Abstract
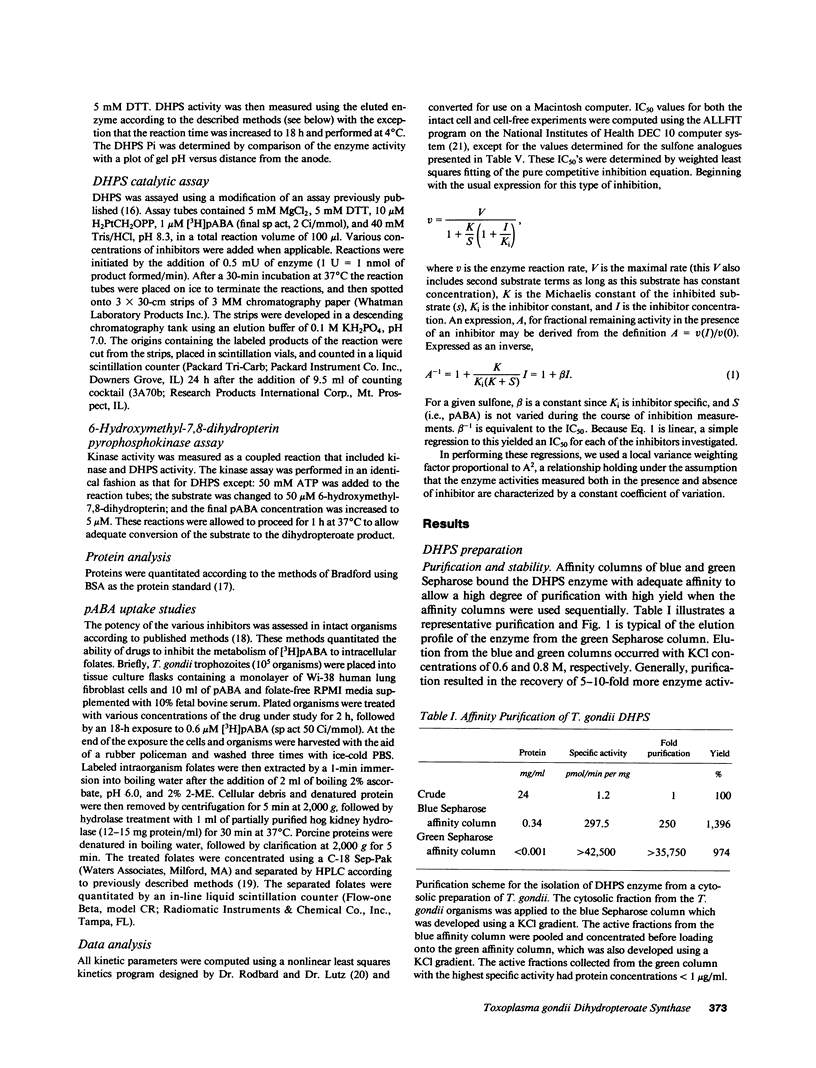
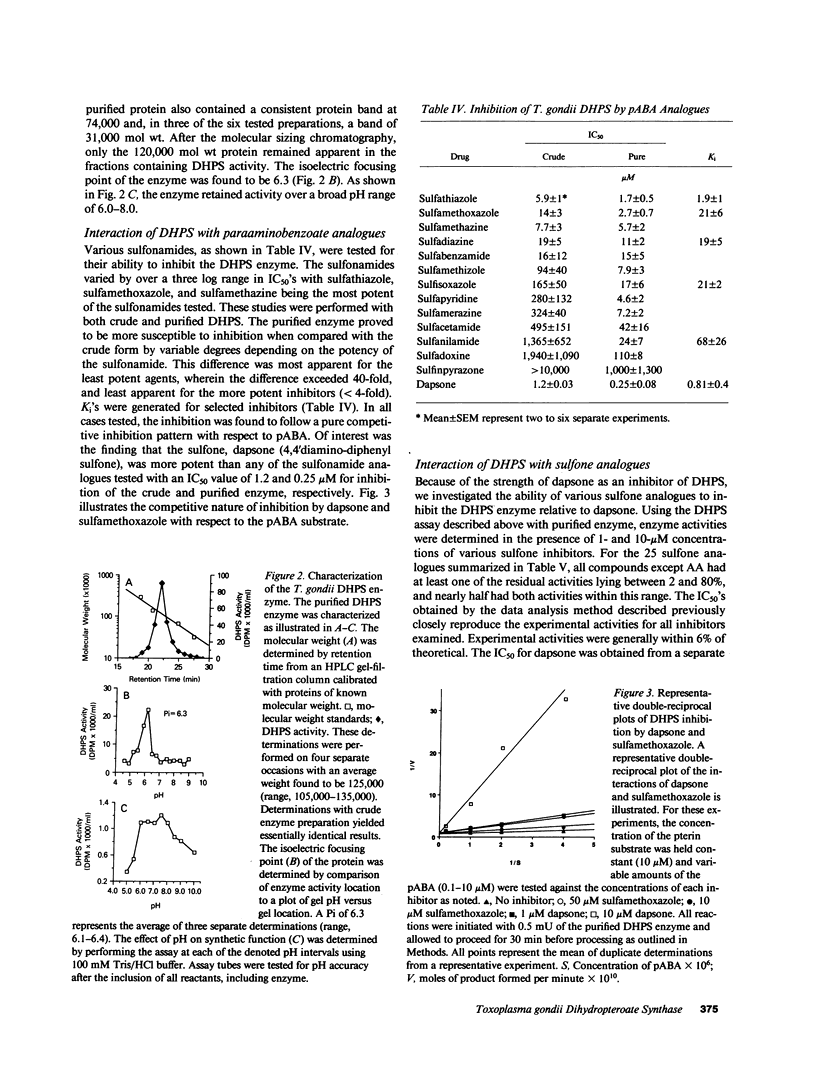
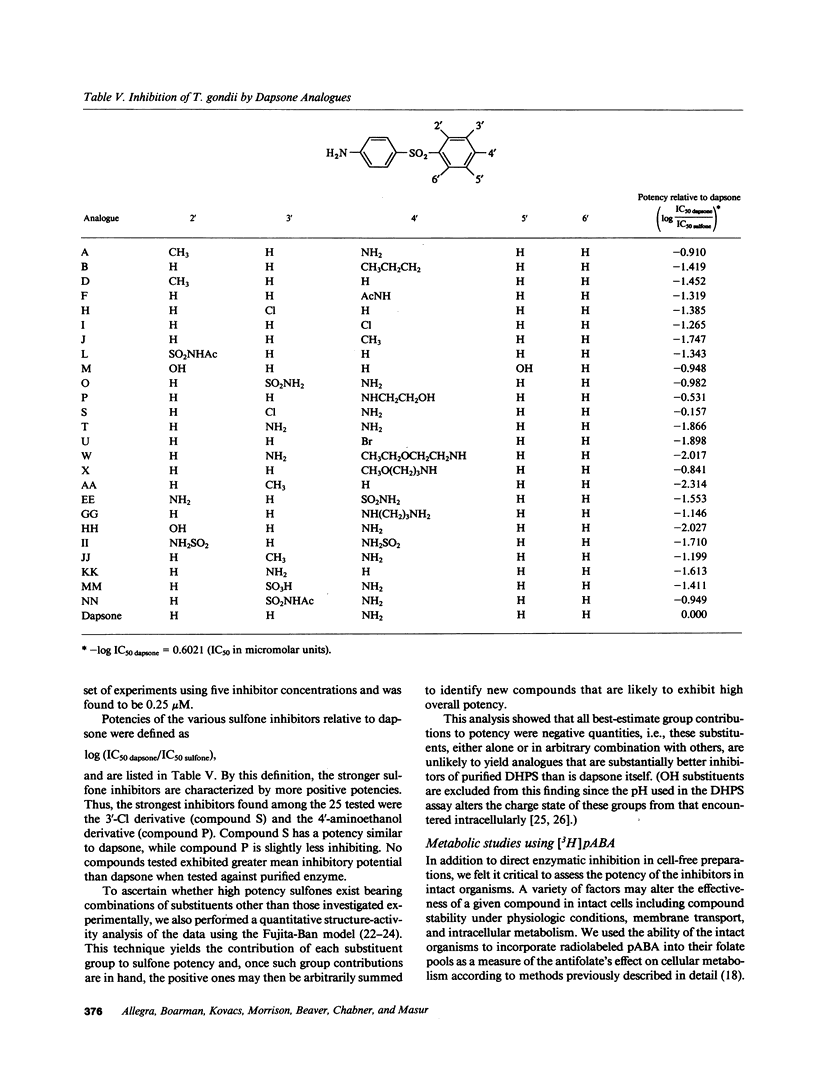
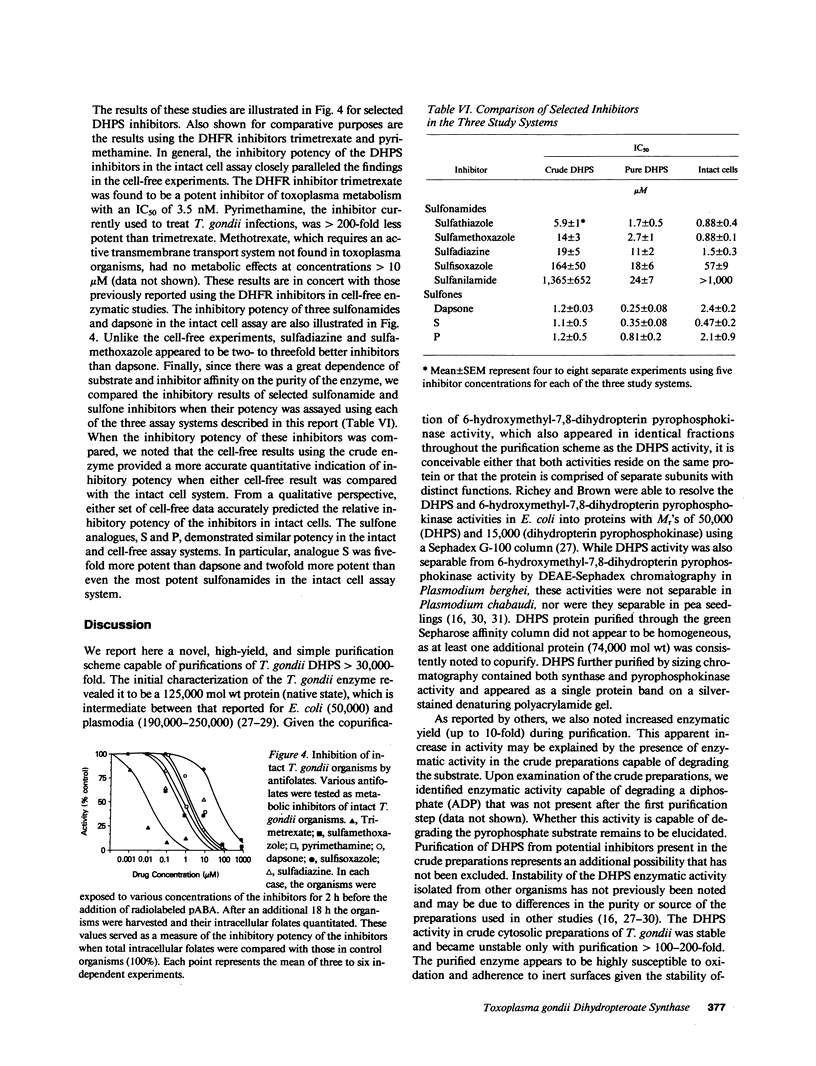
Toxoplasma gondii is a common protozoan disease that often causes life-threatening disease, particularly among patients with the acquired immunodeficiency syndrome. This study demonstrates that the dihydropteroate synthase in T. gondii is kinetically distinct from the enzyme characterized from other sources and can be highly purified with a high yield using sequential dye-affinity chromatography. Conditions have been identified that allow for stabilization of the purified enzyme, and its physical characteristics have been elucidated. The molecular weight of the native protein was 125,000 and the protein appeared to contain both dihydropteroate synthase and 6-hydroxymethyl-dihydropterin pyrophosphokinase activities. The sulfonamide class of compounds vary in inhibitory potency by more than three orders of magnitude. Sulfathiazole, sulfamethoxazole, and sulfamethazine, with 50% inhibitory concentrations (IC50's) of 1.7, 2.7, and 5.7 microM, respectively, represent the most potent of this class of inhibitors. Several sulfone analogues, including dapsone, were identified as highly potent inhibitors with IC50's less than 1 microM. The results of these cell-free experiments were corroborated by investigating the metabolic inhibition produced by the various inhibitors in intact organisms. The qualitative and quantitative relations among the inhibitors were preserved in both the cell-free and intact cell assay systems. These studies suggest that the sulfones may be important therapeutic agents for the treatment of toxoplasmosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra C. J., Chabner B. A., Drake J. C., Lutz R., Rodbard D., Jolivet J. Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates. J Biol Chem. 1985 Aug 15;260(17):9720–9726. [PubMed] [Google Scholar]
- Allegra C. J., Fine R. L., Drake J. C., Chabner B. A. The effect of methotrexate on intracellular folate pools in human MCF-7 breast cancer cells. Evidence for direct inhibition of purine synthesis. J Biol Chem. 1986 May 15;261(14):6478–6485. [PubMed] [Google Scholar]
- Allegra C. J., Kovacs J. A., Drake J. C., Swan J. C., Chabner B. A., Masur H. Potent in vitro and in vivo antitoxoplasma activity of the lipid-soluble antifolate trimetrexate. J Clin Invest. 1987 Feb;79(2):478–482. doi: 10.1172/JCI112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- De Benedetti P. G., Iarossi D., Menziani C., Caiolfa V., Frassineti C., Cennamo C. Quantitative structure-activity analysis in dihydropteroate synthase inhibition by sulfones. Comparison with sulfanilamides. J Med Chem. 1987 Mar;30(3):459–464. doi: 10.1021/jm00386a004. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- EYLES D. E., COLEMAN N. The relative activity of the common sulfonamides against experimental toxoplasmosis in the mouse. Am J Trop Med Hyg. 1953 Jan;2(1):54–63. doi: 10.4269/ajtmh.1953.2.54. [DOI] [PubMed] [Google Scholar]
- EYLES D. E. The present status of the chemotherapy of toxoplasmosis. Am J Trop Med Hyg. 1953 May;2(3):429–444. doi: 10.4269/ajtmh.1953.2.429. [DOI] [PubMed] [Google Scholar]
- FREE S. M., Jr, WILSON J. W. A MATHEMATICAL CONTRIBUTION TO STRUCTURE-ACTIVITY STUDIES. J Med Chem. 1964 Jul;7:395–399. doi: 10.1021/jm00334a001. [DOI] [PubMed] [Google Scholar]
- Ferone R. The enzymic synthesis of dihydropteroate and dihydrofolate by Plasmodium berghei. J Protozool. 1973 Aug;20(3):459–464. doi: 10.1111/j.1550-7408.1973.tb00926.x. [DOI] [PubMed] [Google Scholar]
- Fujita T., Ban T. Structure-activity study of phenethylamines as substrates of biosynthetic enzymes of sympathetic transmitters. J Med Chem. 1971 Feb;14(2):148–152. doi: 10.1021/jm00284a016. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Allegra C. J., Beaver J., Boarman D., Lewis M., Parrillo J. E., Chabner B., Masur H. Characterization of de novo folate synthesis in Pneumocystis carinii and Toxoplasma gondii: potential for screening therapeutic agents. J Infect Dis. 1989 Aug;160(2):312–320. doi: 10.1093/infdis/160.2.312. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Hiemenz J. W., Macher A. M., Stover D., Murray H. W., Shelhamer J., Lane H. C., Urmacher C., Honig C., Longo D. L. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984 May;100(5):663–671. doi: 10.7326/0003-4819-100-5-663. [DOI] [PubMed] [Google Scholar]
- Kubinyi H. Quantitative structure-activity relationships. V. A simple simple algorithm for Fujita-Ban and Free-Wilson analyses. Arzneimittelforschung. 1977;27(4):750–758. [PubMed] [Google Scholar]
- Leport C., Raffi F., Matheron S., Katlama C., Regnier B., Saimot A. G., Marche C., Vedrenne C., Vilde J. L. Treatment of central nervous system toxoplasmosis with pyrimethamine/sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome. Efficacy of long-term continuous therapy. Am J Med. 1988 Jan;84(1):94–100. doi: 10.1016/0002-9343(88)90014-9. [DOI] [PubMed] [Google Scholar]
- McCabe R., Remington J. S. Toxoplasmosis: the time has come. N Engl J Med. 1988 Feb 4;318(5):313–315. doi: 10.1056/NEJM198802043180509. [DOI] [PubMed] [Google Scholar]
- McCullough J. L., Maren T. H. Dihydropteroate synthetase from Plasmodium berghei: isolation, properties, and inhibition by dapsone and sulfadiazine. Mol Pharmacol. 1974 Jan;10(1):140–145. [PubMed] [Google Scholar]
- Okinaka O., Iwai K. The biosynthesis of folic acid compounds in plants. IV. Purification and properties of the dihydropteroate-synthesizing enzyme from pea seedlings. J Vitaminol (Kyoto) 1970 Sep;16(3):201–209. doi: 10.5925/jnsv1954.16.201. [DOI] [PubMed] [Google Scholar]
- Richey D. P., Brown G. M. The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroic acid. J Biol Chem. 1969 Mar 25;244(6):1582–1592. [PubMed] [Google Scholar]
- Roland S., Ferone R., Harvey R. J., Styles V. L., Morrison R. W. The characteristics and significance of sulfonamides as substrates for Escherichia coli dihydropteroate synthase. J Biol Chem. 1979 Oct 25;254(20):10337–10345. [PubMed] [Google Scholar]
- SHIOTA T., DISRAELY M. N., MCCANN M. P. THE ENZYMATIC SYNTHESIS OF FOLATE-LIKE COMPOUNDS FROM HYDROXYMETHYLDIHYDROPTERIDINE PYROPHOSPHATE. J Biol Chem. 1964 Jul;239:2259–2266. [PubMed] [Google Scholar]
- Walter R. D., Königk E. Biosynthesis of folic acid compounds in plasmodia. Purification and properties of the 7,8-dihydropteroate-synthesizing enzyme from Plasmodium chabaudi. Hoppe Seylers Z Physiol Chem. 1974 Apr;355(4):431–437. doi: 10.1515/bchm2.1974.355.1.431. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Kakeya N., Morishita T., Kamada A., Aoki M. Biological activity of drugs. X. Relation of structure to the bacteriostatic activity of sulfonamides. (1). Chem Pharm Bull (Tokyo) 1970 Apr;18(4):702–707. doi: 10.1248/cpb.18.702. [DOI] [PubMed] [Google Scholar]



