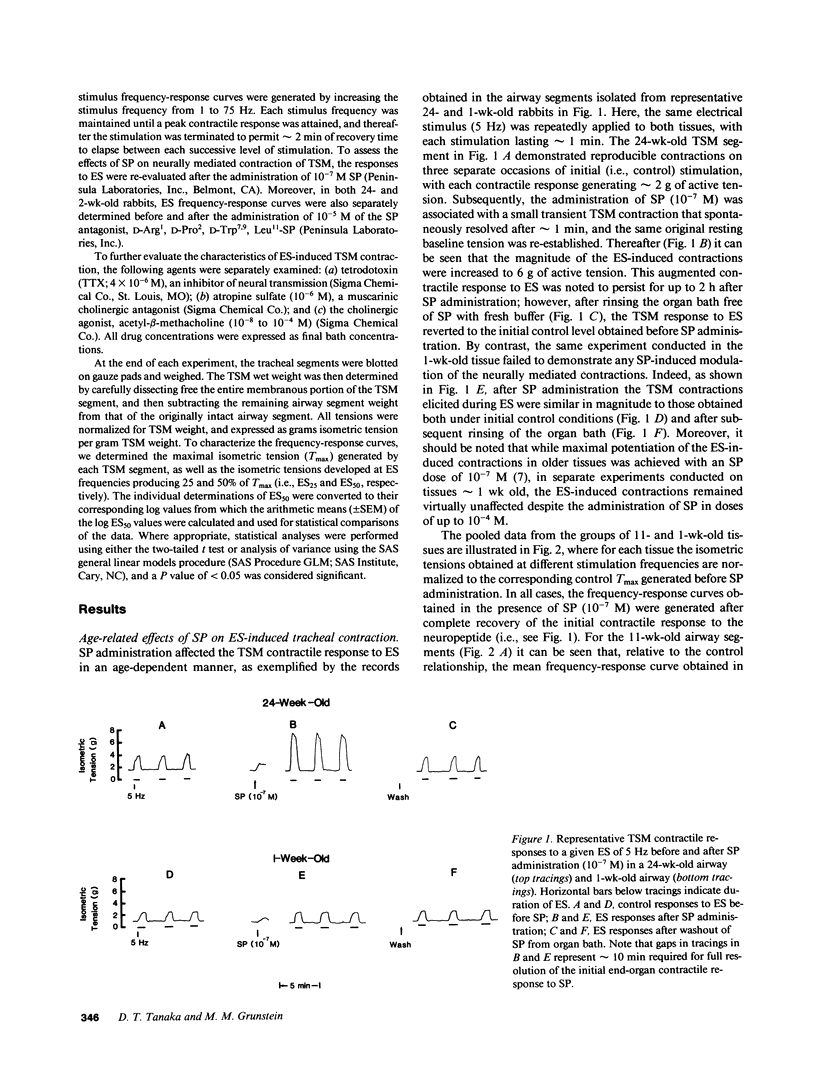
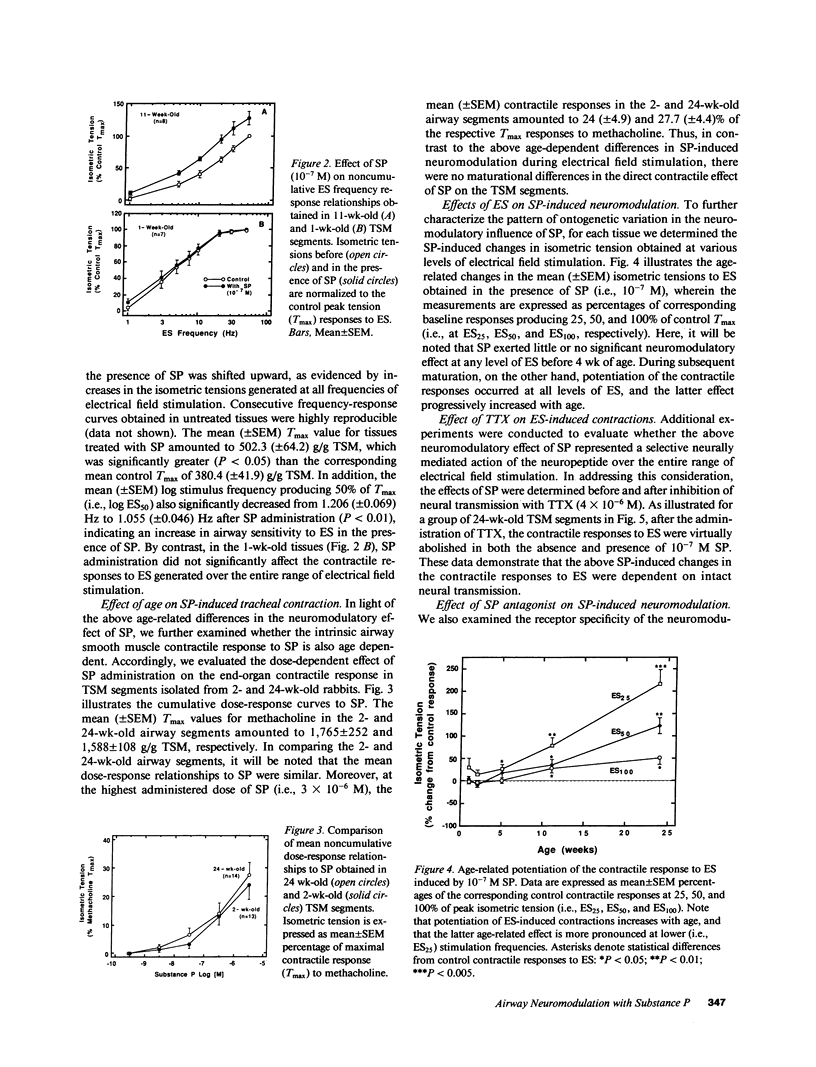
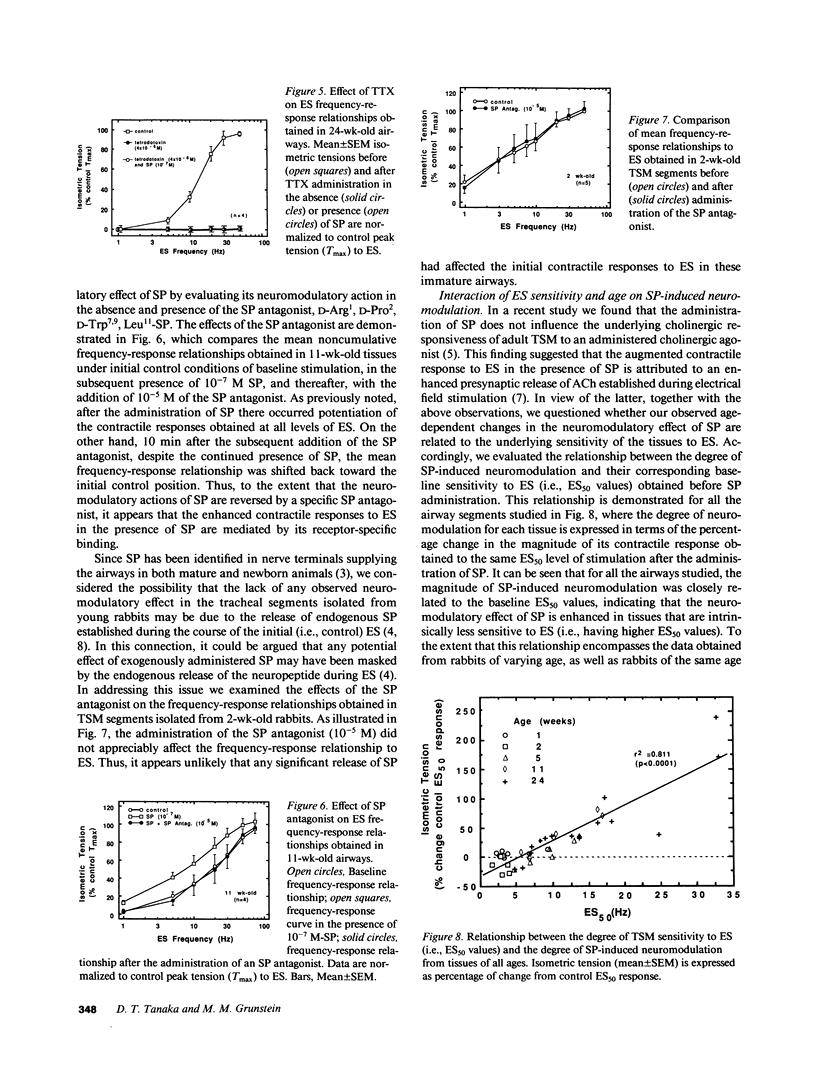
The maturation of the neuromodulatory action of substance P (SP) was investigated in tracheal smooth muscle (TSM) segments isolated from rabbits aged 2-24 wk. The tissues were placed in baths containing Krebs-Ringer solution and contracted with electrical field stimulation (ES) with ES frequencies ranging from 1 to 75 Hz. In tissues greater than 1 mo of age, the ES frequency-response relationships were progressively shifted in the presence of a maximally effective neuromodulatory SP dose (10(-7) M) such that by 24 wk of age the mean (+/- SEM) maximal tension (Tmax) significantly increased from 380.4 (+/- 41.9) to 502.3 (+/- 64.2) g/g TSM, and the corresponding mean (+/- SEM) log ES frequency producing 50% of Tmax (log ES50) significantly decreased from 1.209 (+/- 0.069) to 1.055 (+/- 0.046) Hz. By contrast, relative to methacholine, the direct contractile effects of SP did not significantly vary with age. In further analyzing the basis for the above age-related difference in the neuromodulatory action of SP, we found that the magnitude of SP-induced neuromodulation was highly correlated to the tissue's intrinsic sensitivity to ES. Indeed, after accounting for the tissue's sensitivity to ES, the effect of age alone on the magnitude of SP-induced neuromodulation was not statistically significant. These findings provide new evidence that: (a) SP-induced neuromodulation of acetylcholine release at the airway neuromuscular junction is significantly enhanced during postnatal development; and (b) that the latter age-dependent action of SP is based on a close coupling of the magnitude of SP-induced neuromodulation to the tissue's intrinsic sensitivity to neurally mediated contraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bazzaz F. J., Kelsey J. G., Kaage W. D. Substance P stimulation of chloride secretion by canine tracheal mucosa. Am Rev Respir Dis. 1985 Jan;131(1):86–89. doi: 10.1164/arrd.1985.131.1.86. [DOI] [PubMed] [Google Scholar]
- Baker A. P., Hillegass L. M., Holden D. A., Smith W. J. Effect of kallidin, substance P, and other basic polypeptides on the production of respiratory macromolecules. Am Rev Respir Dis. 1977 May;115(5):811–817. doi: 10.1164/arrd.1977.115.5.811. [DOI] [PubMed] [Google Scholar]
- Chesselet M. F. Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neuroscience. 1984 Jun;12(2):347–375. doi: 10.1016/0306-4522(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Fogel R., Battisti L., Asarkof N. The neurohumoral secretagogues carbachol, substance P and neurotensin increase Ca++ influx and calcium content in rabbit ileum. Life Sci. 1982 Nov 1;31(18):1929–1937. doi: 10.1016/0024-3205(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Gamse R., Lembeck F., Cuello A. C. Substance P in the vagus nerve. Immunochemical and immunohistochemical evidence for axoplasmic transport. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jan;306(1):37–44. doi: 10.1007/BF00515591. [DOI] [PubMed] [Google Scholar]
- Gashi A. A., Borson D. B., Finkbeiner W. E., Nadel J. A., Basbaum C. B. Neuropeptides degranulate serous cells of ferret tracheal glands. Am J Physiol. 1986 Aug;251(2 Pt 1):C223–C229. doi: 10.1152/ajpcell.1986.251.2.C223. [DOI] [PubMed] [Google Scholar]
- Giorguieff-Chesselet M. F., Kemel M. L., Glowinski J. The presynaptic stimulating effect of acetylcholine on dopamine release is suppressed during activation of nigro-striatal dopaminergic neurons in the cat. Neurosci Lett. 1979 Oct;14(2-3):177–182. doi: 10.1016/0304-3940(79)96144-5. [DOI] [PubMed] [Google Scholar]
- Grunstein M. M., Tanaka D. T., Grunstein J. S. Mechanism of substance P-induced bronchoconstriction in maturing rabbit. J Appl Physiol Respir Environ Exerc Physiol. 1984 Oct;57(4):1238–1246. doi: 10.1152/jappl.1984.57.4.1238. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Iversen L. L., Hanley M. R., Sandberg B. E. The possible existence of multiple receptors for substance P. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):281–287. doi: 10.1007/BF00501166. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Martling C. R., Saria A. Substance P and capsaicin-induced contraction of human bronchi. Acta Physiol Scand. 1983 Sep;119(1):49–53. doi: 10.1111/j.1748-1716.1983.tb07304.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A., Brodin E., Rosell S., Folkers K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1120–1124. doi: 10.1073/pnas.80.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983 Mar 17;302(5905):251–253. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
- Schwieler G. H., Douglas J. S., Bouhuys A. Postnatal development of autonomic efferent innervation in the rabbit. Am J Physiol. 1970 Aug;219(2):391–397. doi: 10.1152/ajplegacy.1970.219.2.391. [DOI] [PubMed] [Google Scholar]
- Sekizawa K., Tamaoki J., Nadel J. A., Borson D. B. Enkephalinase inhibitor potentiates substance P- and electrically induced contraction in ferret trachea. J Appl Physiol (1985) 1987 Oct;63(4):1401–1405. doi: 10.1152/jappl.1987.63.4.1401. [DOI] [PubMed] [Google Scholar]
- Shore S. A., Stimler-Gerard N. P., Coats S. R., Drazen J. M. Substance P-induced bronchoconstriction in the guinea pig. Enhancement by inhibitors of neutral metalloendopeptidase and angiotensin-converting enzyme. Am Rev Respir Dis. 1988 Feb;137(2):331–336. doi: 10.1164/ajrccm/137.2.331. [DOI] [PubMed] [Google Scholar]
- Tanaka D. T., Grunstein M. M. Effect of substance P on neurally mediated contraction of rabbit airway smooth muscle. J Appl Physiol (1985) 1986 Feb;60(2):458–463. doi: 10.1152/jappl.1986.60.2.458. [DOI] [PubMed] [Google Scholar]
- Tanaka D. T., Grunstein M. M. Mechanisms of substance P-induced contraction of rabbit airway smooth muscle. J Appl Physiol Respir Environ Exerc Physiol. 1984 Nov;57(5):1551–1557. doi: 10.1152/jappl.1984.57.5.1551. [DOI] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Bloom S. R., Will J. A., Brown M. R., Pearse A. G. Substance P-like immunoreactive nerves in mammalian lung. Invest Cell Pathol. 1979 Jan-Mar;2(1):3–10. [PubMed] [Google Scholar]
- Yau W. M., Dorsett J. A., Youther M. L. Calcium-dependent stimulation of acetylcholine release by substance P and vasoactive intestinal polypeptide. Eur J Pharmacol. 1986 Jan 21;120(2):241–243. doi: 10.1016/0014-2999(86)90547-9. [DOI] [PubMed] [Google Scholar]



