Abstract
There are at least three major African haplotype backgrounds on which the beta s mutation arises. Sequence changes in the immediate 5' flanking area of the gamma-globin genes may account for differences in fetal hemoglobin expression among the three haplotypes. We determined the sequence from -350 to 10 bp 5' of the G gamma and A gamma fetal globin genes from one beta s-containing chromosome on each of the three major haplotype backgrounds. The Senegal chromosome had a T at -158 5' to the G gamma gene; the Benin (BEN) chromosome had an A to G change at -309 5' to the G gamma gene; and the Central African Republic (CAR) chromosome had a C to T change at -271 5' to the A gamma gene. Genomic DNA from patients with sickle cell disease was analyzed using the polymerase chain reaction and radiolabeled allele-specific oligonucleotide probes. The -309 G variant 5' to the G gamma gene is associated with BEN chromosomes, and the -271 T variant 5' to A gamma with CAR. The -309 change was also found on beta A-containing chromosomes, while the -271 change was not. The -309 change may have predated the beta s mutation on the BEN chromosome.
Full text
PDF

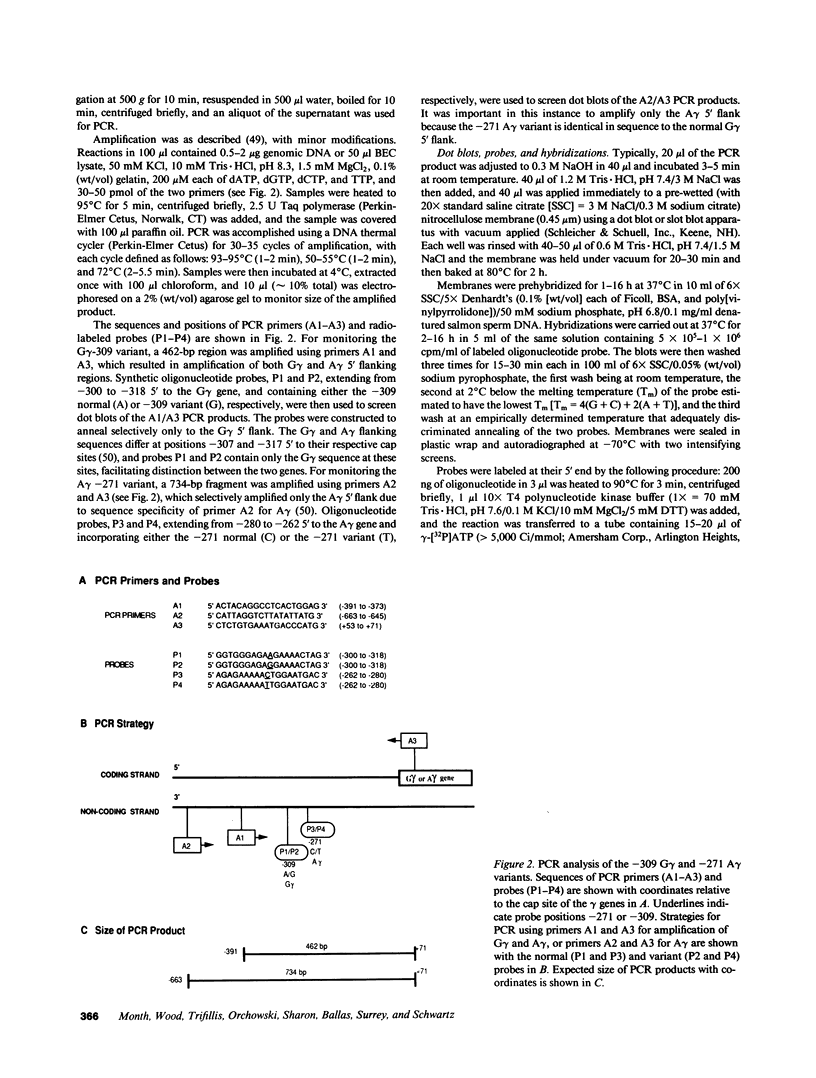
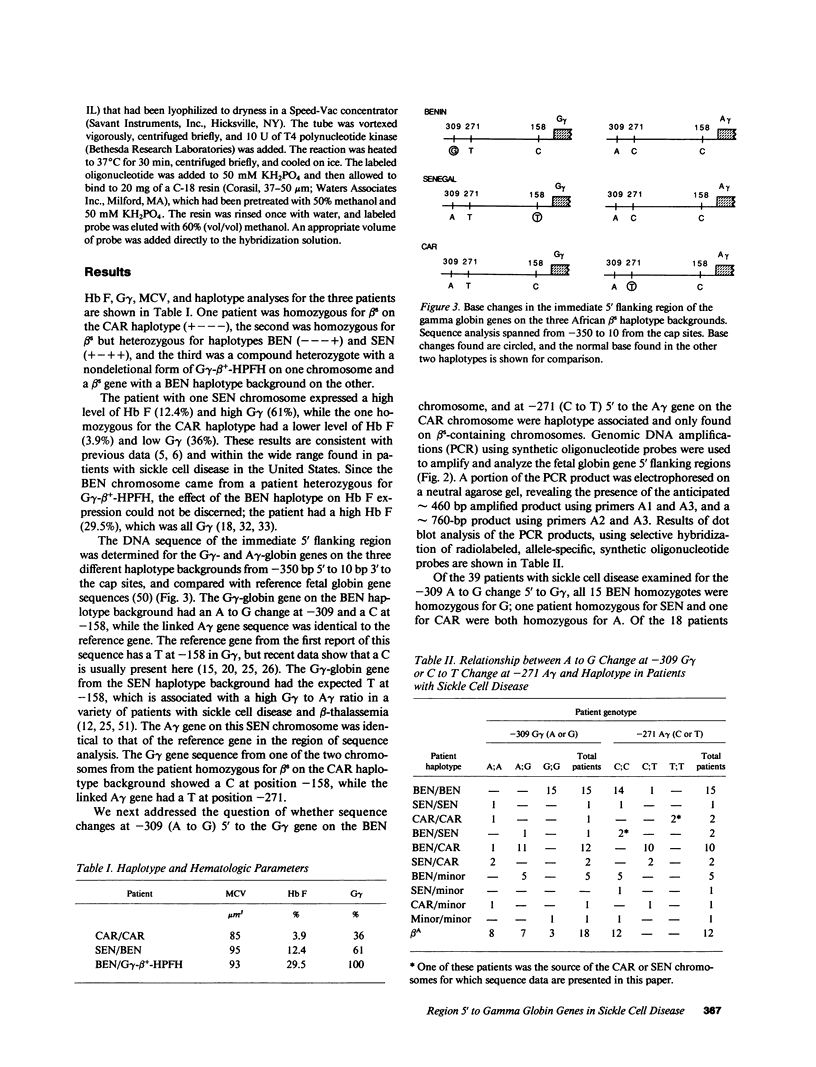



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. W., Kaufman R. E., Kretschmer P. J., Harrison M., Nienhuis A. W. A family of long reiterated DNA sequences, one copy of which is next to the human beta globin gene. Nucleic Acids Res. 1980 Dec 20;8(24):6113–6128. doi: 10.1093/nar/8.24.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnou N. P., Karlsson S., Moulton A. D., Keller G., Nienhuis A. W. Promoter sequences required for function of the human gamma globin gene in erythroid cells. EMBO J. 1986 Jan;5(1):121–126. doi: 10.1002/j.1460-2075.1986.tb04185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Boehm C. D., Serjeant G. R., Theisen C. E., Dover G. J., Kazazian H. H., Jr Origin of the beta S-globin gene in blacks: the contribution of recurrent mutation or gene conversion or both. Proc Natl Acad Sci U S A. 1984 Feb;81(3):853–856. doi: 10.1073/pnas.81.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsley J. F., Rappaport E., Schwartz E., Surrey S. The gamma-delta-beta-globin gene region in G gamma-beta +-hereditary persistence of fetal hemoglobin. Blood. 1982 Apr;59(4):828–831. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bouhassira E. E., Lachman H., Krishnamoorthy R., Labie D., Nagel R. L. A gene conversion located 5' to the A gamma gene in linkage disequilibrium with the Bantu haplotype in sickle cell anemia. J Clin Invest. 1989 Jun;83(6):2070–2073. doi: 10.1172/JCI114118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S. H., Dover G. J., Serjeant G. R., Smith K. D., Antonarakis S. E., Embury S. H., Margolet L., Noyes A. N., Boyer M. L., Bias W. B. Production of F cells in sickle cell anemia: regulation by a genetic locus or loci separate from the beta-globin gene cluster. Blood. 1984 Nov;64(5):1053–1058. [PubMed] [Google Scholar]
- Collins F. S., Boehm C. D., Waber P. G., Stoeckert C. J., Jr, Weissman S. M., Forget B. G., Kazazian H. H., Jr Concordance of a point mutation 5' to the G gamma globin gene with G gamma beta +. Hereditary persistence of fetal hemoglobin in the black population. Blood. 1984 Dec;64(6):1292–1296. [PubMed] [Google Scholar]
- Collins F. S., Metherall J. E., Yamakawa M., Pan J., Weissman S. M., Forget B. G. A point mutation in the A gamma-globin gene promoter in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):325–326. doi: 10.1038/313325a0. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Stoeckert C. J., Jr, Serjeant G. R., Forget B. G., Weissman S. M. G gamma beta+ hereditary persistence of fetal hemoglobin: cosmid cloning and identification of a specific mutation 5' to the G gamma gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4894–4898. doi: 10.1073/pnas.81.15.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Friedman S., Schwartz E. Hereditary persistence of foetal haemoglobin with beta-chain synthesis in cis position (Ggamma-beta+-HPFH) in a negro family. Nature. 1976 Jan 15;259(5539):138–140. doi: 10.1038/259138a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Gelinas R., Endlich B., Pfeiffer C., Yagi M., Stamatoyannopoulos G. G to A substitution in the distal CCAAT box of the A gamma-globin gene in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):323–325. doi: 10.1038/313323a0. [DOI] [PubMed] [Google Scholar]
- Giglioni B., Casini C., Mantovani R., Merli S., Comi P., Ottolenghi S., Saglio G., Camaschella C., Mazza U. A molecular study of a family with Greek hereditary persistence of fetal hemoglobin and beta-thalassemia. EMBO J. 1984 Nov;3(11):2641–2645. doi: 10.1002/j.1460-2075.1984.tb02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J. G. Expression of G gamma and A gamma globin genes in human adults. Hemoglobin. 1988;12(5-6):707–716. doi: 10.3109/03630268808991664. [DOI] [PubMed] [Google Scholar]
- Gilman J. G., Harano T., Nakatsuji T., Bakioglu I., Reese A. L., Gardiner M. B., Huisman T. H. The ratio of the G gamma and A gamma chains: variations due to anomalies at the molecular level. Ann N Y Acad Sci. 1985;445:235–247. doi: 10.1111/j.1749-6632.1985.tb17193.x. [DOI] [PubMed] [Google Scholar]
- Gilman J. G., Huisman T. H. DNA sequence variation associated with elevated fetal G gamma globin production. Blood. 1985 Oct;66(4):783–787. [PubMed] [Google Scholar]
- Gilman J. G., Huisman T. H. Two independent genetic factors in the beta-globin gene cluster are associated with high G gamma-levels in the HbF of SS patients. Blood. 1984 Aug;64(2):452–457. [PubMed] [Google Scholar]
- Groudine M., Kohwi-Shigematsu T., Gelinas R., Stamatoyannopoulos G., Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the beta-globin gene locus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7551–7555. doi: 10.1073/pnas.80.24.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio D. L., Rood K. L., Gray T. A., Riordan M. F., Sartor C. I., Collins F. S. Nuclear proteins that bind the human gamma-globin gene promoter: alterations in binding produced by point mutations associated with hereditary persistence of fetal hemoglobin. Mol Cell Biol. 1988 Dec;8(12):5310–5322. doi: 10.1128/mcb.8.12.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y., Kutlar F., Kutlar A., McKie V. C., Huisman T. H. Haplotypes of beta S chromosomes among patients with sickle cell anemia from Georgia. Hemoglobin. 1986;10(6):623–642. doi: 10.3109/03630268609036566. [DOI] [PubMed] [Google Scholar]
- Labie D., Dunda-Belkhodja O., Rouabhi F., Pagnier J., Ragusa A., Nagel R. L. The -158 site 5' to the G gamma gene and G gamma expression. Blood. 1985 Dec;66(6):1463–1465. [PubMed] [Google Scholar]
- Labie D., Pagnier J., Lapoumeroulie C., Rouabhi F., Dunda-Belkhodja O., Chardin P., Beldjord C., Wajcman H., Fabry M. E., Nagel R. L. Common haplotype dependency of high G gamma-globin gene expression and high Hb F levels in beta-thalassemia and sickle cell anemia patients. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2111–2114. doi: 10.1073/pnas.82.7.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lench N., Stanier P., Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988 Jun 18;1(8599):1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Ley T. J., Chiang Y. L., Haidaris D., Anagnou N. P., Wilson V. L., Anderson W. F. DNA methylation and regulation of the human beta-globin-like genes in mouse erythroleukemia cells containing human chromosome 11. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6618–6622. doi: 10.1073/pnas.81.21.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Nicolis S., Ronchi A., Giglioni B., Ottolenghi S. The effects of HPFH mutations in the human gamma-globin promoter on binding of ubiquitous and erythroid specific nuclear factors. Nucleic Acids Res. 1988 Aug 25;16(16):7783–7797. doi: 10.1093/nar/16.16.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miller B. A., Olivieri N., Salameh M., Ahmed M., Antognetti G., Huisman T. H., Nathan D. G., Orkin S. H. Molecular analysis of the high-hemoglobin-F phenotype in Saudi Arabian sickle cell anemia. N Engl J Med. 1987 Jan 29;316(5):244–250. doi: 10.1056/NEJM198701293160504. [DOI] [PubMed] [Google Scholar]
- Miller B. A., Salameh M., Ahmed M., Wainscoat J., Antognetti G., Orkin S., Weatherall D., Nathan D. G. High fetal hemoglobin production in sickle cell anemia in the eastern province of Saudi Arabia is genetically determined. Blood. 1986 May;67(5):1404–1410. [PubMed] [Google Scholar]
- Milner P. F., Leibfarth J. D., Ford J., Barton B. P., Grenett H. E., Garver F. A. Increased HbF in sickle cell anemia is determined by a factor linked to the beta S gene from one parent. Blood. 1984 Jan;63(1):64–72. [PubMed] [Google Scholar]
- Nagel R. L., Fabry M. E., Pagnier J., Zohoun I., Wajcman H., Baudin V., Labie D. Hematologically and genetically distinct forms of sickle cell anemia in Africa. The Senegal type and the Benin type. N Engl J Med. 1985 Apr 4;312(14):880–884. doi: 10.1056/NEJM198504043121403. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Rao S. K., Dunda-Belkhodja O., Connolly M. M., Fabry M. E., Georges A., Krishnamoorthy R., Labie D. The hematologic characteristics of sickle cell anemia bearing the Bantu haplotype: the relationship between G gamma and HbF level. Blood. 1987 Apr;69(4):1026–1030. [PubMed] [Google Scholar]
- Pagnier J., Baudin V., Labie D., Wajcman H., Jaeger G., Girot R. Sickle cell anemia in Bantu speaking Africa. Hemoglobin. 1986;10(1):73–76. doi: 10.3109/03630268609072472. [DOI] [PubMed] [Google Scholar]
- Pagnier J., Mears J. G., Dunda-Belkhodja O., Schaefer-Rego K. E., Beldjord C., Nagel R. L., Labie D. Evidence for the multicentric origin of the sickle cell hemoglobin gene in Africa. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1771–1773. doi: 10.1073/pnas.81.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Analysis of human hemoglobin switching in MEL x human fetal erythroid cell hybrids. Cell. 1986 Aug 1;46(3):469–476. doi: 10.1016/0092-8674(86)90667-7. [DOI] [PubMed] [Google Scholar]
- Poncz M., Ballantine M., Solowiejczyk D., Barak I., Schwartz E., Surrey S. beta-Thalassemia in a Kurdish Jew. Single base changes in the T-A-T-A box. J Biol Chem. 1982 Jun 10;257(11):5994–5996. [PubMed] [Google Scholar]
- Poncz M., Henthorn P., Stoeckert C., Surrey S. Globin gene expression in hereditary persistence of fetal haemoglobin and (delta beta) (0)-thalassaemia. Oxf Surv Eukaryot Genes. 1988;5:163–203. [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Harpel B., Mory Y., Schwartz E., Surrey S. Construction of human gene libraries from small amounts of peripheral blood: analysis of beta-like globin genes. Hemoglobin. 1982;6(1):27–36. doi: 10.3109/03630268208996930. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalenghe F., Turco E., Edström J. E., Pirrotta V., Melli M. Microdissection and cloning of DNA from a specific region of Drosophila melanogaster polytene chromosomes. Chromosoma. 1981;82(2):205–216. doi: 10.1007/BF00286105. [DOI] [PubMed] [Google Scholar]
- Semenza G. L., Malladi P., Surrey S., Delgrosso K., Poncz M., Schwartz E. Detection of a novel DNA polymorphism in the beta-globin gene cluster. J Biol Chem. 1984 May 25;259(10):6045–6048. [PubMed] [Google Scholar]
- Sharon B., Poncz M., Surrey S., Schwartz E. Non-random association of the Rsa I polymorphic site 5' to the beta-globin gene with major sickle cell haplotypes. Hemoglobin. 1988;12(2):115–124. doi: 10.3109/03630268808998018. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Stoming T. A., Stoming G. S., Lanclos K. D., Fei Y. J., Altay C., Kutlar F., Huisman T. H. An A gamma type of nondeletional hereditary persistence of fetal hemoglobin with a T----C mutation at position -175 to the cap site of the A gamma globin gene. Blood. 1989 Jan;73(1):329–333. [PubMed] [Google Scholar]
- Surrey S., Delgrosso K., Malladi P., Schwartz E. A single-base change at position -175 in the 5'-flanking region of the G gamma-globin gene from a black with G gamma-beta+ HPFH. Blood. 1988 Mar;71(3):807–810. [PubMed] [Google Scholar]
- Tate V. E., Wood W. G., Weatherall D. J. The British form of hereditary persistence of fetal hemoglobin results from a single base mutation adjacent to an S1 hypersensitive site 5' to the A gamma globin gene. Blood. 1986 Dec;68(6):1389–1393. [PubMed] [Google Scholar]
- Wainscoat J. S., Thein S. L., Higgs D. R., Bell J. I., Weatherall D. J., Al-Awamy B. H., Serjeant G. R. A genetic marker for elevated levels of haemoglobin F in homozygous sickle cell disease? Br J Haematol. 1985 Jun;60(2):261–268. doi: 10.1111/j.1365-2141.1985.tb07412.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Wilson L. B., deRiel J. K., Villa-komaroff L., Efstratiadis A., Forget B. G., Weissman S. M. Insertion of synthetic copies of human globin genes into bacterial plasmids. Nucleic Acids Res. 1978 Feb;5(2):563–581. doi: 10.1093/nar/5.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]



