Abstract
Background
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized pathologically by the presence in the brain of intracellular protein inclusions highly enriched in aggregated alpha-synuclein (α-Syn). Although it has been established that progression of the disease is accompanied by sustained activation of microglia, the underlying molecules and factors involved in these immune-triggered mechanisms remain largely unexplored. Lately, accumulating evidence has shown the presence of extracellular α-Syn both in its aggregated and monomeric forms in cerebrospinal fluid and blood plasma. However, the effect of extracellular α-Syn on cellular activation and immune mediators, as well as the impact of familial PD-linked α-Syn mutants on this stimulation, are still largely unknown.
Methods and Findings
In this work, we have compared the activation profiles of non-aggregated, extracellular wild-type and PD-linked mutant α-Syn variants on primary glial and microglial cell cultures. After stimulation of cells with α-Syn, we measured the release of Th1- and Th2- type cytokines as well as IP-10/CXCL10, RANTES/CCL5, MCP-1/CCL2 and MIP-1α/CCL3 chemokines. Contrary to what had been observed using cell lines or for the case of aggregated α-Syn, we found strong differences in the immune response generated by wild-type α-Syn and the familial PD mutants (A30P, E46K and A53T).
Conclusions
These findings might contribute to explain the differences in the onset and progression of this highly debilitating disease, which could be of value in the development of rational approaches towards effective control of immune responses that are associated with PD.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder, after Alzheimer's disease. It is characterized pathologically by the presence of deposits of aggregated α-synuclein (α-Syn) in intracellular inclusions, known as Lewy bodies, in the substantia nigra pars compacta (SN) of the brain [1], [2], and by the loss of dopaminergic neurons [3], [4]. There is considerable evidence indicating a role of α-Syn in the etiology of PD, in which the conversion of α-Syn from soluble monomers to aggregated amyloid-like insoluble forms is a key event in PD pathogenesis [5]. However, the cellular and molecular mechanisms underlying the pathological actions of α-Syn are still not completely understood. Traditionally, α-Syn has been viewed as an exclusively intracellular, cytoplasmic protein which is highly expressed in dopaminergic neuronal cells. Lately, accumulating evidence showing the uptake of extracellular α-Syn by glia and neurons via endocytosis [6], [7], the release and exocytosis of α-Syn to the medium [8], [9], and the presence of α-Syn in cerebrospinal fluid [10], [11] and blood [11] both in its aggregated and non-aggregated forms has pointed at the importance of studying the effects of extracellular α-Syn on surrounding cells in the brain.
Alpha-Syn is a 140-amino acid protein that is highly enriched in presynaptic neuronal terminals, in particular in the neocortex, hippocampus, and SN [12], as well as within astrocytes and oligodendroglia [13], [14]. The physiological role of α-Syn is still being established, but its interaction with pre-synaptic membranes suggests that one function may be the regulation of synaptic vesicle pools, including control of dopamine levels [15]. Alpha-Syn belongs to the group of proteins described as natively unfolded [16], meaning that it does not adopt a well-defined globular structure, but instead a broad ensemble of dynamically interacting and largely disordered conformations [17], [18]. Three missense mutations, A53T, A30P and E46K, as well as multiple copies of wild-type (Wt) α-Syn, are linked to hereditary, early-onset PD [19]–[22]. In vitro studies have shown that the ensemble of α-Syn conformers is perturbed by the mutations [23], at least in the cases of A30P and A53T studied. Presumably as a result of the differences in their structural, biophysical and biochemical characteristics, the various mutants have been reported to have different cytotoxic effects, and this cytotoxicity to be mediated by different pathways (reviewed in [24]). Nevertheless, the factors contributing to both familial and sporadic cases of PD are not understood in any detail.
Even though the central nervous system (CNS) has been traditionally seen as an immune-privileged organ, it has become increasingly evident that inflammation is actively involved in the pathogenesis of various degenerative diseases including multiple sclerosis, Alzheimer's disease and PD (reviewed in [25]). Indeed, accumulating evidence indicates that the onset and progression of PD is accompanied by a robust and highly localized inflammatory response mediated by reactive astrocytes and activated microglia in affected areas in the brain of PD patients [26]–[30]. Whether microglial activation protects or exacerbates neuronal loss is currently the subject of debate [31]–[34]. Significantly, a link was established a few years ago between extracellular, aggregated α-Syn and activation of microglia [35] leading to dopaminergic neurotoxicity, and a few recent in vivo studies have shown that microglial activation and neurodegeneration can be directly caused by α-Syn overexpression [36]–[40]. Even though evidence has accumulated pointing at the importance of the immunological features of α-Syn related to the pathogenesis of Parkinson's disease (reviewed in [2], [41]), the effects of extracellular α-Syn on the cellular and molecular components of the immune system linked to PD pathology [42], remain largely unexplored.
Up to this point, research on α-Syn-mediated cell response has focused primarily on the effects of aggregated α-Syn on neuroinflammation [43] or on activation of microglia [24], [35], [44]–[46]. In turn, most of these studies have focused on nitrated α-Syn [43], [45], [46], assuming that extracellular α-Syn has been modified in a similar manner to α-Syn found in Lewy bodies [47], [48] −a typically pro-oxidative environment− an assumption that is still uncertain [42] and might not be valid for secreted α-Syn. Moreover, it has been recently shown that non-aggregated, exogenous α-Syn can regulate the key brain cytoactive molecules matrix metalloproteinase-9 and tissue plasminogen activator in glial cells [49], [50], and induces higher TNF-α, IL-1β and ROS release levels than aggregated α-Syn in microglia [50]. Furthermore, it has also been observed that, in contrast to the aggregated form, monomeric α-Syn enhances microglial phagocytosis [51]. These results and other recent findings point at the importance of exploring the effects on the immune response of non-aggregated/monomeric as well as aggregated extracellular α-Syn. Despite the fact that some investigations in this direction have been done using monocytic cell lines [52], human astrocytes [53], or microglia [37], nothing has been reported about the cytokine expression profile of primary microglial cells induced by non-aggregated α-Syn, under conditions where the aggregation state of the protein has been characterized. Likewise, apart from a recent article focusing on the pro-inflammatory effects of an α-Syn double mutant which does not exist in nature [54], a comparative study of wild-type α-Syn and pathologically relevant α-Syn mutants is still lacking. Moreover, so far there are no data available on key chemokines that might control the differential homing and activation of T cell subsets, monocytes and glial cells in this context.
In this work, we have compared the activation profile of non-aggregated, extracellular Wt α-Syn and its PD-linked variants, by measuring the release of key interleukins and chemokines in glial cells. Our findings demonstrate significant differences in the immune response profiling of Wt α-Syn and PD-related mutants that might indicate the existence of different pathways towards PD onset and progression.
Results and Discussion
Characterization of α-Synuclein preparations
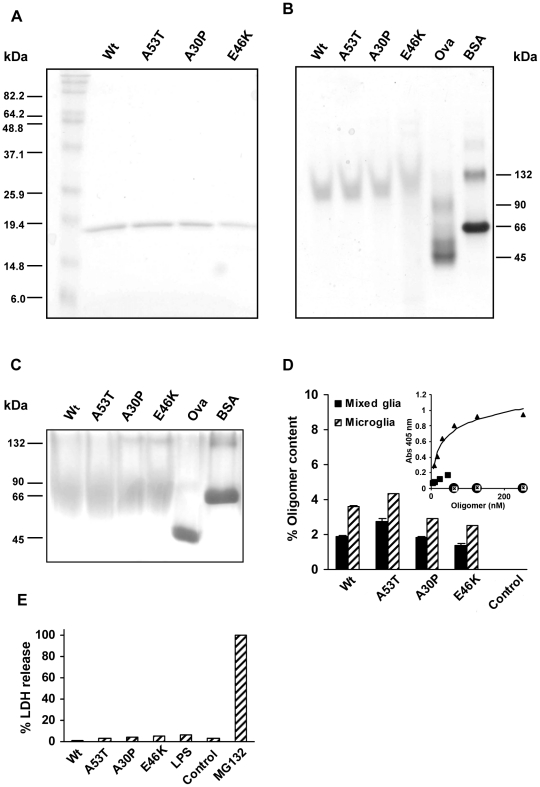
It is known that α-Syn has a tendency to self-assemble under certain conditions to form dimers [55], [56] and higher order oligomeric species in addition to amyloid-like fibrils [57]. In order to assess the purity and oligomerization state of the α-Syn preparations used in this study, we subjected the purified Wt and mutant α-Syn protein variants to electrophoretic analysis ( Figure 1 ). As expected from additional analysis by mass spectrometry (not shown), each of the four α-Syn preparations migrated as a single, well defined band corresponding to ca. 14.5 kDa as analysed by SDS-PAGE ( Figure 1A ). However, when the samples were subjected to native PAGE ( Figure 1B ), they migrated as less defined bands of ca. 110–120 kDa, as found previously for monomeric α-Syn a natively unfolded protein with a large charge/mass ratio under these conditions [58]. Finally, the rather smeared bands corresponding to ca. 50–60 kDa observed by Blue Native PAGE (BN-PAGE) ( Figure 1C ), in which proteins migrate solely as a function of their apparent mass [59], indicates that the α-Syn preparations are monomeric. Taken together, our data demonstrate a well defined monomeric state for the functional characterization of immune-elicited reponses by the α-Syn protein variants. In order to analyse the oligomeric state of α-Syn present in the cell cultures after 20 hours, a specific and sensitive ELISA assay [60] was used. As can be observed, a low amount of α-Syn oligomers were formed by the end of the incubation step with cells, with ≤4.3% of oligomers relative to the initially added α-Syn ( Figure 1D ). In addition, in order to assess the total amount of α-Syn still present in the medium by the end of the incubation step with cells, a time-course quantification of α-Syn in the culture supernatants was performed after 1, 6 and 20 hours ( Figure S1 ). We found that after 20 hours, the α-Syn content was still ca. 60% of the exogenously added amount.
Figure 1. Determination of the purity, oligomeric state and cytotoxic effects of preparations of α-synucleins.
(A) SDS-PAGE electrophoresis and (B) native PAGE electrophoresis of wild type (Wt) and mutant (A53T, A30P and E46K) α-Syn preparations; (C) BN-PAGE of wild type (Wt) and mutant (A53T, A30P and E46K) α-Syn preparations; (D) α-Syn oligomer content after 20 hr incubation with cells (on a protomer basis), relative to the initial amount of exogenously added α-Syn, as determined by sandwich ELISA. (E) LDH release in mixed glial cultures following incubation for 20 hours with the highest concentration of α-Syn used in our experiments, i.e. 5 µg/ml. MG132, a proteasome inhibitor, was used at 6 µM as a control. Values are means from triplicate measurements.
In order to assess the level of cytotoxicity induced by α-Syn at the concentrations used in this study, we next performed lactate dehydrogenase (LDH) release assays with microglial cells. After incubating primary mixed cultures for 20 hours with the α-Syn variants at 5 µg/ml (the highest concentrations used in this work), the cytotoxicity levels displayed by all four α-Syn variants were found to be very low (≤8%) and similar to basal control levels ( Figure 1E ). Therefore, it can be concluded that the parameters measured in this study after α-Syn treatment of cell cultures are not linked to alterations in cellular viability and therefore represent a specific α-Syn immune mediated-response.
Characterization of primary microglial cultures
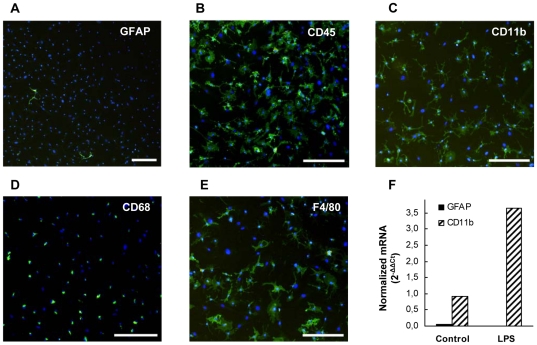
Microglial cells were purified from long-term cultures of neonatal mouse brains as described in the Materials and Methods section. The purity of the isolated microglial fraction was evaluated by two independent approaches. First, immunofluorescence procedures were used as shown in Figure 2 , where the absence of contaminating macroglial cells after purification was confirmed since less than 1% of the total cells stained positively for GFAP, an intermediate filament specifically expressed in macroglial cells ( Figure 2A ). As a positive control, most of the cells detached by trypsinization of the long-term cultures from neonatal mouse brains –expected to be macroglia– were found to be strongly immunostained for GFAP (not shown). As a second measure of the purity of microglial cell cultures, the majority of cells were found to express the pan haematopoietic lineage marker CD45 ( Figure 2B ), the monocyte-macrophage marker CD11b ( Figure 2C ), and the mature macrophage markers CD68 and F4/80 ( Figures 2D and 2E , respectively). In addition, quantitative RT-PCR was employed to amplify specifically the GFAP and CD11b genes ( Figure 2F ), and the results indicate no detectable mRNA expression of the GFAP macroglial marker, and a clear up-regulation of the microglia-specific CD11b gene expression after stimulation by lipopolyssacharide (LPS). Taken together, the cell marker profiles observed confirms the high purity of the microglial cultures and hence to the reliability of the results obtained using our purified microglial cell culture model.
Figure 2. Evaluation of the purity of isolated microglial cell fractions.
Immunofluorescence characterization of purified microglial cell cultures for the specific macroglial lineage marker GFAP (A), the pan haematopoietic lineage marker CD45 (B), and the mature macrophages markers CD11b (C), CD68 (D), and F4/80 (E). Nuclei are counterstained in blue with Hoechst 33342. Scale bar: 100 µm. mRNA expression (qRT-PCR) from isolated microglial preparations after no stimulation (control) or after stimulation with 1 µg/ml LPS for 20 hours (F). Specific primers for amplifying the GFAP and CD11b genes were used.
Pro-inflammatory response of α-Syn-stimulated glia and microglia
In the normal CNS, brain tissue provides an immunosuppressive environment, which seems to be important for the proper function of the CNS. Under circumstances that cause disruption of this environment, including chronic inflammatory conditions in neurodegenerative diseases, a variety of immune regulatory and inflammatory mediators can be activated [25]. Although various types of cells have been identified as sources of cytokines in the CNS, microglia appear to be a principal source of pro-inflammatory and immune regulatory cytokines [25], [61]. In order to explore the immunological properties of extracellular, non-aggregated α-Syn, as well as to evaluate the importance of the cellular context in this process, we measured by ELISA the release of a series of key cytokines after incubation for 20 hours of mouse primary cultures of mixed glia and isolated microglia, with exogenously added Wt α-Syn or the early onset PD-linked α-Syn variants A30P, E46K, and A53T (at 0.2, 1 or 5 µg/ml).
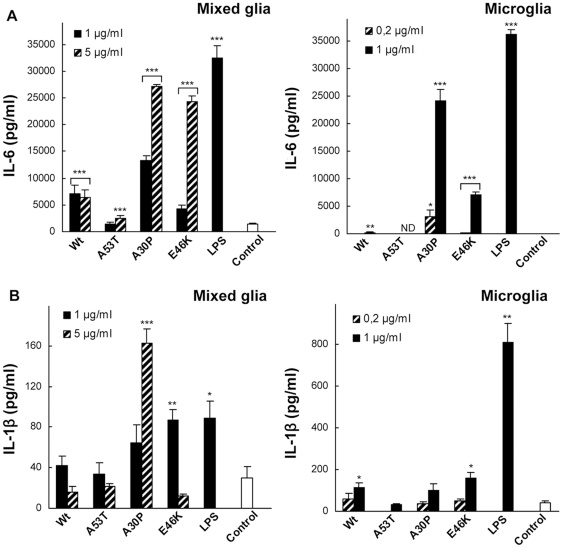
First, we assayed the release of IL-6, TNF-α, IFN-γ and IL-1β, four key pro-inflammatory cytokines ( Figure 3 and S2 ). Wild-type α-Syn moderately stimulated the release of IL-6 in mixed glial cultures ( Figure 3A, left panel), but contrary to findings for aggregated Wt α-Syn [62], this effect was hardly detectable on isolated microglia ( Figure 3A, right panel). And in contrast to the comparable IL-6 response observed for the four α-Syn variants reported in a study using the human U-373 MG astrocytoma cell line [53], we found remarkable differences in their behaviour, notably a very strong IL-6-mediated pro-inflammatory response induced by the A30P and E46K variants after stimulation of both mixed glial and microglial cultures. These differences could be explained by the nature of the cell line used by Klegeris and coworkers. Alternatively, given that astrocytes are the most abundant glial cell population of the CNS participating in local innate immune responses; this result could be consistent with our finding of less marked differences ( Figure 3A ) between the α-Syn variants in mixed glial cultures. The A53T variant, however, caused a weak but significant increase in IL-6 levels on total glia but not on isolated microglia, similar to Wt α-Syn but even less prominent. In the case of TNF-α and IFN-γ levels measured in microglial cultures, only stimulation with A30P produced a significant increase ( Figure S2 ), partially coinciding with the IL-6 observed profile. Therefore, A30P and E46K appear to drive IL-6, TNF-α, and IFN-γ cytokine secretion in the context of PD-affected glia.
Figure 3. Pro-inflammatory interleukin profile of α-Syn-stimulated primary mixed glial and isolated microglial cultures.
IL-6 (A) and IL-1β (B) release was measured by ELISA in culture supernantants of mixed glia (left) and microglia (right) after a 20-hour treatment with monomeric Wt or mutant α-Syn variants or lipopolysaccharide (LPS). Values are mean ± S.E.M. (n = 4). * P<0.05, ** P<0.01, *** P<0.001. The results shown are representative of two or three independent experiments with microglia and mixed glial cultures, respectively.
When we assayed the levels of IL-1β from mixed glial cultures ( Figure 3B, left panel), only the A30P and E46K α-Syn variants showed a significant stimulatory effect. Interestingly, when tested on isolated microglial cells, while A30P did not cause a significant rise in IL-1β levels relative to the control, Wt α-Syn −in agreement with a previous report of mRNA levels in a comparable experiment design [37]− and especially E46K, showed a very significant increase in cytokine release ( Figure 3B, right panel). Taken together, these results suggest that A30P and E46K, as opposed to either Wt α-Syn or the A53T variant, induce a pro-inflammatory response in glia. Remarkably, only two of the three PD-linked variants of α-synuclein were IL-1β inducers in primary glia, and contrary to expectations from previous studies performed using cell line cultures and other studies involving modified α-synuclein species, our results show that the A53T α-Syn variant has instead a very modest activity in regulating the innate immune response by primary glia and microglia. These findings indicate the need for further detailed studies on the role of the glia in early PD onset before detailed conclusions as to the role of the latter in PD can be drawn.
Previously, the available data had shown that the A30P and A53T α-Syn variants, but not the Wt and the E46K forms, efficiently induced the release of IL-1β when added to THP-1 macrophage cell line cultures [52] and that all the α-Syn variants were able to increase IL-1β secretion in THP-1 cells only when co-treatments with INF-γ were included, suggesting a pro-inflammatory response in already immune-primed THP-1 cells [52]. Our data, obtained with primary microglial cultures, indicate a very different behaviour. Thus, Wt and E46K α-Syn in our study appear to be pro-inflammatory in primary microglia, while the A30P and A53T variants seem to be unable to produce a significant response. These differential responses of these two studies point at the importance of the differentiation/maturation status of the primary microglia, which suggests that there could be multiple subtle but important differences in immune responses during the establishment of PD. It is interesting that these differences were only revealed in primary settings that were not over-primed, as was the case of IFN-γ treated THP-1 cells.
The observed effects on IL-1β secretion by primary glia and microglia are of particular interest considering the emerging role of an adaptive immune response in PD, in particular by CD4+ T cells [63]. Given IL-1's known effects to promote T cells responses, our findings on IL-1β regulation by α-Syn variants in innate immunocompetent cells require further attention in view of the potential effects of the latter in mediating immune tolerance and T effector responses.
IL-10 regulation by α-Syn-stimulated glia and microglia
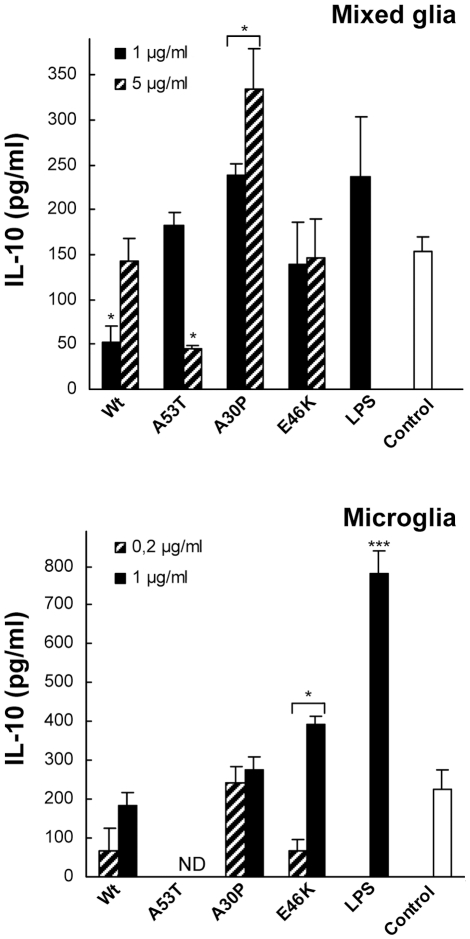
Although most studies in the past have focused on microglial production of pro-inflammatory cytokines, a large body of evidence has supported the notion that microglia also produce cytokines with anti-inflammatory or regulatory activities [25]. Indeed, a strong induction of IL-10 −recognized as an anti-inflammatory cytokine− had been observed for microglial cells stimulated with nitrated, aggregated Wt α-Syn [62]. We therefore investigated the effects of non-aggregated and unmodified α-Syn on glial secretion of IL-10 −a Th2 immunoregulator− which reduces cytokine production by Th1 cells ( Figure 4 ). Our results show that only the A30P variant produced a significant increase in IL-10 levels in mixed glial cells (top panel) whilst, on the contrary, the A53T variant caused a significant reduction of IL-10 basal levels, also observed in microglia, likely suggesting a lack of microglial response, a differential uptake by microglia, or an effect of the uptaken α-Syn on the endogenous IL-10 when A53T is present. In this sense, it has been reported a link between α-Syn and the microglial activation features [64], including phagocytic ability.
Figure 4. Immunoregulatory effect of α-Syn-stimulation in primary mixed glial and isolated microglial cultures.
IL-10 release was measured by ELISA in supernatants of α-Syn-stimulated mixed-glial cultures (top) and microglia (bottom) after a 20-hour treatment with monomeric Wt or mutant α-Syn variants, or lipopolysaccharide (LPS). Values are mean ± S.E.M. (n = 4). * P<0.05, ** P<0.01, *** P<0.001. The results shown are representative of two or three independent experiments with microglia and mixed glial cultures, respectively.
In microglial cells, on the other hand, only the E46K variant produced an increase in IL-10 levels as compared to the control (bottom panel). These results might suggest that microglial cells, in order to produce α-Syn-driven endogenous IL-10, require both IL-6 and IL-1β secretion. In this sense, while Wt α-Syn increased only IL-1β production in microglial cells, A30P increased only the IL-6 production. These facts could reflect the requirement of a doubly activated state of the microglia for IL-10 production. Although the general mechanism that generate IL-10 production within the CNS during neuroinflammation is still not well enough understood, our results support a role for α-Syn in the modulation of the microglial phenotype as suggested by Austin and collaborators [64].
Chemokine release profiles by α-Syn-stimulated glia and microglia
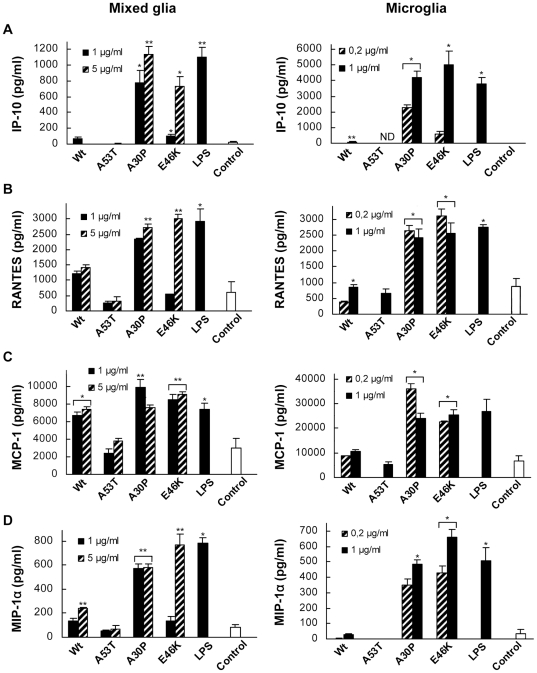
Chemokines are involved in a wide variety of disorders in the CNS and their actions contribute to reactive glial changes and neuronal injury in neuroinflammatory conditions [65]–[67]. We therefore sought to determine the chemokine release profiles of glial and microglial cells induced by non-aggregated Wt and PD-linked α-Syn variants. In particular, we assayed the release of IP-10/CXCL10, RANTES/CCL5, MCP-1/CCL2 and MIP-1α/CCL3 chemokines ( Figure 5 ).
Figure 5. Chemokine release profile of α-Syn-stimulated primary mixed glial cultures and isolated microglia cultures.
IP-10 (A), RANTES (B), MCP-1 (C), and MIP-1α (D) were measured in supernantants of mixed-glial cultures (left) and microglia (right) after a 20-hour treatment with monomeric Wt or mutant α-Syn variants, or lipopolysaccharide (LPS). All chemokines were assayed by ELISA as described in Materials and Methods. Values are mean ± S.E.M. (n = 2). * P<0.05, ** P<0.01. The results shown are representative of two and three independent experiments with microglia and mixed glial cultures, respectively.
The regulation by α-Syn of IP-10/CXCL10 in CNS cells has not been previously reported. In addition, the only data available regarding RANTES/CCL5 levels in PD are from a transgenic rat model for A53T α-Syn [38], or from sera and thus relate to peripheral dysregulation in the cytokine network associated with PD patients [68]–[70], and although no information is given on the genetic background of these PD patients, these studies reported increased levels of circulating RANTES/CCL5. Our results show large and comparable effects for A30P and E46K variants on IP-10/CXCL10 and RANTES/CCL5 release, both by mixed glial cultures and by microglia ( Figures 5A and 5B ). Remarkably, neither Wt nor A53T in both glial cultures and microglia produced a relevant increase in IP-10/CXCL10 or RANTES/CCL5 secretion, the latter result in agreement with the one reported for the striatum and SN in the (A53T) rat model [38]. These data suggest a specific role for A30P and E46K α-Syn variants associated with enhancement of Th1-cell recruitment, activation, and effector potential.
Besides their role as chemoattractants, IP-10/CXCL10 and RANTES/CCL5 are known to induce T-cell proliferation and cytokine production [71], suggesting a role for A30P and E46K α-Syn variants beyond purely innate pro-inflammatory responses. In this sense, IP-10/CXCL10 is pivotal in generating antigen-specific T-cells [71]. As the possible roles of adaptive immune responses in PD is gaining increasing attention [72], [73], our data should contribute to a better understanding of the differential immune responses exerted by the different α-Syn variants in terms of augmented microglia and macrophage recruitment and/or activation. The strong pro-inflammatory species (IL-1β, IL-6, IP-10/CXCL10, and RANTES/CCL5) whose release the A30P and E46K variants seem to promote in the CNS could lead to augmented macrophage recruitment and/or activation. This view is supported by the observation of upregulation of MCP-1/CCL2 and MIP-1α/CCL3 by A30P and E46K ( Figures 5C and 5D ). Interestingly, the large effects observed for A30P and E46K were much more pronounced in microglia, indicating a context-dependent stimulation mechanism. Further studies to asses the extent to which peripheral inflammation may amplify the neuroinflammation contributing to PD are needed, but our results raise the possibility of the need for a more personalised manipulation of the different PD situations in terms of immunosupressory treatments and/or immunomodulatory therapeutic approaches.
Previously, in addition to increased levels of TNF-α, IL-6, and INF-γ, stimulation of microglia with nitrated, aggregated α-Syn had been shown to enhance the secretion of MCP-1/CCL2 [46], [62]. In this work, we also found significantly higher levels of MCP-1/CCL2 induced by non-aggregated Wt α-Syn, as well as a similar MCP-1 release profile in mixed glial cultures for Wt α-Syn and its A30P and E46K variants, while A53T caused no detectable chemokine release ( Figure 5C ).
In summary, stimulation of total glia and microglia with A30P and E46K variants show large increases of IP-10/CXCL10, RANTES/CCL5, MCP-1/CCL2 and MIP-1α/CCL3 levels. Wt α-Syn, however, only induced the release of MCP-1/CCL2 and MIP-1α/CCL3, but not in isolated microglia, suggesting potential differential effects of Wt α-Syn and its variants on Th1/Th2 activation and/or recruitment, and a more prominent effect for A30P and E46K α-Syn variants in adaptive immune responses in PD.
Effect of non-aggregated α-Syn stimulation on microglial phagocytosis
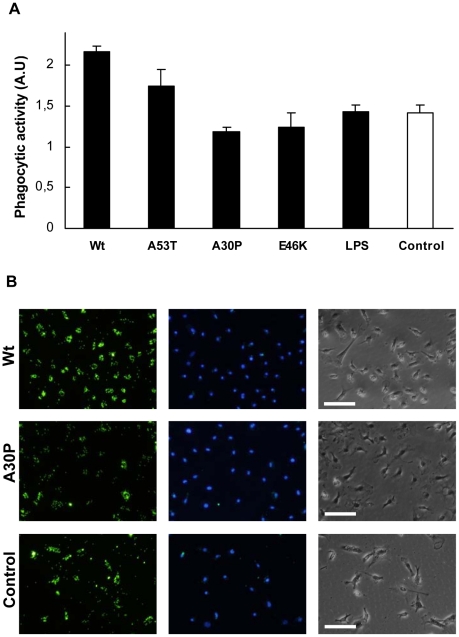
Phagocytosis is believed to be involved in steady-state tissue homeostasis, via the clearance of apoptotic cells, and the promotion of tissue repair and resolution of the wound [66], [74]. These aspects are related to the ‘alternative activation’ and acquired deactivation of the microglia, as a counter phenotype to the ‘classically activated’, pro-inflammatory, microglia [74]. Therefore, in order to assess the role of Wt α-Syn and the PD-linked variants on phagocytosis, we used fluorescein-conjugated tracker microparticles for measuring the phagocytosis capacity of differentially activated primary microglial cells ( Figure 6 ).
Figure 6. Effect of α-Syn-stimulation on microglial phagocytosis.
(A) After treatment of the primary microglial cell cultures with α-Syn (1 µg/ml) for 20 hours, cells were incubated with fluorescent microspheres for 1 hour. After fixing the cells, phagocytosis was assessed by fluorescence microscopy analysis. The phagocytic index was calculated by dividing the fluorescence from the phagocytosed microspheres by the total number of cells in the images. Four images were analysed for each sample in each experiment, and the results shown are representative of three independent experiments. A.U.: arbitrary units. (Representative microscopy images used to determine the phagocityc activity of microglial cultures stimulated with Wt (top) an A30P (centre) α-Syn, or non stimulated microglial cells (bottom). From left to right, green fluorescent microspheres, Hoechst-stained cells, and cells as observed in the absence of fluorescence. Scale bar: 50 µM.
It has been previously reported that monomeric Wt α-Syn enhances phagocytosis of a microglial cell line [51], and indeed, our results show that Wt and A53T α-Syn promote phagocytosis of microglial cells ( Figure 6B ), while the opposite effect was observed for cell cultures stimulated by the A30P and E46K α-Syn variants ( Figures 6A and 6B ). Interestingly, the phagocytic capacity measured for the stimulated microglia was observed to be inversely correlated to the IL-6 release levels induced by the different α-Syn variants ( Figure 3A ). Our findings point to induction of differential microglial phenotypes by α-Syn variants. Thus, A53T α-Syn, which was not associated with a robust pro-inflammatory activity, produced an increase in the phagocytic capacity that could reflect an alternative activation state of the microglia, as observed with CNS damage [61]. However, Wt α-Syn, which was associated with a moderate proinflammatory response in our model, promoted phagocytosis in microglia, indicating a combination of alternative and classical activation states, a scenario that has been related to chronic inflammatory processes such as those observed in Alzheimer's disease [74]. On the other hand, the A30P and E46K variants of α-Syn induced a strong pro-inflammatory response, combined with reduced phagocytic capacity, reflecting a classical activation state, which is clearly associated with the most cytotoxic situation.
Taken together, our results suggest that extracellular, non-aggregated Wt α-Syn produces a moderate to low pro-inflammatory response in glia, together with a reduction of the immunoregulatory response, and a moderate stimulation of Th1 chemokine secretion. The A30P and E46K pathological variants, on the other hand, can induce strong pro-inflammatory and immunoregulatory responses, together with marked increases in chemokine release levels, both in total glia and microglia. This exacerbated native immune response generated by these two α-Syn variants might explain the earlier onset and more rapid evolution of these two genetic forms of PD as compared to the sporadic variety. Intriguingly, our results from the pathologically-linked A53T variant, apart from a weak effect on IL-6 levels, did not provoke a significant native immune response. This finding suggests that there are other mechanisms of neurodegeneration that can contribute to the pathogenesis of PD, perhaps involving adaptive immune responses that might be promoted specifically by the A53T variant.
In comparison to the classical sporadic form of PD, the clinical phenotypes associated with mutations in α-Syn are characterized by an earlier disease onset but a reduced prevalence of tremor [75]–[79]. Studies that help to correlate the different α-Syn variants with the mechanism of neurodegeneration, and ultimately with disease progression, are therefore of considerable importance. However, we should be cautious about preclinical studies in animal models relating to translational research, as immune responses might vary between mice and human systems [80]. Equally important is to mention the lack of physiologically oriented studies employing human primary microglia, rather than immortalised cell lines. In this context, our results on the effects on glial native immunity exerted by extracellular, non-aggregated Wt α-Syn and the various familial PD-linked variants could be of value in the development of rational approaches towards effective control of immune responses that are associated with PD.
Materials and Methods
α-synuclein overexpression, purification, and preparation
Human wild-type α-Syn and the A30P, E46K and A53T mutants were overexpressed in E. coli BL21(DE3) cells using plasmid pT7-7 and purified as described previously [81] with minor modifications, as follows. After cell transformation, BL21(DE3)-competent cells were grown in LB in the presence of ampicillin (100 µg/ml). Protein expression was induced with 1 mM IPTG, and cells were harvested by centrifugation at 3,500 g after shaking at 37°Cfor 4 hours. The cell pellet was resuspended in 10 mM Tris–HCl (pH 8.0), 1 mM EDTA, and EDTA-free protein inhibitor cocktail (Roche Diagnostics, Burgess Hill, UK), and lysed by multiple freeze–thaw cycles and sonication. The cell suspension was boiled for 20 min and centrifuged at 20,000 g. Streptomycin sulphate was added to the supernatant to a final concentration of 10 mg/ml and the mixture was stirred for 15 min at 4°C. After centrifugation at 13,500 rpm, the supernatant was collected and ammonium sulphate was added (to 0.36 g/ml). The solution was stirred for 30 min at 4°C and centrifuged again at 13,500 rpm. The pellet was resuspended in 25 mM Tris–HCl (pH 7.7), and loaded onto an HQ/M-column on a BioCAD (Applied Biosystems, Foster City, USA) workstation. The synuclein proteins were eluted at ca. 300 mM NaCl with a salt gradient from 0 mM to 600 mM NaCl. The pure α-Syn fractions were pooled together and dialyzed extensively at 4°C against water. Protein purities were >95% as determined by SDS-PAGE, and molecular masses were confirmed by electrospray mass spectrometry.
In order to get rid of any possible bound contaminants, the purified proteins were subjected to denaturation with 6 M urea in 20 mM Tris/HCl (pH 8.0) for 1 hour at room temperature and subsequently dialyzed at 4°C against 20 mM Tris/HCl (pH 8.0) containing 3 M, 1.5 M, 0.75 M, and 0.375 M urea, and finally against water. Afterwards, the protein preparations were concentrated by centrifugation using a 10 k Amicon Ultra-Centrifugal Filter Device (Millipore Iberica, Madrid, Spain), and passed under sterile conditions through a 0.22-µm filter, and stored at −80°C.
PAGE electrophoresis analyses of the α-Syn preparations
Ten µg of protein were loaded onto a 15% SDS-PAGE gel (the loading buffer containing 0.1 M DTT), or 25 µg onto a 4–12% gradient Tris-glycine native PAGE gel (Lonza Group, Basel, Switzerland), and subjected to electrophoresis at 200 V. Gels were stained with Blue Silver [82], and destained with water. For Blue-Native PAGE (BN-PAGE), samples were prepared according to the protocol described previously for purified samples [83] with some modifications. Briefly, 12 µg of protein for experimental samples and 20 µg for protein markers were loaded on a continuous 15% poly-acrylamide gel. Ovalbumin and bovine serum albumin (Sigma-Aldrich, St. Louis, USA) were used as protein markers for native PAGE and BN-PAGE assays. After electrophoresis, gels were destained in a solution of 50% methanol and 10% acetic acid until the bands were clearly visible.
Time-course determination of total α-Syn and oligomer content in the medium after incubation with cells
For a quantitative assessment of the oligomeric content of culture supernatants, a specific sandwich ELISA assay [60] and a calibration curve with oligomers of an α-Syn variant carrying six point mutations (V63A, T64S, N65H, V66L, V71F, T72S), were used. Such oligomers were prepared as follows: a 700 µM solution in PBS of the mutant α-Syn was incubated overnight at RT, and subjected to centrifugation with a 100 kDa cut-off centrifugal filter (Millipore #UFC510024). The resulting retentate was rinsed with 150 µl of cold PBS and subjected to centrifugation as before. This procedure was repeated three more times, in order to eliminate the non-oligomerized protein. The oligomeric nature of the prepared α-Syn fraction was confirmed by native PAGE and Western blot (not shown), and the α-synuclein protomer concentration was determined with the BCA assay kit (Pierce, Rockford, USA.).
Quantification of total α-Syn species in culture supernatants after 0, 1, 6 and 20 hours of incubation with cells at 37°C was performed by ELISA. Briefly: individual wells of 96-well ELISA plates were coated with 100 µl of cell-free culture supernatants. Plates were incubated at 37°C for 2 hours and washed with 0.5% Tween20/PBS, pH 7.2 (PBST). Plates were blocked with 200 µl of 1% BSA in PBS and incubation at 37°C for 1 hour. After washing with PBST, 100 µl/well of a 1 µg/ml biotinylated anti-α-Syn 211 mouse mAb [Santa Cruz Biotechnology, Santa Cruz, USA; biotinylated as previously described [60] diluted in 1% BSA in PBS were added and plates were incubated at 37°C for 1 hour. After washing with PBST, 100 µl/well of 1∶5000 dilution of ExtrAvidin-alkaline phosphatase (Sigma-Aldrich, St. Louis, USA) in 1% BSA in PBS were added, and plates were incubated at 37°C for 30 min. After washing with PST, 100 µl/well of pNPP substrate (Sigma, St. Louis, USA) were added, and absorbance at 405 nm was measured within 30 minutes.
Cell cultures
Mixed glial cultures were prepared from the cerebral cortices of 1–3 days-old C57BL/6 mice (University of Seville Animal Core Facility, Seville, Spain) according to previously described methods [33] with some modifications. After mechanical, trypsin-mediated (BioWhittaker, Verviers, Belgium) dissociation, followed by filtration in DMEM-F12 with 10% inactivated FBS (BioWhittaker, Verviers, Belgium), cells were cultured at 37°C onto 12-well plates treated with poly-D-lysine (Sigma-Aldrich, St. Louis, USA). After 2 days, half of the volume of culture medium was carefully changed, and completely changed after 4 days of culture. Cells were used for stimulation or microglial isolation at day 18–22 of culture.
Microglial isolation was carried out according to previously described methods [84] with some modifications. The supernatant was removed and the wells were washed with DMEM-F12 without inactivated FBS; this conditioned medium was stored to be used later. Cells were incubated for 30–45 min with DMEM-F12/trypsin 0.25% solution at 37°C and complete medium was added to inactivate trypsin and the supernatant containing microglial cells collected. Conditioned medium was added to attached microglia; the following day the medium was changed for normal medium, and microglial cultures were stimulated after 5 days post isolation.
Immunofluorescence analysis
Purified microglia were characterized by immunocytochemistry on the basis of their expression of the pan haematopoietic marker CD45 and of the monocyte/macrophage markers CD11b, F4/80 and CD68. Additionally, the absence of Glial Fibrillary Acidic Protein (GFAP)-positive astrocytes in purified microglial cell cultures was also established. By contrast, trypsin-detached cells, largely astrocytes but containing some microglial cells, were plated back into 12-multiwell tissue culture plates and immunocytochemically processed as mentioned above.
For detection of CD45, CD11b and CD68 cell-surface antigens, cells were fixed for 10 min at room temperature (RT) in PBS containing 4% paraformaldehyde, washed twice in PBS and finally blocked overnight at 4°C in PBS containing 3% bovine serum albumin (BSA). The cells were then incubated for 1 hour at 4°C with a fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD45 monoclonal antibody (Leukocyte Common Antigen, Ly-5, clone 30-F11, BD Pharmingen, Franklin Lakes, USA) and a FITC-conjugated rat anti-mouse CD11b monoclonal antibody (clone M1/70, BD Pharmingen, Franklin Lakes, USA). Cells were exposed to primary rat anti-mouse F4/80 monoclonal antibodies (clone Cl:A3-1, Serotec, Oxford, UK) for 1 hour at RT at a final dilution of 1∶100 in PBS. For detection of CD68 and GFAP intracellular antigens, 4% PFA-fixed cells were permeabilized and blocked overnight at 4°C in PBS containing 3% BSA and 0.5% of the permeabilizing detergent Triton-X-100. The following day, cells were incubated for 1 hour at 4°C in PBS containing a dilution of 1∶100 rat anti-mouse monoclonal CD68 antibodies (clone FA-11, Serotec, Oxford, UK) and a 1∶300 dilution of anti-GFAP mouse monoclonal antibodies (clone G-A-5, Sigma-Aldrich, Saint Louis, USA). Labelling with F4/80 and CD68 primary antibodies was detected from fluorescence measurements after incubating cells for 30 min at RT in PBS containing 1∶100 diluted FITC-conjugated goat anti-rat IgG secondary antibodies (Jackson ImmunoResearch, West Grove, USA). For GFAP detection, an Alexa Fluor 488 nm goat anti-mouse IgG secondary antibody solution (Invitrogen, Paisley, UK) was added for 30 min at RT at a final dilution of 1∶300 in PBS. Immunofluorescence images were captured with an inverted fluorescence microscope Olympus IX71 using the digital image processing softwares DP Controller and DP Manager (Olympus Europa, Hamburg, Germany).
Phagocytosis assays
FluoresbriteTM carboxylate 0.75 µ microspheres (2.64% Solid-Latex; Polysciences Inc, Warrington, USA) were used as fluorescein-conjugated tracker microparticles for measuring the phagocytosis capacity of differentially activated microglial cells. One hour before starting the phagocytosis assay, fluorescent microspheres (1.08×1011 particles/ml) were mixed at a ratio of 1 µl/20 µl inactivated FBS (BioWhittaker, Verviers, Belgium) and incubated for 1 hour at 37°C in order to opsonise fully the carboxylate groups. The mixture of microspheres and FBS was then resuspended in fresh DMEM-F12 medium (BioWhittaker, Verviers, Belgium), with L-glutamine and P/S antibiotics supplements to obtain normal 10% FBS-supplemented media containing 5.4×108 microspheres/ml.
After removal of 400 µl of supernantant from the α-Syn-stimulated microglial cell cultures, a volume of 150 µl of resuspended microspheres was added to each well to obtain a final concentration of 1.08×108 particles/ml. The particles were then homogenously distributed throughout each well by gentle movements of the plate and incubated for 1 hour at 37°C. The medium containing non-phagocytosed microspheres was then removed and the cells were washed with PBS prior to their fixation with 4% paraformaldehyde in PBS for 30 min at 4°C. One ml of PBS containing the nuclear fluorescent dye Hoechst 33342 (1 µg/ml) was then added to the cells, and the plates were stored at 4°C for a minimum of 24 hours before being analyzed.
Determination of the phagocytic index
For each cell culture condition, the phagocytic capacity of microglial cells was determined by analysing fluorescent images of phagocytosed FITC-labelled microspheres and by staining cell nuclei with Hoechst 33342. An Olympus IX71 fluorescence microscope equipped with the digital image processing softwares DPController and DPManager (Olympus Europa, Hamburg, Germany) was used.
For each random field, a mean phagocytic index was calculated by determining the intensity of specific green and blue fluorescence emissions with digital imaging analysis software MetaMorph (MDS Analytical Technologies, Toronto, Canada). Specific green fluorescence emitted by FITC-microspheres was determined by subtracting the mean background fluorescence calculated from three different areas of the image where no cells were present, from the overall mean green fluorescence of the entire image. Specific blue emitted nuclear fluorescence was then calculated in a similar manner. The phagocytic index corresponds to the specific green/blue ratio. The mean phagocytic index was calculated from 4 random fields of cells (>100 cells) and was considered as a representative value of the phagocytic capacity of the microglial cells, as previously determined for mouse peritoneal macrophages within normal media for 24 hours with 1 µl/ml of lipopolysacharide (LPS).
qRT-PCR
Expression of GFAP and CD11b was determined by using a two-step quantitative real-time PCR. Total RNA from two microglial culture wells, one without treatment and one treated with LPS for 16 hours, was extracted using the RNeasy Micro Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocol. One µg of RNA was reverse-transcribed using the Quantitect Reverse Transcription kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocol. qPCR was performed with SYBR® Premix EX TaqTM (Perfect Real Time) (Takara Bio Inc., Otsu, Shiga, Japan) on an ABI Prism 7500 Real Time PCR System. Primers used were: HPRT_For: 5′-GTAATGATCAGTCAACGGGGGAC-3′, HPRT_Rev: 5′-CCAGCAAGCTTGCAACCTTAACCA-3′; GFAP_For: 5′-ATCGAGATCGCCACCTACAG-3′, GFAP_Rev: 5′-CTCACATCACCACGTCCTTG-3′; CD11b_For: 5′-CAGATCAACAATGTGACCGTATGG-3′, CD11b_Rev: 5′-CATCATGTCCTTGTACTGCCGC-3′). Multiple transcripts were analyzed simultaneously for 40 cycles using an optimized qRT-PCR thermal profile. Changes in gene expression were determined using the 2−ΔΔCt method with normalization to endogenous hypoxanthinephophoribosyltransferase (HPRT) control.
Cytotoxicity assays
The cytotoxic effect of the α-Syn variants under study was evaluated from the extent of LDH release with by using the LDH Cytoxicity Detection Kit (Roche, Basel, Switzerland) in MGC, following stimulation with the maximum concentration of wild-type α-Syn, its mutants or LPS (Sigma-Aldrich, St. Louis, USA), used in the present experiments (5 µg/ml for α-synuclein samples and 1 µg/ml for LPS). The limiting value in each case was determined using 6 µM MG132 (Sigma-Aldrich, St. Louis, USA), which is known to be lethal for cells at the concentration used, again using the manufacturer's protocol.
Cytokine release measurements
Glial mixed cultures and isolated microglial cultures were stimulated with Wt α-Syn and its mutational variants at different concentrations (5 µg/ml and 1 µg/ml for MGC and 1 µg/ml and 0.2 µg/ml for MiG) for 20 hours. LPS at a concentration of 1 µg/ml, and culture medium alone were used as positive and negative controls, respectively. Culture supernatants were harvested and centrifuged at 700 g for 5 min. and cell-cleared supernatants were recovered and stored at −80°C before cytokine measurement. IL-6, IL-1β, TNF-α, IFN-γ and IL-10 levels were assayed using Mouse IL-6/IL-1β/TNF-α/IFN-γ/IL-10 BD OptEIA ELISA set (BD Biosciences, Madrid, Spain) according to the manufacturer's protocol. Chemokine levels in the culture supernatants were determined by a specific sandwich ELISA by using capture/biotinylated detection antibodies obtained from Peprotech (London, UK) according to the manufacturer's recommendations. Cytokine profiles shown are representative of three independent experiments.
Ethics statement
All animals were handled in strict accordance with good animal practice as defined by the relevant national/EU guidelines and the CEA-CABIMER Experimental Animal Committee, and all animal work was approved by the appropriate committee (file CEA-2010-14).
Data analysis
All values are expressed as mean ± S.E.M. Statistical significance (Student's test, two-tailed) was evaluated using SPSS Statistics 17.0 (IBM Company, Chicago, USA).
Supporting Information
Time-course quantitation of total α-Syn in cell culture supernatants. Total α-Syn content measured by direct ELISA in culture supernatants recovered after addition of wild-type α-Syn to mixed glial cultures and incubation for 0, 1, 6 and 20 hours at 37°C.
(0.06 MB TIF)
TNF-α (A) and IFN-γ (B) levels were measured by ELISA in culture supernantants of microglia after a 20-hour treatment with exogeneously added α-Syn variants, or lipopolysaccharide (LPS). Values are mean ± S.E.M. (n = 3). The results shown are representative of two independent experiments.
(0.13 MB TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: CR held a Federation of the Societies of Biochemistry and Molecular Biology Long-Term Fellowship during part of this work, and currently holds a postdoctoral fellowship by the Spanish Ministry of Science and Innovation (Juan de la Cierva Programme). RFM holds an FPI fellowship at the University of Seville Department of Normal and Pathological Cytology and Histology. This work was supported by Instituto de Salud Carlos III - Fund for Health of Spain (to MD and DP); Junta de Andalucia BIO-323 (DP) and CTS-541 (MD); Alicia Kloplowitz Foundation (to MD and DP); and Wellcome Trust and Leverhulme Trust grants (CMD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksen JL, Wszolek Z, Petrucelli L. Molecular pathogenesis of Parkinson disease. Arch Neurol. 2005;62:353–357. doi: 10.1001/archneur.62.3.353. [DOI] [PubMed] [Google Scholar]
- 4.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 5.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 6.Sung JY, Kim J, Paik SR, Park JH, Ahn YS, et al. Induction of neuronal cell death by Rab5A-dependent endocytosis of alpha-synuclein. J Biol Chem. 2001;276:27441–27448. doi: 10.1074/jbc.M101318200. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Zhou Y, Wang Y, Fong H, Murray TM, et al. Identification of proteins involved in microglial endocytosis of alpha-synuclein. J Proteome Res. 2007;6:3614–3627. doi: 10.1021/pr0701512. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung JY, Park SM, Lee CH, Um JW, Lee HJ, et al. Proteolytic cleavage of extracellular secreted α-synuclein via matrix metalloproteinases. J Biol Chem. 2005;280:25216–25224. doi: 10.1074/jbc.M503341200. [DOI] [PubMed] [Google Scholar]
- 10.Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, et al. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- 11.El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, et al. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Seo JH, Suh YH. Alpha-synuclein, Parkinson's disease, and Alzheimer's disease. Parkinsonism Relat Disord. 2004;10:S9–13. doi: 10.1016/j.parkreldis.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM. alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res. 2000;62:9–14. doi: 10.1002/1097-4547(20001001)62:1<9::AID-JNR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- 15.Perez RG, Hastings TG. Could a loss of alpha-synuclein function put dopaminergic neurons at risk? J Neurochem. 2004;89:1318–1324. doi: 10.1111/j.1471-4159.2004.02423.x. [DOI] [PubMed] [Google Scholar]
- 16.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 17.Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM. Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J Am Chem Soc. 2005;127:476–477. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]
- 18.Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, et al. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc Natl Acad Sci USA. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 20.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 21.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 22.Gasser T. Genetics of Parkinson's disease. Curr Opin Neurol. 2005;18:363–369. doi: 10.1097/01.wco.0000170951.08924.3d. [DOI] [PubMed] [Google Scholar]
- 23.Bertoncini CW, Fernandez CO, Griesinger C, Jovin TM, Zweckstetter M. Familial mutants of alpha-synuclein with increased neurotoxicity have a destabilized conformation. J Biol Chem. 2005;280:30649–30652. doi: 10.1074/jbc.C500288200. [DOI] [PubMed] [Google Scholar]
- 24.Cookson MR. alpha-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:9. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 26.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 27.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord. 1998;13:221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- 29.Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- 30.Mirza B, Hadberg H, Thomsen P, Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson's disease. Neuroscience. 2000;95:425–432. doi: 10.1016/s0306-4522(99)00455-8. [DOI] [PubMed] [Google Scholar]
- 31.Vila M, Jackson-Lewis V, Guegan C, Wu DC, Teismann P, et al. The role of glial cells in Parkinson's disease. Curr Opin Neurol. 2001;14:483–489. doi: 10.1097/00019052-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson's disease by blocking microglial activation. FASEB J. 2003;17:944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, et al. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J Neuroinflammation. 2004;1:6. doi: 10.1186/1742-2094-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Wang T, Pei Z, Miller DS, Wu X, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 36.Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, et al. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson's disease. PLoS One. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu XL, Long CX, Sun L, Xie C, Lin X, et al. Astrocytic expression of Parkinson's disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roodveldt C, Christodoulou J, Dobson CM. Immunological features of alpha-synuclein in Parkinson's disease. J Cell Mol Med. 2008;12:1820–1829. doi: 10.1111/j.1582-4934.2008.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson's disease. J Mol Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- 43.Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, et al. Nitrated alpha-Synuclein Immunity Accelerates Degeneration of Nigral Dopaminergic Neurons. PLoS One. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Dallas S, Zhang D, Guo JP, Pang H, et al. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55:1178–1188. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- 45.Thomas MP, Chartrand K, Reynolds A, Vitvitsky V, Banerjee R, et al. Ion channel blockade attenuates aggregated alpha-synuclein induction of microglial reactive oxygen species: relevance for the pathogenesis of Parkinson's disease. J Neurochem. 2007;100:503–519. doi: 10.1111/j.1471-4159.2006.04315.x. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, et al. Nitrated alpha-synuclein-activated microglial profiling for Parkinson's disease. J Neurochem. 2008;104:1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- 47.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 48.Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 49.Joo SH, Kwon KJ, Kim JW, Hasan MR, Lee HJ, et al. Regulation of matrix metalloproteinase-9 and tissue plasminogen activator activity by alpha-synuclein in rat primary glial cells. Neurosci Lett. 2010;469:352–356. doi: 10.1016/j.neulet.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 50.Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, et al. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- 51.Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric alpha-synuclein, not aggregated alpha-synuclein: implications for Parkinson's disease. Glia. 2008;56:1215–1223. doi: 10.1002/glia.20691. [DOI] [PubMed] [Google Scholar]
- 52.Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, et al. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 53.Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, et al. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20:2000–2008. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- 54.Su X, Federoff HJ, Maguire-Zeiss KA. Mutant alpha-Synuclein overexpression mediates early proinflammatory activity. Neurotox Res. 2009;16:238–254. doi: 10.1007/s12640-009-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen PH, Hojrup P, Hager H, Nielsen MS, Jacobsen L, et al. Binding of Abeta to alpha- and beta-synucleins: identification of segments in alpha-synuclein/NAC precursor that bind Abeta and NAC. Biochem J. 1997;323:539–546. doi: 10.1042/bj3230539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, et al. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 57.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 58.Moussa CE, Wersinger C, Rusnak M, Tomita Y, Sidhu A. Abnormal migration of human wild-type alpha-synuclein upon gel electrophoresis. Neurosci Lett. 2004;371:239–243. doi: 10.1016/j.neulet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 60.El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 61.Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson's disease. Prog Neurobiol. 2009;89:277–287. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Nitrated α-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J Immunol. 2009;182:4137–4149. doi: 10.4049/jimmunol.0803982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson's disease. J Leukoc Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- 64.Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barreiro O, Martin P, Gonzalez-Amaro R, Sanchez-Madrid F. Molecular cues guiding inflammatory responses. Cardiovasc Res. 2010;86:174–182. doi: 10.1093/cvr/cvq001. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz M. “Tissue-repairing” blood-derived macrophages are essential for healing of the injured spinal cord: From skin-activated macrophages to infiltrating blood-derived cells? Brain Behav Immun. 2010;24:1054–1057. doi: 10.1016/j.bbi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 67.Rich RR, Fleisher TA, Shearer WT, Shroeder HW, Jr, Frew AJ, Weyand CM. Clinical Immunology. In: TA F, editor. Principles and Practice. Elsevier Inc; 2008. [Google Scholar]
- 68.Rentzos M, Nikolaou C, Andreadou E, Paraskevas GP, Rombos A, et al. Circulating interleukin-15 and RANTES chemokine in Parkinson's disease. Acta Neurol Scand. 2007;116:374–379. doi: 10.1111/j.1600-0404.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 69.Gangemi S, Basile G, Merendino RA, Epifanio A, Di Pasquale G, et al. Effect of levodopa on interleukin-15 and RANTES circulating levels in patients affected by Parkinson's disease. Mediators Inflamm. 2003;12:251–253. doi: 10.1080/09629350310001599701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, et al. Peripheral cytokines profile in Parkinson's disease. Brain Behav Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, et al. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 72.Stone DK, Reynolds AD, Mosley RL, Gendelman HE. Innate and adaptive immunity for the pathobiology of Parkinson's disease. Antioxid Redox Signal. 2009;11:2151–2166. doi: 10.1089/ars.2009.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 74.Colton CA, Wilcock DM. Assessing Activation States in Microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 75.Bonifati V, Oostra BA, Heutink P. Unraveling the pathogenesis of Parkinson's disease: the contribution of monogenic forms. Cell Mol Life Sci. 2004;61:1729–1750. doi: 10.1007/s00018-004-4104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA. Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha-synuclein mutation. Ann Neurol. 2001;49:313–319. [PubMed] [Google Scholar]
- 77.Golbe LI, Di Iorio G, Sanges G, Lazzarini AM, La Sala S, et al. Clinical genetic analysis of Parkinson's disease in the Contursi kindred. Ann Neurol. 1996;40:767–775. doi: 10.1002/ana.410400513. [DOI] [PubMed] [Google Scholar]
- 78.Papapetropoulos S, Paschalis C, Athanassiadou A, Papadimitriou A, Ellul J, et al. Clinical phenotype in patients with alpha-synuclein Parkinson's disease living in Greece in comparison with patients with sporadic Parkinson's disease. J Neurol Neurosurg Psychiatry. 2001;70:662–665. doi: 10.1136/jnnp.70.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bostantjopoulou S, Katsarou Z, Papadimitriou A, Veletza V, Hatzigeorgiou G, et al. Clinical features of parkinsonian patients with the alpha-synuclein (G209A) mutation. Mov Disord. 2001;16:1007–1013. doi: 10.1002/mds.1221. [DOI] [PubMed] [Google Scholar]
- 80.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 81.Hoyer W, Antony T, Cherny D, Heim G, Jovin TM, et al. Dependence of alpha-synuclein aggregate morphology on solution conditions. J Mol Biol. 2002;322:383–393. doi: 10.1016/s0022-2836(02)00775-1. [DOI] [PubMed] [Google Scholar]
- 82.Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, et al. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- 83.Wittig I, Braun HP, Schagger H. Blue native PAGE. Nat Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 84.Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time-course quantitation of total α-Syn in cell culture supernatants. Total α-Syn content measured by direct ELISA in culture supernatants recovered after addition of wild-type α-Syn to mixed glial cultures and incubation for 0, 1, 6 and 20 hours at 37°C.
(0.06 MB TIF)
TNF-α (A) and IFN-γ (B) levels were measured by ELISA in culture supernantants of microglia after a 20-hour treatment with exogeneously added α-Syn variants, or lipopolysaccharide (LPS). Values are mean ± S.E.M. (n = 3). The results shown are representative of two independent experiments.
(0.13 MB TIF)