Abstract
The most significant long-term complication of radiotherapy in the head and neck region is hyposalivation and its related complaints, particularily xerostomia. This paper addresses the pathophysiology underlying irradiation damage to salivary gland tissue, the consequences of radiation injury, and issues contributing to the clinical management of salivary gland hypofunction and xerostomia. These include ways to: (1) prevent or minimize radiation injury of salivary gland tissue, (2) manage radiation-induced hyposalivation and xerostomia, and (3) restore the function of salivary gland tissue damaged by radiotherapy.
Keywords: radiotherapy, hyposalivation, xerostomia, prevention, palliative care, gene transfer, stem cell therapy
Introduction
Radiotherapy plays a pivotal role in the curative treatment of the majority of patients with head and neck cancer, either as single modality or in combination with surgery and/or chemotherapy. Despite the beneficial effects of radiotherapy in loco-regional tumor control, the damage inflicted to normal tissues surrounding the tumor may cause severe complications. In particular, co-irradiation of the salivary glands during the treatment of head and neck cancer results in a progressive loss of gland function (hyposalivation) beginning early in the course of radiotherapy.1
Oral sequelae resulting from radiation injury of salivary gland tissue
Quantitative and qualitative salivary changes predispose the irradiated patient to a variety of problems that develop either directly or indirectly as a result of diminished salivary output. 2–4 These include oral dryness, impairment of normal oral functions (speech, chewing and swallowing) because of insufficient wetting, and decreased lubrication of the mucosal surfaces and of ingested food. Furthermore, the oral mucosa can become dry and atrophic, leading to frequent ulceration and injury. Finally, the shift in oral microflora towards cariogenic bacteria, the reduced salivary flow (oral clearance), and changes in saliva composition (decreased buffer capacity, pH, immunoprotein concentrations), may result in rapidly progressing radiation caries.2,5
Although most studies focus on salivary flow, other endpoints related to salivary function, such as patient-rated xerostomia and physician-rated RTOG xerostomia, are probably of even more clinical relevance.6,7 Importantly, the subjective symptom of xerostomia may not always correlate with salivary flow rates. For understanding this phenomenon, one should be aware that saliva enters the mouth at several locations, and that the different glandular secretions are not well mixed.8 For example, the contribution of parotid saliva to (un)stimulated whole saliva varies from site to site, ranging from being the major contributor to whole saliva collected buccally from the maxillary molars to being almost non-contributing to whole saliva collected in the incisor region.9 The wide variation in local contribution of the various salivary glands to whole saliva is also obvious when assessing mucosal wetness as the thickness of the salivary layer on the oral mucosa is much thinner in the labial and anterior hard palatal region than on the buccal mucosa and anterior tongue.10 These phenomana might explain, at least in part, the differences reported in the literature about level of hyposalivation and sensation of oral dryness.
Pathophysiology of radiation damage to salivary gland tissue
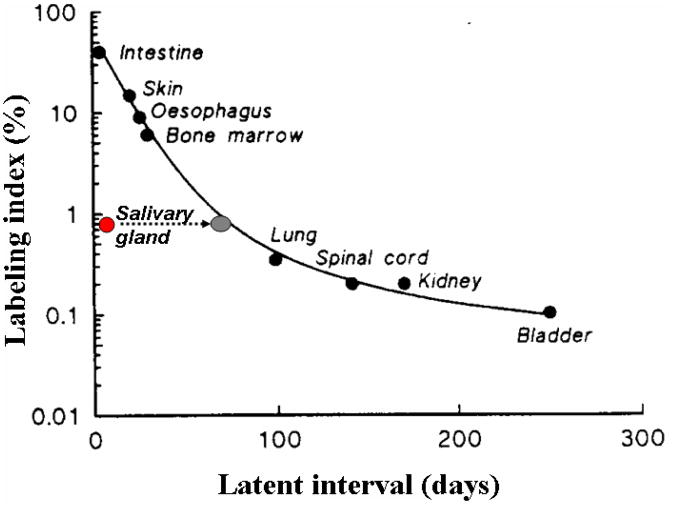
Radiation-induced DNA damage impairs proper cell division, resulting in cell death or senescence of cells that attempt to divide. Based on the slow turnover rates of their cells (60–120 days), the salivary glands would be expected to be late responding tissue (>60 days) (Fig. 1).11 However, the changes in quantity and composition of saliva that occur shortly after radiotherapy indicate that these glands respond acutely.1,12 Radiation injury leads primarily to the loss of saliva-producing acinar cells, however interestingly, the ducts, although deprived of function, mostly remain intact.13 A human postmortem study suggests that in the lower dose range (< 30 Gy, in 2 Gy fractions) damage is reversible to a certain extent, but with cumulative doses (> 75 Gy) extensive degeneration of acini is observed along with inflammation and fibrosis in the interstitium.14 The role of apoptotic cell death in early salivary gland dysfunction after radiotherapy remains unclear. Paardenkooper et al.15 did not observe a dose-related increase in apoptotic cells very early after radiotherapy, while Avila et al.16 found that early radiation-induced salivary gland dysfunction resulted from p53-dependent apoptosis.
Fig. 1.
Based on the slow turnover rates of their cells, salivary glands are expected to be late responding, but the changes in quantity and composition of saliva already occur shortly after radiotherapy (red circle). This resembles an immediacy of a radiation response (short latent interval) that is normally observed for cells with a higher labelling index (such as intestine). However, when one looks at the actual kill of acinar cells, the curve behaves just like any other late responding tissue (gray circle) (adapted from Stewart and van de Kogel, 2002).7
Next to the suggestion of massive apoptosis, the leakage of granules and subsequent lysis of acinar cells has been suggested as an alternative explanation for the acute radiation-induced dysfunction of the salivary glands.17,18 However several studies show no cell loss during the first days after irradiation, although saliva flow is dramatically reduced, and water secretion is selectively hampered.19–22 One mechanism of action to explain the early effects and the enigmatic high radiosensitivity of salivary cells is selective radiation damage to the plasma membrane of the secretory cells resulting in disruption of muscarinic receptor-stimulated water secretion. Based on their studies in the rat model, Coppes et al.21 have proposed that radiation-induced loss of salivary gland function occurs over four phases. The first phase (0–10 days) is characterised by a rapid decline in flow rate without changes in amylase secretion or acinar cell number. The second phase (10–60 days) consists of a decrease in amylase secretion paralleled by acinar cell loss. Flow rate, amylase secretion and acinar cell numbers do not change in the third phase (60–120 days). In the fourth phase (120–240 days) further deterioration of gland function is seen, but is accompanied by an increase in acinar cell number, albeit with poor tissue morphology. Comparable changes have been observed in rat submandibular tissue; however similar studies are not available in humans.12,22
Prevention of radiation-induced injury to the salivary gland
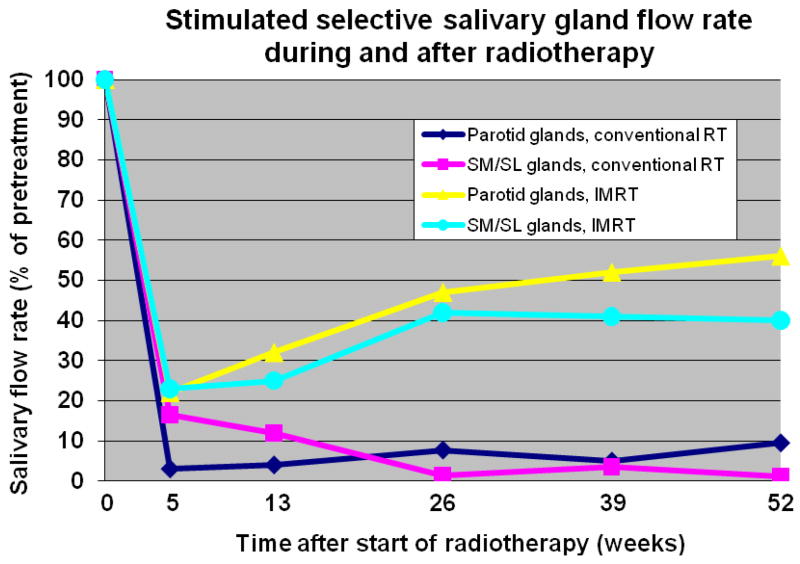
In humans, depending on the localization of the radiation portals, a rapid decrease of the salivary flow rate is observed during the first week of radiotherapy, after which there is a continuing gradual decrease to less than 10% of the initial flow rate (Fig. 2).1,23,24 Although in the older literature the submandibular gland was thought to be less radiosensitive than the parotid gland, both glands have been shown to be as sensitive to radiotherapy, at least with respect to their function.1,23,24 The range of the mean doses, which represents the threshold for significant salivary flow reduction, is 26 to 39 Gy.25–28 Recently, it was shown that there is no threshold dose for reduced parotid gland function after radiotherapy and the TD50 is probably equal to 40 Gy,29 comparable to the TD50 (39 Gy) for submandibular gland function.26
Fig. 2.
Flow rate of 2% citric acid-stimulated parotid (single gland) and bilateral submandibular-sublingual (SM/SL) saliva as a function of time after start of radiotherapy (RT) [Conventional RT; both parotid, submandibular and sublingual glands located in the treatment portal, 2 Gy per day, 5 days per week, total dose 60–70 Gy. Parotid-sparing 3-dimensional (3D)/intensity-modulated RT (IMRT); bilateral (the majority) and unilateral RT (scattered radiation to contralateral gland). For parotid IMRT data: 1.8–2.0 Gy per fraction, prescribed dose to primary target 64 Gy (range 57.6–72 Gy) and for SM/SL IMRT data: 2 Gy per day, 70 Gy to gross disease planning target volume]. Initial flow rates are set to 100% (modified after Burlage et al.1 for conventional RT, Eisbruch et al.22 for parotid glands IMRT, and Murdoch-Kinch et al.25 for SM/SL glands IMRT).
Advances in radiation delivery
Intensity modulated radiotherapy (IMRT)
Changing a conventional schedule of fractionated radiot-herapy into a schedule of continuous, hyperfractionated, accelerated radiotherapy (CHART) results in some sparing of salivary gland function; howeverIMRT allows a more accurate delivery of specific radiation dosage and dose distribution to the tumor and thereby brings about the possibility of better sparing of surrounding tissues, e.g. major salivary glands. IMRT is currently recommended as a standard approach in head and neck cancer to limit the cumulated radiation dose to critical normal tissues. IMRT can reduce the dose to parotid, submandibular/sublingual and minor salivary glands while helping maintain adequate whole saliva flow rates and reducing xerostomia (Fig. 2)6,25–27, Although IMRT, when compared to two-dimensional radiotherapy (2D-CRT), results in a significant reduction of patient- and observer-rated xerostomia, still about 40% of patients complain of xerostomia.30
Proton radiotherapy
Further improvement of salivary gland tissue sparing could be achieved by new radiation techniques using charged particles, e.g., protons instead of the currently used photons. The physical and radiobiological properties of protons allow a superior dose distribution as compared to current photon (X-ray) radiotherapy, thereby minimizing the dose to normal tissues and significantly reducing acute and late side effects. The potential benefit of protons have been investigated in a number of planning comparative studies that show that the dose to critical organs can be significantly reduced when protons are used, particularly for tumors originating in the pharynx,31,32 the paranasal sinuses33,34 and in head and neck cancer patients treated with bilateral neck irradiation.35 In some studies, a significant reduction of the risk of side effects was observed in approximately 70% of the cases based on existing and validated NTCP (normal tissue complication probability) models.35
Radioprotectors
Amifostine
Direct radioprotection may be achieved by the use of amifostine, an oxygen radical scavenger, when systemically administered during radiation treatment (Table 1).36,37 Although amifostine has the potential to reduce xerostomia during and post radiation treatment, a significant proportion of patients continue to experience xerostomia.40 Intravenous administration of amifostine is accompanied by many side effects; recently it has been shown that subcutaneous administration of amifostine provides a well-tolerated alternative.41 Unfortunately, amifostine might have the undesirable effect of tumor protection, thus the controversy continues as to whether amifostine is safe for use in cancer patients.37,42 Guidelines for the use of amifostine and other agents are presented in Table 1.
Table 1.
Guidelines for a variety of management strategies aimed to reduce xerostomia and their level of evidence (for details see the systematic review by Jensen et al.6). Quality of recommendations according to the American Society of Clinical Oncology clinical practice guidelines.39
| Treatment approach | Guideline | Level of evidence |
|---|---|---|
| Radical scavenger | ||
| Amifostine | Phase III trials have shown that amifostine might reduce xerostomia after radiotherapy. However, no guidelines are possible due to lack of consensus on interpretation of existing evidence. Most studies did not have the statistical power to evaluate the influence of amifostine on the therapeutic index. Also the trial design of most studies was at least questionable and the outcomes subject to debate. The majority of the trials lacked a placebo in the control arms. | Level II, grade C |
| Muscarinic agonist stimulation | ||
| During radiotherapy | Use of pilocarpine can not be recommended for improvement of xerostomia as the results of the randomized clinical trials were not univocal. Moreover, improvement of salivary gland hypofunction was limited. The dissimilar results on sparing of the salivary gland function are probably highly dependent on the wide range of cumulative doses applied. The only trial providing an analysis of sparing of parotid gland function related to mean parotid dose indicated significant sparing of parotid gland function and reduced xerostomia for mean parotid doses exceeding 40 Gy.38 | Level II, grade C |
| After radiotherapy | The use of pilocarpine after radiotherapy can be recommended for improvement of xerostomia | Level II, grade B |
| Mucosal lubricants/saliva substitutes | The use of oral mucosal lubricants and saliva substitutes for short-term improvement of xerostomia after radiotherapy can be recommended. | Level II, grade B |
| Acupuncture | The use of acupuncture to stimulate salivary gland secretion and to alleviate xerostomia can be suggested to the patient. | Level II, grade C |
Tempol
Tempol, is a stable nitroxide, is thought to provide radioprotection by several mechanisms including oxidizing transition metals, mimicking superoxide dismutase activity, and scavenging free radicals. In a mouse model it was shown that administration of Tempol i.p., i.v., s.c. or even in topical form (mouthwash, gel), all significantly reduced irradiation-induced salivary gland hypofunction.43,44 Moreover, Tempol provided salivary gland protection but did not protect tumor tissue.45 These various studies support further development and consideration of Tempol for human clinical trials as a selective protector against radiation-induced salivary gland damage.
Other preventive agents
Other potential preventive agents include pre- or during radiotherapy administration of insulin growth factor 1 (IGF-1) or keratinocyte growth factor (KGF), both of which have been studied in mice. The rationale for use of growth factors is to suppress apoptosis and/or enhance survival and proliferation of salivary acinar cells post-radiation.16,46 Utilization of IGF-1 has been shown to reduce radiation-induced apoptosis and preserve salivary gland function in a mouse model extending the causal relationship between apoptosis and dysfunction.46 Subcutaneous administration of KGF, either before or directly after irradiation also reduced radiation-induced hyposalivation.47 Post-irradiation administration of KGF seemed to act through accelerated expansion of the pool of progenitor/stem cells that survived the irradiation treatment. Recently, it was shown that pre-irradiation intraglandular administration of botulinum toxin into rat submandibular glands can also significantly reduce radiation injury at a glandular level.48
Strategies for restoring salivary gland function follwing irradiation injury
Stimulation of residual function
Administration of pilocarpine or pure cholinergic sialogogues to stimulate any residual function of the salivary gland post-radiotherapy is worthwhile (Tables 1 and 2), however the functional gain ceases as soon as the administration of the sialogogue is stopped.49,50 A more persistent effect can be observed when pilocarpine is administered before radiotherapy and continued during radiotherapy and then stopped.38 Moreover, in a rat study it was shown that amelioration of early loss of rat salivary gland function after radiation by pilocarpine pretreatment was, at least in part, due to compensatory mechanisms through increased proliferation of undamaged cells.51,52 In a large study by Scarantino et al.,53 no significant differences in xerostomia, mucositis and quality of life were found, but unstimulated whole salivary flow significantly increased. In the study of Burlage et al.38 no statistically significant differences between the pilocarpine and the placebo group could be found overall. However, the results of that study also showed that the beneficial effect of pilocarpine depended on the dose distribution in the parotid glands, indicating that when the mean parotid dose exceeds 40 Gy, pilocarpine might significantly spare parotid flow and reduce patient-rated xerostomia, particularly after 12 months.
Table 2.
Sialogogues.
| Gustatory and tactile sialogogues* | Pharmacological sialogogues |
|---|---|
| Acid-tasting substances | Anetholetrithione |
| acidic (sugar-free) sweets | Benzapyrone |
| acidic or effervescent drinks (lemon juice, citric acid, buttermilk) | Betanechol chloride |
| Carbachol | |
| citric acid crystals | Cevimeline** |
| cotton-wool gauze soaked in a citric acid and glycerine solution | Folia Jaborandi and tinctura Jaborandi |
| Neostigmine, neostigmine bromide, pyridostigmine bromide, destigmine bromide | |
| lemon pastilles | |
| lemon slices | Nicotinamide and nicotine acid |
| vitamin C tablets | Pilocarpine hydrochloride**, pilocarpine nitrate |
| Miscellaneous substances: | Potassium iodide |
| dried pieces of reed root (calami rhizome) | Trithioparamethoxyphenylpropene |
| sugar-free chewing gum | |
| sugar-free sweets | |
| vegetables or fruits |
Not all substances are recommended in dentulous patients as acidic products may induce demineralization of dental hard tissues
Only these agents have been approved for relief of xerostomia in humans and have undergone substantial, controlled clinical testing
Probably, a significant part of the beneficial effect of pilocarpine on post-irradiation xerostomia can be attributed to stimulation of the minor salivary glands as the mucous minor palatal glands have been shown to have a greater resistance to irradiation than serous parotid glands.54 Other drugs that have been reported to be of significance in the treatment of hyposalivation include anetholtrithione55 and cevimeline.56 As the stimulating properties of these sialogogues are short-lived, patients have to use them for the rest of their lives.
Acupunture
Stimulation of residual salivary secretory capacity by acupuncture has shown some promising results in head and neck radiotherapy patients (Table 1).57–59 The effects of acupuncture treatment on secretion of whole saliva and related symptoms are sustained for at least six months57,59,60 and additional acupuncture therapy can maintain this improvement up to 3 years.57 Moreover, Deng et al.61 showed that acupuncture was associated with neuronal activation that was absent during sham acupuncture stimulation.
Oral lubricants and saliva substitutes
When stimulation of residual secretion is insufficient the clinician is left with a purely symptomatic approach. This is most commonly through the frequent use of water, although complex saliva substitutes have been developed that contain agents to impart viscosity and to keep soft tissues moist (Table 2). These substitutes are either based on carboxymethylcellulose (CMC)62, mucin63 or xanthan gum64. Mucin-containing and xanthan gum-containing saliva substitutes are usually preferred over CMC-containing substitutes, both by patients with Sjögren’s syndrome and radiation-induced xerostomia as they have superior rheological and wetting properties.64,65 In addition ‘gel-like’ saliva substitutes have been developed, of which the polyglycerylmethacrylate based substitute holds promise,66 particularly when employed at night and when daily activities are at a low level. It is worthwhile to try different types of saliva substitutes in a particular patient in order to select the most effective substitute for that patient.64,67 Based on the literature, recommendations for the treatment of hyposalivation have been summarized in Table 3.66
Table 3.
Recommendations for the treatment of hyposalivation with oral mucosa lubricants and saliva substitutes.66
| Severe hyposalivation | A saliva substitute with gel-like properties should be used during the night and when daily activities are at a low level. During the day, a saliva substitute with properties resembling the viscoelasticity of natural saliva, such as substitutes which have xanthan gum and mucin (particularly bovine submandibular mucin) as a base should be applied. |
| Moderate hyposalivation | If gustatory, tactile or pharmacological stimulation of the residual salivary secretion does not provide sufficient amelioration, saliva substitutes with a rather low viscoelasticity, such as substitutes which have carboxymethylcellulose, hydroxypropylmethylcellulose, mucin (porcine gastric mucin), or low concentrations of xanthan gum as a base are indicated. During the night or other periods of severe oral dryness, the application of a gel is helpful. |
| Slight hyposalivation | Gustatory, tactile or pharmacological stimulation of the residual secretion is the treatment of choice. Little amelioration is to be expected from the use of saliva substitutes. |
Promising new approaches for restoration of salivary gland function
Gene therapy
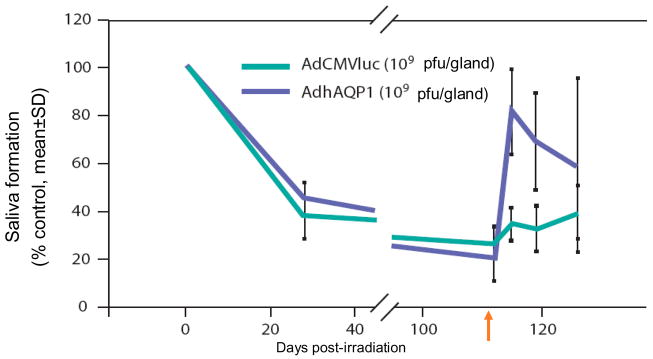
In the future gene therapy might provide a therapeutic option for radiation induced-salivary hypofunction in some patients. The gene transfer strategy pioneered by Baum et al.68 focused on developing a gene transfer event that could elicit fluid secretion from surviving (primarily duct) epithelial cells in an irradiated salivary gland.69 Delporte et al.69 reasoned that surviving duct cells could serve as water secreting cells if there was a pathway for water transport inserted in the duct cell membranes. Their approach utilized the water channel protein, human aquaporin-1 (hAQP1).70 hAQP1 can facilitate the extremely rapid movement of water in response to an osmotic gradient, and expression of hAQP1 protein in cell types in which it is not normally found can lead to dramatic increases in osmotically-obliged water movement (Fig. 3a). There have been two key pre-clinical studies with hAQP1. Both utilized a recombinant serotype 5 adenoviral vector encoding hAQP1, AdhAQP1, which was delivered to salivary glands via intraductal cannulation through the orifice of the main excretory duct.71 In the first in vivo study irradiated rats given AdhAQP1 displayed salivary flow rates approaching those of sham-irradiated animals treated with the control virus, i.e., nearly normal.69 The second key in vivo study was a longitudinal study and targeted parotid glands in a large animal, the miniature pig (~25–30kg).72 Sixteen weeks following irradiation, salivary secretion was decreased by >80%. Administration of AdhAQP1 resulted in a dose-dependent increase in parotid salivary flow to ~80% pre-irradiation levels on day 3 (Fig. 3b). Since the hAQP1 transgene was expressed only in parotid duct cells, the implication is that the increased salivary secretion observed was due to enhanced water permeability in the normally water-impermeable duct cells.72 Currently, a phase I study is underway in individuals with salivary hypofunction to see whether hAQP1 gene transfer is safe and effective in humans (http://www.clinicaltrials.gov/ct/show/NCT00372320).
Fig. 3.
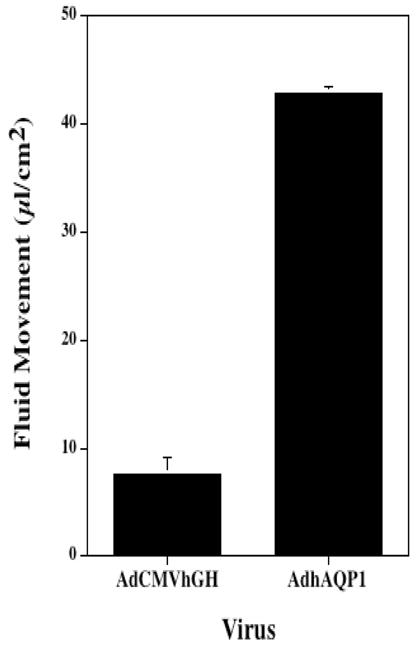
Fig. 3a Effect of AdhAQP1 on net fluid movement across SMIE cell monolayers. Data shown are the mean ± SEM of experiments originally reported in He et al. 73 SMIE cell monolayers were either transduced at a multiplicity of infection of 5 with AdhAQP1 or AdCMVhGH (control vector, encoding human growth hormone), and 24 hours later transepithelial fluid movement was measured for 60 min.73
Fig. 3b Parotid salivary output after delivery of an adenoviral vector containing a water channel or control. The flow rate prior to irradiation was set at 100% and the salivary flow rates obatained at times thereafter are represented as a percentage of this initial value. The arrow indicates the time point when the adenoviral vectors were administered. AdhAQP1 is the vector encoding the water channel transgene hAQP1. AdCMVluc is a control vector (modified after Shan et al., 200574).
Stem cell therapy
Following irradiation, salivary gland recovery is dependent on the radiation dose and on the number of remaining viable stem cells.47 These findings indicate that similar to other organs, in salivary glands, lack of replenishment of functional cells is due to the radiation-induced loss of the tissues’ endogenous gland stem cells.13 Increasing the regenerative potential of salivary glands by stem cell therapy after irradiation should be able to restore tissue homeostasis.
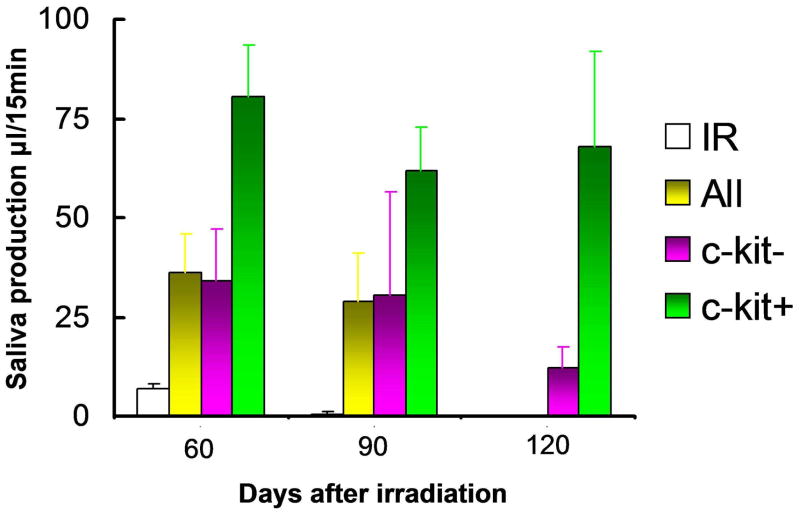
Lombaert et al.73 recently discovered a population of c-Kit+ cells with the capability to restore radiation-induced damage to salivary glands in rodents. Stem cell-containing salispheres were cultured from rodent submandibular glands. In vitro, cells from these spheres expressed many stem cell markers (e.g., Sca-1, c-Kit, Musashi-1) and were able to differentiate into all salivary gland lineages.73 Following stem cell enrichment, c-Kit+ cells were able to regenerate and completely restore submandibular gland function in irradiated secondary recipients three months after transplantation,73 indicative of two essential characteristics of stem cells, the capability to self-renew and to differentiate into all lineages of an organ (Fig. 4). Importantly, salispheres grown from human parotid and submandibular salivary glands also contained c-Kit+ cells and showed self-renewal and differentiation capacities in vitro,73 bringing human clinical application of such therapy within reach.
Fig. 4.
Restored organ function by transplanted progenitor/stem cells. Cells of day 3 old salispheres cultured from dispersed submandibular gland cells were intra-glandularly injected 30 days post-irradiation. Injection of 300–1000 c-Kit+ cells (green bars) restored gland function up to 120 days post-irradiation, whereas 10,000–90,000 c-Kit− only temporarily, and to a much lesser extent, restored function (up to 90 days) indicating that they may also contain some progenitor cells. All (red) all cells of the salispheres; (n.d. not determined).
Epilogue
Despite advances in our understanding of the cellular and biochemical basis for irradiation-induced loss of salivary gland function, options for the clinical management of irradiation-induced salivary gland hypofunction remain largely limited to palliative therapies. Efforts to protect or diminish irradiation-induced damage including IMRT and the use of radioprotectors such as Tempol are progressing; however there is a need for the concurrent pursuit of therapies aimed at restoration of the function of damaged glands. Although most cells in the salivary gland are thought to be post-mitotic, recent studies suggest that an important target of radiation-induced damage may be the relatively small population of salivary stem cells. Identification of this cell population and strategies for the expansion of salivary stem cells for gland regeneration are important goals that may lead to restoration of the functional capacity of damaged glands. Likewise exciting studies are underway to restore the functional capacity of the gland through gene transfer, e.g., hAQP-1, which can enhance fluid formation in damaged salivary gland. This pursual of these multiple complementary strategies is important if improvements in the clinical manangment of the oral complications of irradiation are to advance.
Acknowledgments
Funding for this conference was made possible in part by Award Number R13 DE19330 from the National Institute of Dental and Craniofacial Research. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government. Partial funding was also provided by the National Cancer Institute, the National Institute of Health Office of Rare Diseases, The University of Connecticut School of Dental Medicine, Carolinas Medical Center, The Multinational Association of Supportive Care in Cancer, and the International Society of Oral Oncology. This conference was also supported by unrestricted educational grant funds from Endo Pharmaceuticals, Inc. and from EUSA Pharma, and by funds from Biovitrum and Helsinn Healthcare SA.
COI: RP Coppes declares that partial research support was provided by AMGEN.
Footnotes
Discussed at the Conference on Oral Complications of Emerging Cancer Therapies, Bethesda, Maryland USA, April 14-15th, 2009
No other conflicts are declared by any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burlage FR, Coppes RP, Meertens H, Stokman MA, Vissink A. Parotid and submandibular/sublingual flow during high dose radiotherapy. Radiother Oncol. 2001;61:271–274. doi: 10.1016/s0167-8140(01)00427-3. [DOI] [PubMed] [Google Scholar]
- 2.Vissink A, Jansma J, Spijkervet FKL, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 3.Jellema AP, Slotman BJ, Doornaert P, et al. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:751–760. doi: 10.1016/j.ijrobp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Langendijk JA, Doornaert P, Verdonck-de L, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 5.Jensen SB, Pedersen AM, Reibel J, et al. Xerostomia and hypofunction of the salivary glands in cancer therapy. Support Care Cancer. 2003;11:207–225. doi: 10.1007/s00520-002-0407-7. [DOI] [PubMed] [Google Scholar]
- 6.Jensen SB, Pedersen AML, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010 Mar 25; doi: 10.1007/s00520-010-0837-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 8.Dawes C. Salivary flow patterns and the health of hard and soft oral tissues. J Am Dent Assoc. 2008;139 (Suppl):18S–24S. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- 9.Sas R, Dawes C. The intra-oral distribution of unstimulated and chewing-gum-stimulated parotid saliva. Arch Oral Biol. 1997;42:469–474. doi: 10.1016/s0003-9969(97)00045-9. [DOI] [PubMed] [Google Scholar]
- 10.Osailan S, Pramanik R, Shirodaria S, Challacombe SJ, Proctor GB. Investigating the relationship between hyposalivation and mucosal wetness. Oral Dis. 2010 doi: 10.1111/j.1601-0825.2010.01715.x. (in press) [DOI] [PubMed] [Google Scholar]
- 11.Stewart FA, Van der Kogel AJ. Volume effects in normal tissues. In: Steel GG, editor. Basic clinical radiobiology. 3. London: Arnold; 2002. pp. 42–51. [Google Scholar]
- 12.Coppes RP, Vissink A, Konings AWT. Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother Oncol. 2002;63:321–328. doi: 10.1016/s0167-8140(02)00129-9. [DOI] [PubMed] [Google Scholar]
- 13.Konings AWT, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005;62:1187–1194. doi: 10.1016/j.ijrobp.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer JO, Sakuma Y, Seifert G. Die Strahlen-Sialadenitis. Stadieneinteilung und Immunhistologie. Pathologe. 1989;10:165–170. [PubMed] [Google Scholar]
- 15.Paardenkooper GMRM, Zeilstra LJW, Coppes RP, et al. Radiation induced apoptosis is not the cause of acute impairement of rat salivary gland function. Int J Radiat Biol. 1998;73:641–648. doi: 10.1080/095530098141898. [DOI] [PubMed] [Google Scholar]
- 16.Avila JL, Grundmann O, Burd R, et al. Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J Radiat Oncol Biol Phys. 2009;73:523–529. doi: 10.1016/j.ijrobp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abok K, Brunk U, Jung B, et al. Morphologic and histochemical studies of the deferring radiosensitivity of the ductular and acinar cell of the rat submandibular gland. Virchows Arch Cell Pathol. 1984;45:443–460. doi: 10.1007/BF02889885. [DOI] [PubMed] [Google Scholar]
- 18.Nagler RM, Marmary Y, Fox PC, et al. Irradiation-induced damage to the salivary glands: The role of redox-active iron and copper. Radiat Res. 1997;147:468–475. [PubMed] [Google Scholar]
- 19.Vissink A, ‘s-Gravenmade EJ, Ligeon EE, et al. A functional and chemical study of radiation effects on rat parotid and submandibular/sublingual glands. Radiat Res. 1990;124:259–265. [PubMed] [Google Scholar]
- 20.Vissink A, Kalicharan D, ’s-Gravenmade EJ, et al. Acute irradiation effects on morphology and function of rat submandibular glands. J Oral Pathol Med. 1991;20:449–456. doi: 10.1111/j.1600-0714.1991.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 21.Coppes RP, Zeilstra LJW, Kampinga HH, et al. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br J Cancer. 2001;85:1055–1063. doi: 10.1054/bjoc.2001.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeilstra LJ, Vissink A, Konings AWT, et al. Radiation induced cell loss in rat submandibular gland and its relation to gland function. Int J Radiat Biol. 2000;76:419–429. doi: 10.1080/095530000138763. [DOI] [PubMed] [Google Scholar]
- 23.Liu RP, Fleming TJ, Toth BB, et al. Salivary flow rates in patients with head and neck cancer 0.5 to 25 years after radiotherapy. Oral Surg Oral Med Oral Pathol. 1990;70:724–729. doi: 10.1016/0030-4220(90)90008-g. [DOI] [PubMed] [Google Scholar]
- 24.Valdez JH, Atkinson JC, Ship JA, et al. Major salivary gland function in patients with radiation-induced xerostomia: flow rates and sialochemistry. Int J Radiat Oncol Biol Phys. 1993;25:41–47. doi: 10.1016/0360-3016(93)90143-j. [DOI] [PubMed] [Google Scholar]
- 25.Eisbruch A, Ten Haken RK, Hyungjin MK, et al. Dose, volume and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 26.Murdoch-Kinch CA, Kim HM, Vineberg KA, et al. Dose-effect relationship for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:373–382. doi: 10.1016/j.ijrobp.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roesink JM, Moerland MA, Battermann JJ, et al. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2001;51:938–46. doi: 10.1016/s0360-3016(01)01717-5. [DOI] [PubMed] [Google Scholar]
- 28.Ortholan C, Chamorey E, Benezery K, et al. Modeling of salivary production recovery after radiotherapy using mixed models: determination of optimal dose constraint for IMRT planning and construction of convenient tools to predict salivary function. Int J Radiat Oncol Biol Phys. 2009;73:178–186. doi: 10.1016/j.ijrobp.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 29.Dijkema T, Raaijmakers CPJ, Ten Haken RK, et al. Parotid gland function after radiotherapy: the combined Michigan and Utrecht experience. Int J Radiother Oncol Biol Phys. 2010 Jan 5; doi: 10.1016/j.ijrobp.2009.07.1708. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009;74:1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 31.Steneker M, Lomax A, Schneider U. Intensity modulated photon and proton therapy for the treatment of head and neck tumors. Radiother Oncol. 2006;80:263–267. doi: 10.1016/j.radonc.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Widesott L, Pierelli A, Fiorino C, et al. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72:589–596. doi: 10.1016/j.ijrobp.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 33.Cozzi L, Fogliata A, Lomax A, et al. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol. 2001;61:287–297. doi: 10.1016/s0167-8140(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 34.Lomax AJ, Goitein M, Adams J. Intensity modulation in radiotherapy: photons versus protons in the paranasal sinus. Radiother Oncol. 2003;66:11–18. doi: 10.1016/s0167-8140(02)00308-0. [DOI] [PubMed] [Google Scholar]
- 35.van de Water T, Lomax T, Bijl HP, Schilstra K, Hug E, Langendijk JA. Comparative treatment planning study between Scanned Intensity Modulated Proton Therapy and Photon Therapy in complex oropharyngeal carcinoma. Radiother Oncol. 2008;88(suppl 2):S77–S78. [Google Scholar]
- 36.Wasserman TH, Brizel DM, Henk M, et al. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and-neck cancer: 2 year follow-up of a prospective, randomized, phase III trial. Int J Radiat Oncol Biol Phys. 2005;63:985–990. doi: 10.1016/j.ijrobp.2005.07.966. [DOI] [PubMed] [Google Scholar]
- 37.Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127–1245. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 38.Burlage FR, Roesink JM, Kampinga HH, et al. Protection of salivary function by concomitant pilocarpine during radiotherapy: a double-blind, randomized, placebo-controlled study. Int J Radiat Oncol Biol Phys. 2008;70:14–22. doi: 10.1016/j.ijrobp.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Brennan MT, Elting LS, Spijkervet FKL. Systematic reviews of oral complications from cancer therapies, Oral Care Study Group, MASCC/ISOO: Methodology and quality of the literature. Support Care Cancer. 2010 Mar 20; doi: 10.1007/s00520-010-0856-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Jellema AP, Slotman BJ, Muller MJ, et al. Radiotherapy alone, versus radiotherapy with amifostine 3 times weekly, versus radiotherapy with amifostine 5 times weekly: A prospective randomized study in squamous cell head and neck cancer. Cancer. 2006;107:544–553. doi: 10.1002/cncr.22020. [DOI] [PubMed] [Google Scholar]
- 41.Anné PR, Machtay M, Rosenthal DI, et al. A Phase II trial of subcutaneous amifostine and radiation therapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2007;67:445–452. doi: 10.1016/j.ijrobp.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 42.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 43.Vitolo JM, Cotrim AP, Sowers AL, et al. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res. 2004;10:1807–1812. doi: 10.1158/1078-0432.ccr-03-0194. [DOI] [PubMed] [Google Scholar]
- 44.Cotrim AP, Sowers AL, Lodde BM, et al. Kinetics of tempol for prevention of xerostomia following head and neck irradiation in a mouse model. Clin Cancer Res. 2005;11:7564–7568. doi: 10.1158/1078-0432.CCR-05-0958. [DOI] [PubMed] [Google Scholar]
- 45.Cotrim AP, Hyodo F, Matsumoto K, et al. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin Cancer Res. 2007;13:4928–4933. doi: 10.1158/1078-0432.CCR-07-0662. [DOI] [PubMed] [Google Scholar]
- 46.Limesand KH, Said S, Anderson SM. Suppression of radiation-induced salivary gland dysfunction by IGF-1. PLoS ONE. 4(3):e4663. doi: 10.1371/journal.pone.0004663. Epub 2009 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lombaert IM, Brunsting JF, Wierenga PK, et al. Keratinocyte Growth Factor Prevents Radiation Damage to Salivary Glands by Expansion of the Stem/Progenitor Pool. Stem Cells. 2008;31:2007–1034. doi: 10.1634/stemcells.2007-1034. [DOI] [PubMed] [Google Scholar]
- 48.Teymoortash A, Müller F, Juricko J, et al. Botulinum toxin prevents radiotherapy-induced salivary gland damage. Oral Oncol. 2009;45:737–739. doi: 10.1016/j.oraloncology.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JT, Ferretti GA, Nethery WJ, et al. Oral pilocarpine for postirradiation xerostomia in patients with head and neck cancer. N Engl J Med. 1993;329:390–395. doi: 10.1056/NEJM199308053290603. [DOI] [PubMed] [Google Scholar]
- 50.LeVeque FG, Montgomery F, Potter D, et al. A multi-centre randomized, double-blind, placebo-controlled, dose-titration study of oral pilocarpine for treatment of radiation-induced xerostomia in head and neck cancer patients. J Clin Oncol. 1993;11:1124–1131. doi: 10.1200/JCO.1993.11.6.1124. [DOI] [PubMed] [Google Scholar]
- 51B.urlage FR, Roesink JM, Faber H, et al. Optimum dose range for the amelioration of long term radiation-induced hyposalivation using prophylactic pilocarpine treatment. Radiother Oncol. 2008;86:347–353. doi: 10.1016/j.radonc.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Burlage FR, Faber H, Kampinga HH, et al. Enhanced proliferation of acinar and progenitor cells by prophylactic pilocarpine treatment underlies the observed amelioration of radiation injury to parotid glands. Radiother Oncol. 2009;90:253–256. doi: 10.1016/j.radonc.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Scarantino C, LeVeque F, Swann RS, et al. Effect of pilocarpine during radiation therapy: Results of RTOG 97–09, a phase III randomised study in head and neck cancer patients. J Support Oncol. 2006;4:252–258. [PubMed] [Google Scholar]
- 54.Niedermeier W, Matthaeus C, Meyer C, et al. Radiation-induced hyposalivation and its treatment with oral pilocarpine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:541–549. doi: 10.1016/s1079-2104(98)90343-2. [DOI] [PubMed] [Google Scholar]
- 55.Epstein JB, Schubert MM. Synergistic effect of sialogogues in management of xerosto-mia after radiation therapy. Oral Surg Oral Med Oral Pathol. 1987;64:179–182. doi: 10.1016/0030-4220(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 56.Petrone D, Condemi JJ, Fife R, et al. A double-blind, randomized, placebo-controlled study of cevimeline in Sjögren’s syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum. 2002;46:748–754. doi: 10.1002/art.510. [DOI] [PubMed] [Google Scholar]
- 57.Blom M, Lundeberg T. Long-term follow-up of patients treated with acupuncture for xerostomia and the influence of additional treatment. Oral Dis. 2000;6:15–24. doi: 10.1111/j.1601-0825.2000.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 58.Johnstone PA, Peng YP, May BC, et al. Acupuncture for pilocarpine-resistant xerostomia following radiotherapy for head and neck malignancies. Int J Radiat Oncol Biol Phys. 2001;50:353–357. doi: 10.1016/s0360-3016(00)01530-3. [DOI] [PubMed] [Google Scholar]
- 59.Wong RKW, Jones GW, Sagar SM, et al. A phase I-IIstudy in the use of acupuncture-like transcutaneous nerve stimulation in the treatment of radiation-induced xerostomia in head-and-neck cancer patients treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:472–480. doi: 10.1016/s0360-3016(03)00572-8. [DOI] [PubMed] [Google Scholar]
- 60.Braga FP, Sugaya NN, Hirota SK, et al. The effect of acupuncture on salivary flow rates in patients with radiation-induced xerostomia. Minerva Stomatol. 2008;57:343–348. [PubMed] [Google Scholar]
- 61.Deng G, Hou BL, Holodny AI, et al. Functional magnetic resonance imaging (fMRI) changes and saliva production associated with acupuncture at L1–2 acupuncture point: a randomized controlled study. BMC Complementary Alternative Med. 2008;8:137. doi: 10.1186/1472-6882-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matzker J, Schreiber J. Synthetischer Speichel zur Therapie der Hyposalien insbesondere bei der radiogenen Sialadenitis. Z Laryngol Rinol Otol. 1972;51:422–428. [PubMed] [Google Scholar]
- 63.‘s-Gravenmade EJ, Roukema DA, Panders AK. The effects of mucin-containing artificial saliva on severe xerostomia. Int J Oral Surg. 1974;3:435–439. doi: 10.1016/s0300-9785(74)80009-8. [DOI] [PubMed] [Google Scholar]
- 64.Van der Reijden WA, Van der Kwaak H, Vissink A, Veerman ECI, Van Nieuw Amerongen A. Treatment of xerostomia with polymer-based saliva substitutes in patients with Sjögren’s syndrome. Arthritis Rheum. 1996;39:57–69. doi: 10.1002/art.1780390108. [DOI] [PubMed] [Google Scholar]
- 65.Vissink A, ‘s-Gravenmade EJ, Panders AK, et al. A clinical comparison between commercially available mucin- and CMC-containing saliva substitutes. Int J Oral Surg. 1983;12:232–238. doi: 10.1016/s0300-9785(83)80048-9. [DOI] [PubMed] [Google Scholar]
- 66.Regelink G, Vissink A, Reintsema H, et al. Efficacy of a synthetic polymer based saliva substitute in reducing oral complaints of patients suffering from irradiation-induced xerostomia. Quintessence Int. 1998;29:383–388. [PubMed] [Google Scholar]
- 67.Momm F, Volegova-Neher NJ, Schulte-Mönting J, et al. Different saliva substitutes for treatment of xerostomia following radiotherapy. A prospective crossover study. Strahlenther Onkol. 2005;181:231–236. doi: 10.1007/s00066-005-1333-7. [DOI] [PubMed] [Google Scholar]
- 68.Baum BJ, Zheng C, Cotrim AP, et al. Aquaporin-1 gene transfer to correct radiation-induced salivary hypofunction. In: Beitz E, editor. Aquaporins. Handbook of Experimental Pharmacology; Berlin: Heidelberg: Springer-Verlag; 2009. pp. 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delporte C, O’Connell BC, He X, et al. Increased fluid secretion after adenovira-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci. 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991;88:11110–1114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He X, Kuijpers GA, Goping G, et al. A polarized salivary cell monolayer useful for studying transepithelial fluid movement in vitro. Pflugers Arch. 1998;435:375–381. doi: 10.1007/s004240050526. [DOI] [PubMed] [Google Scholar]
- 72.Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C, Goldsmith CM, Wellner RB, Baum BJ, Wang S. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11:444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Lombaert IM, Brunsting JF, Wierenga PK, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE. 2008 Apr 30;3(4):e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]