Abstract
Glutaredoxin 2 (Grx2) belongs to the oxidoreductase family and is an isozyme of glutaredoxin 1 (Grx1) present in the mitochondria, however its function is not well understood. The purpose of this study is to evaluate the potential anti-apoptotic function of Grx2 by examining its ability to protect complex I in the mitochondrial electron transport system using human lens epithelial cells as a model. We found that cells treated with 200 μM hydrogen peroxide (H2O2) for 24 h exhibited decreased viability and became apoptotic with corresponding Bax up-regulation, Bcl-2 down-regulation, caspase 3 activation and mitochondrial cytochrome c leakage. Grx2 over-expression (OE) could protect cells against H2O2-induced damage while Grx2 knockdown (KD) showed the opposite effect. Under the same conditions, H2O2 treatment caused 50% inactivation of complex I activity in control cells (vector only), 75% in Grx2 KD cells but only 20% in Grx2 OE cells. This antiapoptotic function of Grx2 is specific as rotenone, a complex I specific inhibitor, could block this Grx2-mediated protection of complex I activity. Immunoprecipitation study also revealed that Grx2 co-precipitated with complex I in the mitochondrial lysate. Thus, the mechanism of Grx2 protection against H2O2-induced apoptosis is likely associated with its ability to preserve complex I.
Keywords: Glutaredoxin2, Mitochondria, Apoptosis, Hydrogen peroxide, Reactive oxygen species (ROS), Free radicals, Complex I
Introduction
Reactive oxygen species (ROS) are generated and degraded by all aerobic organisms. A physiological low level of ROS is required for normal cell function while excessive ROS cause damage to cellular proteins, membrane lipids, nucleic acids, and can lead to cell death. Oxidative stress is resulted in an imbalance between the production of ROS and the ability of biological system to readily detoxify ROS or repair the resulting damage [1].
In order to maintain intracellular redox homeostasis, cells have defense systems against oxidation. Besides using high glutathione (GSH) content for protecting protein thiols, the cells also contain ROS detoxification enzymes, including catalase, superoxide dismutase (SOD) and glutathione peroxidase [2]. In addition, the cells have oxidoreductases to dethiolate the oxidized thiols in proteins/enzymes to preserve their respective functions. One of these systems is the thioredoxin (Trx) system, which is effective in reducing protein-protein disulfide bonds. The other is the glutaredoxin (Grx) system that is critical in cleaving protein-thiol mixed disulfide bonds (glutathionylation) formed in the oxidized proteins [3].
Glutaredoxin 2 (Grx2) is a recently identified mitochondrial isozyme of Grx, which has a mass of 18 kDa protein and a vicinyl dithiol motif of Cys-Ser-Tyr-Cys at the active site, similar to other oxidoreductases [4, 5]. It has been reported that Grx2 protects cells against a variety of oxidative insults [6-8]. Conversely, knockdown of Grx2 sensitizes HeLa cells to anticancer drugs [9]. These studies suggest that Grx2 may play an important role in cytoprotection. Our previous studies have shown that Grx2 has both GSH-dependent and thioredoxin reductase-dependent peroxidase activities in addition to the dethiolase activity [10]. Recently there were studies indicating that Grx2 also has antiapoptotic function. However, the mechanism how Grx2 protects apoptosis is largely unknown.
Mitochondria is an organelle in eukaryotes responsible for aerobic respiration, but it is also the most common source of ROS produced during un-efficient respiration or apoptosis [11]. Within the electron transport chain (ETC) in mitochondria, complex I is the first and the largest component of the respiratory chain. It is the entry point for electrons from tricarboxylic acid (TCA) cycle to enter into the respiratory chain by oxidation of NADH and transporting electrons to coenzyme-Q10. In normal resting cells, 1-2% of electrons carried by the mitochondrial ETC may leak from this pathway and forms superoxide free radical anions. Therefore, complex I is considered as one of the major sites of ROS production in the mitochondrion [12]. It has been reported that high concentration of antioxidant enzymes like Mn-superoxide dismutase are present in the mitochondrial matrix to ensure that this basal level of superoxide anion production is neutralized before it can cause any damage to the cell [13].
The eye lens is a unique tissue that is extremely sensitive to oxidative stress and depends on a balanced redox state for maintaining its transparency [14, 15]. Exposure of lens epithelial cells, as well as whole lens, to high level of H2O2 is known to cause protein oxidation and aggregation, lipid peroxidation, decreased ion transport, and perturbs ubiquitin-mediated proteolysis [16], each of which is thought to contribute to cataract formation. H2O2 has also been shown to induce apoptotic cell death in whole lens [17] and to upregulate expression of the proto-oncogenes c-jun, c-fos, and c-myc, and to increase AP-1 transactivation in cultured lens epithelial cells [18]. In this study we examine how H2O2 induces apoptosis to human lens epithelial cells (HLE-B3). By using cells transfected with Grx2 siRNA or Grx2 gene over-expression, we test whether Grx2 plays any role in protecting the cells from apoptosis. We examined various apoptotic factors and the activity of complex I under these conditions. Based on our results we hypothesize that one of the major functions of Grx2 is to protect cells from oxidative stress-induced apoptosis and this function is accomplished by the preservation of the activity of complex I of ETC in the mitochondria.
Material and Methods
Cell culture and treatment
Human lens epithelial cell line (HLE-B3), immortalized by infecting with adenovirus 12-SV40, was generously provided by Dr. Usha Andley (Washington University, St. Louis, MO, USA). The cells were grown in MEM medium supplemented with 20% FBS, 50 μg/ml gentamicin and 50μg/ml penicillin (all from Gibco, Carlsbad, CA, USA) in humid atmosphere with 5% CO2 at 37°C.
For the H2O2-induced apoptotic studies, HLE-B3 cells were deprived serum gradually. They were first cultured overnight in MEM with 2% FBS followed by incubating in serum-free medium for 30 min before exposing to a bolus of 200 μM H2O2 for 24 h.
Over-expression of Grx2 in HLE-B3 cells
Sense cDNA for human Grx2 was introduced into the multi-cloning site of geneticin (G418 sulfate)-resistant mammalian expression vector pcDNA3.1 (+) to construct sense plasmids. The plasmids were then transfected into HLE-B3 cells using jetPEI™-RGD reagent (Polyplus-transfection, San Marcos, CA, USA) following the manufacturer’s protocol. Cells were incubated with transfection medium for 1 day and then passaged into new dishes with fresh culture medium. The cells were grown for two days and then changed to fresh medium containing 1mg/ml geneticin for selection. Thereafter, the cells were fed every four days. After 4 weeks of selection, the cells were passaged into new dishes containing fresh medium with 400 μg/ml geneticin and grown to confluence before use.
SiRNA transfection
The short interfering RNA (siRNA) duplexes used in this study were chemically synthesized by Santa Cruz Biotechnology (Santa Cruz, CA, USA). 5′-GGAGAGCAAUACAUCAUCAtt -3′ and 5′- UGAUGAUGUAUUGCUCUCCtt were used for repressing Grx2 expression. Non-silencing siRNA (5′-UUAAGUAGCUUGGCCUUGATdT-3′and 5′-UCAAGGCCAAGCUACUUAATdT-3′) was used as a negative control. SiRNA duplexes were transfected into HLE-B3 cells with siRNA transfection reagent (Polyplus-transfection, San Marcos, CA, USA) according to the manufacturer’s instructions.
Mitochondria isolation
Mitochondrial fraction was isolated according to the method described by Christian et al. [19]. Briefly, HLE-B3 cells were trypsinized and centrifuged. The resultant cell pellet was suspended in 3 ml ice-cold isolation buffer containing 0.2 M sucrose, 10 mM MOPS, 10 mM EGTA and 10 mM Tris-HCl (pH 7.4) and homogenized using glass homogenizer followed by centrifuge at 600 g for 10 min. The supernatant was saved and centrifuged at 7,000 g for 10 min. The pellets were collected and washed with 200 μl of isolation buffer followed by centrifugation at 7,000 g for another 10 min. The final fraction enriched in mitochondria was resuspended in the isolation buffer and immediately used for measurement of Grx2 and complex I activities.
Grx2 activity assay
Grx2 activity was assayed according to a previously described method [4]. Briefly, the reaction mixture contained 0.2 mM NADPH, 0.5 mM GSH, 0.1 M potassium phosphate buffer (pH 7.4), 0.4 units of GSSG reductase and an aliquot of mitochondrial fraction in a total volume of 1 ml. The reaction was carried out at 30 °C following a 5-min pre-incubation with 2 mM hydroxyethyldisulfide (HEDS). The decrease in absorbance of NADPH at 340 nm was monitored for 3 min using Beckman DU 640 Spectrophotometer (Beckman, Fullerton, CA). To determine the Grx2 activity, the slope of the linear portion of the time course for 340-nm absorption loss in a control (Grx2-free) sample was subtracted from the slope of the samples containing Grx2.
Cytotoxicity assays
Cell viability was measured by a colorimetric cell viability kit (PromoKine, Heidelberg, Germany). The kit uses tetrazolium salts WST-8 that is reduced to water-soluble, orange formazan dye by dehydrogenases present in the metabolically active cells. The absorbance of the formazan dye is proportional to the number of viable cells. HLE-B3 cells were treated with 200μM of H2O2 for 24 h. After treatment, the cells were incubated with 10 μl WST-8 solution at 37°C for another 4 h. The transmission was evaluated at 450 nm using a 96-well microplate reader (Bio-Rad, Richmond, CA).
Complex I activity assay
The enzymatic activity of complex I was assayed by using the sensitive spectrophotometric method developed recently [20]. In brief, 50 μg (20μl) of mitochondrial proteins isolated from HLE-B3 cells were re-suspended in 960 μl of medium containing 20 mM KH2PO4, 3.5 mg/ml BSA, 60 μM, 2,6-dichloroindophenol (DCIP), 70μM decylubiquinone (prepared in dimethyl sulfoxide, DMSO), 1 μM antimycin-A at 30°C. After 3 min, 20μl of 10 mM NADH was added and the absorbance was measured at 30 sec intervals for 4 min at 37°C. After that, 1.0 μl rotenone (1 mM, in DMSO) was immediately added and the absorbance was measured again at 30 seconds intervals for additional 4 min to confirm it is the rotenone-sensitive complex I. The enzyme activity was expressed as nmol DCIP reduced per min/mg protein. The substrate DCIP, which is a water-soluble final electron acceptor, is specific for complex I as DCIP only receives electron from complex I but not other non-mitochondrial NADH dehydrogenases. BSA is a necessary component in the assay mixture as it facilitates as a solubilization agent for rotenone and decylubiquinone.
Immunoprecipitation
HEL-B3 cells were treated with or without H2O2 for 24 h and total mitochondrial proteins were isolated as described above. A specific antibody against the 75 KDa unit, a Fe-S protein in NADH dehydrogenase, was used to co-precipitated Grx2 protein. The 75 kDa antibody (5 μg, Santa Cruz, Santa Cruz, CA, USA) was incubated with 50 μl of protein A/G agarose beads (Exalpha Biologicals, Shirley, MA, USA) for 60 min at room temperature. The 75 kDa antibody was replaced by IgG (Santa Cruz, Santa Cruz, CA, USA) as the control. The protein A/G agarose–anti-75 kDa complex was washed three times with PBS containing 0.1% Triton X-100 followed by incubation overnight at 4□ with the mitochondrial lysate (1 mg protein). The immunoprecipitated (IP) complex was collected and washed three times with PBS containing 0.1% Triton X-100. The immunocomplex was analyzed for the levels of Grx2 or the 75 kDa unit of complex I by western blot analysis using anti-Grx2 or anti-75 kDa specific antibody, respectively. Probing the IP complex with IgG was done as a negative control.
Western Blot Analysis
Equal amounts of protein was subjected to SDS-PAGE on a 12% polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare, Boulder, CO, USA). The membrane with blotted protein was blocked for 1 h with blocking buffer containing 5% nonfat dry milk and 0.05% Tween 20 in Tris-buffered saline (TBS-T), followed by incubation with indicated antibodies diluted 1:1000 in blocking buffer overnight at 4°C. Then, the membrane was washed three times with TBS-T for 30 mins and incubated at room temperature for 1 h with diluted (1:2000) secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Santa Cruz, Santa Cruz, CA, USA). Detection was done using the ECL Western Blotting Detection System (Thermo Scientific, Rockford, IL, USA). The immunoblot was analyzed with an imaging system (Versadoc 5000 MP Imaging System, Bio-Rad, Richmond, CA). Voltage dependent anion channel protein (VDAC) was used as marker and loading control for mitochondrial fraction (Cell Signaling, Danvers, MA, USA).β-actin was used as loading control for cytosolic fraction (Santa Cruz, Santa Cruz, CA, USA). GAPDH was used as loading control for the whole cell lysates (Santa Cruz, Santa Cruz, CA, USA).
Statistics
Each experiment was performed at least three times and statistical analyses were performed using one-way ANOVA followed by Tukey’s test as a post hoc test with the SPSS software. The number of experimental samples used in each group was presented in the figure legends. All data were expressed as means ± S.D. and the differences were considered significant at P < 0.05.
Results
Effect of Grx2 overexpression on H2O2-induced HEL-B3 cell apoptosis
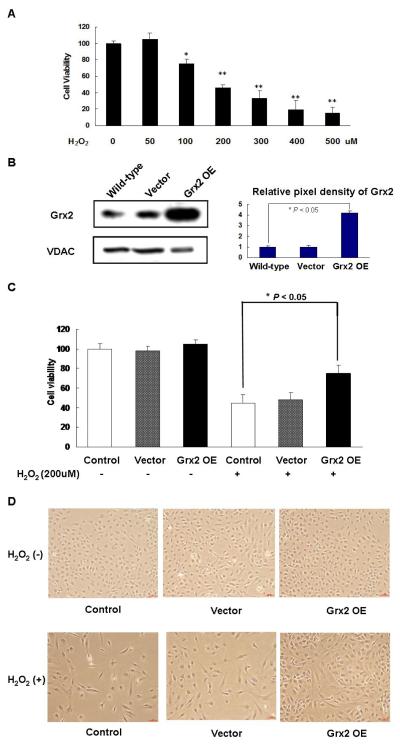
To examine the effect of H2O2 on cell viability, HLE-B3 cells were exposed to H2O2 (50–500 μM) for 24 h and the cells were evaluated using the WST-8 assay. H2O2 at low dose of 50 μM showed no effect but doses between 100-500 μM caused extensive loss in cell viability and the toxicity increased in a dose-and time-dependent manner (Fig. 1A). In the presence of 200 μM of H2O2, there were only 46 ± 3.3% (mean ± S.E.M., n=3) viable cells as compared to that of the control untreated cells. Therefore, the treatment of 200 μM H2O2 for 24 h was used to induce HLE-B3 cell injury and the potential protection by Grx2 over-expression was examined in the subsequent experiments.
Fig. 1. Over-expression of Grx2 and its protective effect on H2O2-induced cytotoxicity in HLE-B3 cells.
(A) The dose-dependent effect of H2O2 on the viability of HLE-B3 cells. Cells were exposed to 0, 50, 100, 200, 300, 400, 500 μM H2O2, respectively for 24 h. The viable cells were quantified by WST-8 assay. *p < 0.05, **P < 0.01, comparing with 0 μM H2O2 group. (B) Western blot analysis of Grx2 expression in the mitochondrial lysates of control (wild-type), vector-transfected (vector) and Grx2 over-expressed (Grx2 OE) HLE-B3 cells. VDAC level was also analyzed for comparison (left panel). The right panel depicts the relative pixel density of Grx2 over VDAC. Data presented are a typical representation of triplicate experiments. (C) Effects of Grx2 overexpression on H2O2-induced cytotoxicity in HLE-B3 cells. Normal HLE-B3 cells (control), vector-transfected cells (vector) and Grx2 over-expressed cells (Grx2 OE) were exposed to medium with and without H2O2 (200 μM) for 24 h. After treatment, cell viability was determined by WST-8 assay. The data were expressed as means ± S.D. of three experiments, n=6. *P < 0.05 (comparison between the control and the Grx2 OE groups). (D) Comparison of the cell density and morphology in (C).
Next, we tested if H2O2-induced reduction in cell viability could be rescued with enriched Grx2 in cells. Grx2 over-expression was carried out and the results are shown in Figure 1B, in which the western blot analysis indicated the mitochondrial Grx2 was over-expressed in HLE-B3 cells to nearly 5-fold over the non-transfected control wild type HLE-B3 cells. The cells transfected with vector only showed the same level of Grx2 as the control. The mitochondrial-specific protein called the voltage dependent anion channel (VDAC) was probed and confirmed that equal amount of proteins was applied to each lane on the gel (Fig 1B).
As summarized in Figure 1C, when comparing cell viability with and without the presence of H2O2 (200 μM), the cells exposed to H2O2 showed extensive (40-50%) mortality within 24 h in cells without transfection (control) or cells transfected with vector only (vector). However, the toxic effect of H2O2 in the Grx2-transfected cells was significantly reduced by 20% in comparison with that of the wild-type or vector-transfected cells (P < 0.05). The protective effect of Grx2 over-expression could also be confirmed by the morphological observation (Fig. 1D), in which the cell population was sparse and the cells were elongated in shape in the H2O2-treated control or H2O2-treated vector group while the H2O2-treated Grx2 over-expressed cells remained relatively dense with a healthy rounded shape (lower panel), similar to that of the untreated Grx2 over-expressed, vector or control group (upper panel).
Effect of Grx2 knockdown on H2O2-induced HEL-B3 cell injury
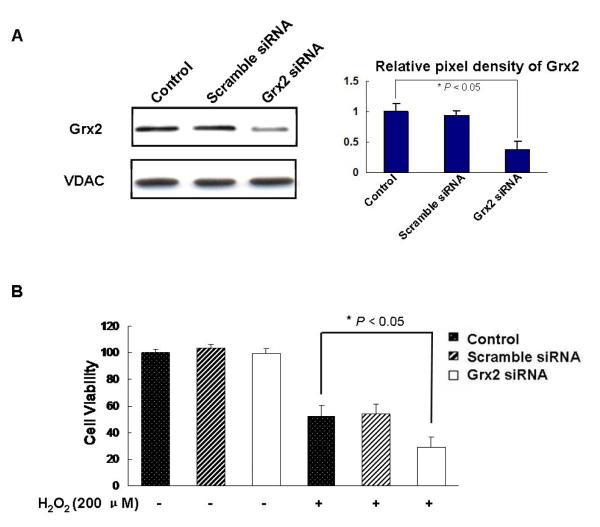
By using the siRNA technique, we were able to knockdown nearly 60% of the Grx2 protein while the scramble siRNA (negative control) showed no change in Grx2 expression in comparison to the control (untrasfected) HLE-B3 cells (Fig. 2A). The same intensity of VDAC indicated that equal amount of proteins were used for the Western blot analysis (Fig. 2A, lower panel).
Fig. 2. Grx2 siRNA enhanced H2O2-induced cellular damage.
HLE-B3 cells were transfected with Grx2 siRNA, scramble siRNA or without Grx2 siRNA (control) for 48 h. Then the cells were incubated with or without 200 μM H2O2 for another 24 h. (A) Western blot analysis of Grx2 and VDAC in control, scramble siRNA and Grx2 siRNA groups (left panel). The right panel depicts the relative pixel density of Grx2 over VDAC. Data presented are a typical representation of triplicate experiments. (B) Cell viability was determined by WST-8 assay in control, scramble siRNA and Grx2 siRNA groups after treatment with and without 200 μM H2O2. The data were expressed as means ± S.D., n=6 from three separate experiments, *P < 0.05 (comparison between H2O2-treated control and Grx2 siRNA).
To examine if suppressed cellular Grx2 expression would compromise the cell viability and sensitize cells to oxidative stress, we compared the following three lines of cells: control, scramble siRNA and Grx2 siRNA and subjected them to H2O2 stress (200 μM for 24 h) in comparison with the unstressed conditions. As shown in Figure 2B, knocking down the gene expression of Grx2 does not affect cell viability but it enhances the sensitivity of cells to oxidative stress. Both the control and the scramble siRNA cells showed 50% suppression of cell viability after treatment with H2O2 while cells with Grx2 KD showed 70% reduction in cell viability (P < 0.05).
Prevention of H2O2 –induced apoptosis by Grx 2 overexpression in HLE-B3 cells
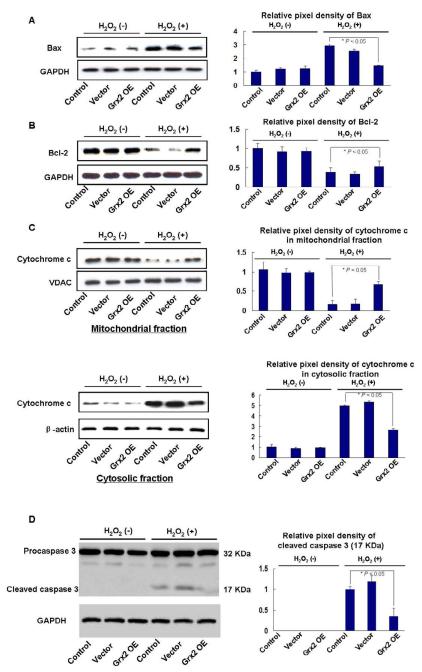
Because cell viability loss is closely related to cell apoptosis, we examined if treating the cells with H2O2 would activate the apoptotic signaling pathways, including suppression of Bcl-2, activation of Bax and caspase 3 and initiating the release of cytochrome c from mitochondria. Again, control, vector and Grx2 over-expressed cell lines were compared with and without treatment with 200 μl of H2O2 for 24 h. As shown in Figure 3A, Bax protein levels are elevated extensively after oxidative stress in both control and vector groups but much less in cells with enriched Grx2. In contrast, the protein levels of anti-apoptotic factor Bcl-2 were suppressed 70-80% after H2O2 treatment. However, cells with Grx2 over-expression sustained Bcl-2 to nearly the original level even after oxidative stress (Fig. 3B).
Fig. 3. Grx2 over-expression protected HLE-B3 cells against H2O2-induced apoptosis.
Non-transfected (control), vector-transfected (vector) and Grx2 over-expressed (Grx2 OE) HLE-B3 cells were treated with or without 200 μM H2O2 for 24 h. Whole cell or mitochondrial lysates (40μg proteins) from control, vector, and Grx2 OE groups were separated by 12% SDS–PAGE and hybridized with indicated specific antibodies. (A) Effects of Grx2 over-expression on the Bax protein expression in HLE-B3 cells (left panel). GAPDH was analyzed to ensure equal amount of proteins was applied on the gel. Right panel depicts the relative pixel density of Bax over GAPDH. (B) Effects of Grx2 over-expression on the Bcl-2 protein expression in HLE-B3 cells. The right panel depicts the relative pixel density of Bcl-2 over GAPDH. (C) Effects of Grx2 over-expression on the cytosolic and mitochondrial cytochrome c protein levels in H2O2-treated HLE-B3 cells. VDAC and β-actin were also analyzed in the mitochondrial or cytosolic fractions (left panels). The relative pixel density of cytochrome c over VDAC for the mitochondrial fraction and cytochrome c over beta-actin in the cytosolic fraction are shown in the respective right panel. (D) Effects of Grx2 over-expression on caspase 3 cleavage in HLE-B3 cells. Samples from both H2O2 treated and H2O2 untreated groups were immunoblotted against antibodies specific for procaspase 3 and caspase 3, respectively. GAPDH was also probed as a control. The relative pixel density of the cleaved caspase 3 at 17 kDa over GAPDH is shown in the right panel. The above data presented are a typical representation of triplicate experiments.
As cytochrome c is an essential component in the electron transport chain resides inside the mitochondria, its leakage from mitochondria to cytosol is considered as a hallmark of cell apoptosis [11]. Therefore, we examined the protein levels of cytochrome c in cells with and without oxidative stress and compared its presence in isolated mitochondrial and cytosolic fractions. By using the cells in groups of control, vector-expressed and Grx2 over-expressed, we found that after H2O2 treatment, cytochrome c level in the mitochondrial fraction was extensively compromised in both the control and the vector groups, but there was little change in the Grx2 over-expressed group. In contrast, the cytosolic fraction showed corresponding increase in cytochrome c both in the control and the vector groups while minimal change was seen in the Grx 2 OE group (Fig. 3C). These data indicate that Grx2 can prevent the release of cytochrome c from mitochondria.
Caspase 3 is another marker of cell apoptosis. Caspase 3 is normally present as the inactive form procaspase 3 in the cells until stress-induced apoptotic signaling converts procaspase 3 to the active form [21]. Therefore, measuring caspase 3 cleavage or activity is another confirmation for apoptosis. As shown in Figure 3D, caspase 3 protein is not seen in the untreated control, vector and Grx2 over-expressed cells, however, the oxidative stress condition induces caspase 3 cleavage as seen by the appearance of caspase 3 positive band at 17 kDa in the control and vector groups but not in the Grx2 OE group. These results indicate that 200 μM H2O2 induced cell apoptosis and Grx2 enrichment protected the cells from H2O2 induced damage.
In the above experiments, GAPDH or β-actin was used for the cytosolic samples and VDAC was used for the mitochondrial samples in Western blotting to ensure that equal amount of proteins were loaded in the gels.
Grx2 over-expression modulates mitochondrial ETC complex activities
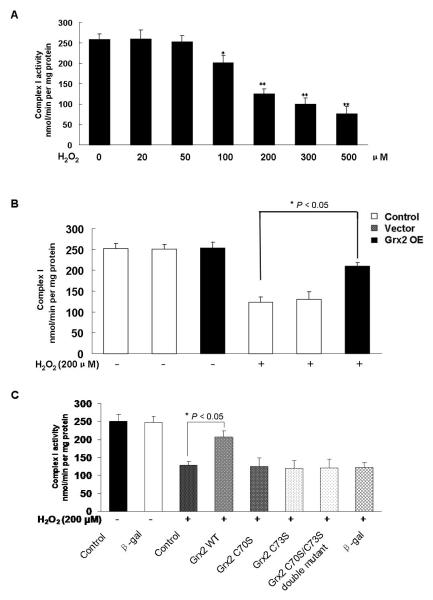
Complex I is the rate limiting enzyme in the chain of electron transport system of the mitochondria for ATP production and molecular oxygen reduction [22]. This enzyme is highly sensitive to oxidative stress [23]. We next examined if complex I catalytic integrity has any association with H2O2-induced apoptosis, and if there is a potential protective effect of Grx2 on complex I by conducting the following experiments. HLE-B3 cells were treated with various concentrations of H2O2 for 24 h and complex I activity was measured in the mitochondrial fractions. We observed that complex I was not inhibited by H2O2 at low levels between 20-50 μM, but the activity was progressively suppressed by H2O2 between 100-500 μM, with nearly 50% loss at 200 μM and 70% at 500 μM (Fig. 4A). Therefore, we chose 200 μM H2O2 to examine if Grx2 has any protective function for complex I. As shown in Figure 4B, complex I shows similar activity in control (non-transfected), vector and Grx2 over-expressed cells, but after oxidative stress (200 μM H2O2 for 24 h), complex I lost over 60% activity in the control and vector groups while complex I is inactivated only 20% in the Grx2 enriched cells. This finding suggests that Grx2 has protective function for complex I.
Fig. 4. Grx2 over-expression protected mitochondria from H2O2-induced loss in complex I activity.
(A) Inhibitory effects of H2O2 on complex I activity in HLE-B3 cells. Cells were incubated in medium containing 0, 20, 50, 100, 200, 500 μM H2O2, respectively for 24 h and the mitochondrial lysate of each time point was used to measure the activity of complex I as described under “Materials and Methods”. *P < 0.05; **P < 0.01, each compared with the 0 μM H2O2 group. (B) Effect of Grx2 over-expression on H2O2-induced complex I activity loss in HLE-B3 cells. Control, vector-transfected (vector) and Grx2 over-expressed (Grx2 OE) HLE-B3 cells were treated with and without H2O2 (200 μM) for 24 h. After treatment, the mitochondrial lysate of each group was analyzed for complex I activity. The data are expressed as mean ± S.D. with n = 6; *P < 0.05 (comparison of control with Grx2 OE in the H2O2 –treated groups). (C) Inhibitory effects of rotenone on HLE-B3 complex I activity. HLE-B3 cells were treated with 0, 100, 200, 300, 400, 500, 1,000, 5,000 nM rotenone, respectively for 24 h. Mitochondrial fractions were isolated and complex I activity was measured as described under “Material and Methods”. *P < 0.05; ** P < 0.01, both compared with rotenone-free group. (D) Effects of rotenone on caspase-3 cleavage in HLE-B3 cells. Cells were incubated with rotenone for 24 h, and the cell lysates (40μg proteins) were subjected to Western blot analysis with antibodies against caspase 3, and GAPDH, respectively (left panel). The right panel depicts the relative pixel density of the cleaved caspase 3 (17 kDa) over GAPDH. Data presented are a typical representation of triplicate experiments. (E) Rotenone partially blocks Grx2-mediated protective effects in HLE-B3 cells. Cell viability was quantified by WST-8 analysis. Control, vector and Grx2 OE HLE-B3 cells were pretreated with or without 500 nM rotenone for 60 min followed by exposing to 200μM H2O2 for 24 h. Mitochondria fractions were assayed for complex I activity as described under “Material and Methods”. Values were expressed as means ± S.D. n=3 from three separate experiments, *P < 0.05 (comparison of Grx2 OE in the H2O2 treated group with the rotenone + H2O2 treated group).
Next we used rotenone, a specific inhibitor to the electron transfer function of complex I, to examine if the protective function of Grx2 could be blocked or neutralized by inhibiting complex I. To ensure that a proper concentration of rotenone was chosen, we conducted a dose-dependent study by treating HLE-B3 cells with 100-5,000 nM of rotenone for 24 h. Figure 4C clearly indicates that rotenone is a very potent inhibitor for complex I, as complex I activity is inhibited in a concentration-dependent manner, with a loss of 20% at 100 nM, 50% at 200 nM, 80% at 300 nM and then >90% at 400-500nM. Complex I activity was un-measurable in cells treated with rotenone above 500 nM. Additionally, we examined the profile of activated caspase 3 in the above rotenone-treated cells and found that rotenone at concentrations of 300-500 nM did not show any effect but concentrations at 1,000 and 5,000 nM induced caspase-3 activation (Fig. 4D).
If the anti-apoptotic function of Grx2 is mediated by its ability to preserve complex I activity, then, it is likely that cells pretreated with rotenone in a concentration that can inactivate complex I but does not induce apoptosis, should eradicate the potential anti-apoptotic function of Grx2. To address this question, we conducted the following experiment in which cell viability was compared in cells pretreated 60 min with or without 500 nM rotenone (> 90% complex I inactivation without apoptosis) before exposing to H2O2 (200 μM, 24 h). It was observed that the control, vector and Grx2 over-expressed cells all showed the same level of viability in the no treatment and the rotenone pre-treatment conditions (Fig. 4E). Cells treated with H2O2 showed lower cell viability to nearly 30% of the control and the vector cells in comparison with the no treatment conditions. However, under the same oxidative stress conditions, the Grx2 over-expressed cells were able to protect the cells and sustained about 50% of their original viability. This protective function of Grx2-enriched cells was lost when the cells were pretreated with rotenone. Rotenone pre-treatment specifically attenuated the viability of Grx2 over-expressed cells, as it did not affect the control and vector cells (Fig. 4E). These data strongly suggest that H2O2-induced cell viability loss or apoptosis can be rescued by Grx2 through its preservation of complex I activity but the site of protection is different from the inhibitory site of rotenone.
Effect of Grx2 knockdown on mitochondrial ETC complex activities
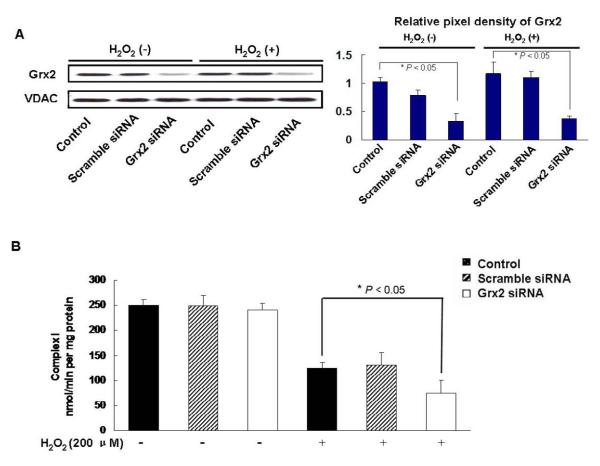
To examine if cells with Grx2 knockdown (Grx siRNA) could have an influence on complex I activity, we carried out the following series of experiments. We first evaluated the effect of H2O2 (200 μM, 24 h) treatment on Grx2 expression in control, scramble siRNA and Grx2 siRNA cells. As shown in the results of Western blot analysis (Fig. 5A), the oxidative stress conditions (200 μM H2O2, 24 h) did not change Grx2 expression in all three cell lines. However, the complex I activity was inhibited extensively in the control and the scramble siRNA cells (lost 50%), but more so in the Grx2 siRNA cells, in which only 25% of the activity was remained (Fig. 5B).
Fig. 5. Grx2 siRNA sensitized H2O2-induced complex I activity loss in HLE-B3 cells.
HLE-B3 cells were transfected with Grx2 siRNA, scramble siRNA or without Grx2 siRNA (control) for 48 h, and then incubated with or without 200 μM H2O2 for another 24 h. (A) Western blot analysis of Grx2 protein levels. Mitochondrial lysates of each group was subjected to Western blot analysis with indicated antibodies. The relative pixel density of Grx2 over VDAC is depicted in the right panel. (B) Grx2 siRNA sensitized H2O2-induced complex I activity loss in HLE-B3 cells. HLE-B3 cells were transfected with Grx2 siRNA, scramble siRNA or without Grx2 siRNA for 48 h, and then incubated with or without 200 μM H2O2 for another 24 h. Mitochondria fractions were assayed for complex I activity. Values are expressed as means±S.D., n=3 from three separate experiments. *P < 0.05, comparison of the H2O2-treated Grx2 siRNA and control groups.
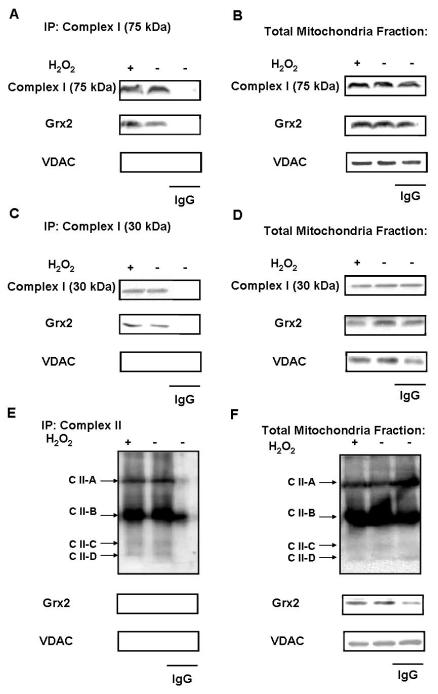
Interaction of Grx2 with Complex I
The 75 kDa unit in NADH dehydrogenase (ubiquinone), also called NDUFS1, is a Fe-S protein and the largest subunit of complex I. When mitochondria is incubated with H2O2, complex I is known to be glutathionylated at the 75kDa subunit, which suggests that this 75 kDa subunit may be a primary site of oxidative damage in complex I [23]. Since Grx2 has a function in dethiolation, it is likely that 75 kDa of complex I could be a target of Grx2 for de-glutathionylation. To test if Grx2 can interact with complex I, mitochondria fractions from cells treated with or without H2O2 were immunoprecipitated with anti-complex I 75 kDa antibody and then immunoblotted for Grx2. As shown in Figure 6A, the immunocomplex showed the presence of both complex I (75 kDa) and Grx2 in the mitochondrial fractions isolated from H2O2 treated or untreated human lens epithelial cells. Oxidative stress with H2O2 appeared to elevate more Grx2 binding to the complex (first lane). For negative control some of the mitochondrial fractions from cells without oxidative stress were immunoprecipitated with IgG only to ensure that no specific binding by IgG was present. As expected, no 75 kDa and Grx could be found in these immunoprecipitants under such conditions.
Fig. 6. Interaction between Grx2 and Complex I.
HLE-B3 cells were treated with or without 200 μM H2O2 for 24 h. (A) Mitochondrial lysates from cells treat with or without H2O2 were immunoprecipitated with anti-complex I 75 kDa antibody. The immunocomplex was analyzed for Grx2 and complex I 75 kDa levels by western blot using antibodies sequentially. Goat IgG was used as a control for immunoprecipitation. (B) Part of the mitochondrial lysates (without immunoprecipitation) was also analyzed Grx2 and complex I 75 kDa expression by western blot using antibodies sequentially.
In the same mitochondrial lysates used for the above study, we also removed a portion and analyzed for the general presence of complex I (75 kDa) and Grx2 without conducting complex I (75 kDa)-immunoprecipitation. As shown in Figure 6B, the samples with and without H2O2 treatment as well as in the sample treated with only IgG all showed the presence of 75 kDa and Grx2.
Discussion
In the present study, we established the role of Grx2 in protecting cells against H2O2-induced apoptosis. And the mechanism of this anti-apoptotic function is likely mediated through its ability to protect complex I in the mitochondrial electron transport chain. Our speculation is based on the following observations: (1) Grx2 expression level correlated with HLE-B3 cell viability; (2) Grx2 overexpression could protect cells against H2O2-induced apoptosis by modulating the associated mitochondrial apoptotic signals; (3) Grx2 overexpression also protected complex I activity loss caused by H2O2; (4) Rotenone, a selective blocker of complex I, attenuated the protective effect of Grx2 against H2O2-induced cell injury and (5) Grx2 can directly interact with the key subunit (75 kDa) in complex 1. Therefore our study may have identified a new regulatory mechanism of Grx2 on protecting complex I from oxidative damage.
Oxidative stress-induced cell damage is associated with aging and development of many degenerative disorders, including ocular degeneration such as cataract [16]. It is generally believed that H2O2 is the major reactive oxygen species that produces stress in the ocular and other tissues. In order to counteract the oxidant effects, cells have the ability to restore the state of redox balance by activating the protein/enzyme repair systems. By using cells with Grx2 gene over-expression or knockdown, we also showed that Grx2 appeared to play an important protective role to H2O2-induced damage in cell viability or cell apoptosis, as Grx2 over-expression preserved cell viability, slowed down the apoptotic signals of Bax activation, cytochrome c and caspase 3 release, and lowered apoptotic cell population (Fig. 1 and Fig. 3). In contrast, Grx2 knockdown accelerated H2O2-induced damage (Fig. 2). These results agree with the findings in HeLa cells in which over-expressing Grx2 inhibited cytochrome c release and caspase activation caused by 2-deoxyglucose or doxorubicin [7] while silencing Grx2 dramatically enhanced cell death in the presence of doxorubicin [9]. In our previous studies, we have shown that over-expression of Grx2 prevented H2O2-induced damage in mitochondrial membrane and preserved the mitochondrial transmembrane potential (MTP) [10]. Alteration in MTP is known to be an early event of apoptotic process [24]. Taking together, these data strongly suggest the importance of Grx2 in the general health of mitochondria and cells.
Complex I is a major enzyme in the electron transport chain (ETC), controlling its activity is of vital importance for the function of this chain in providing energy supply through oxidative phosphorylation and reduction of molecular oxygen. However, damage of complex I is also a major source of ROS production within the cells, that can lead to a wide range of pathologies, such as Parkinson’s diseases [25]. Complex I is a large protein complex of 45 subunits, with hydrophilic peripheral arm extending into the matrix for NADH oxidation and electron transport, and a hydrophobic membrane-embedded arm in the inner membrane for proton-transport activity [26]. Additionally, it is a cysteine-rich protein complex and recent studies have shown that the redox status of these thiols may influence complex I activity and the ETC function. The studies of Hurd et al. [23] have shown that glutathionylation of Cys-531 and Cys-704 of the 75 kDa subunit may play a role in decreasing oxidative damage while Chen et al. [27] showed that site specific S-glutathionylation of the FMN-containing 51 kDa NADH dehydrogenase unit inhibited complex I activity. The mitochondrial Grx2 apparently plays a major role in this reversible glutathionylation process, and the studies of Beer et al. [28] have clearly demonstrated in vitro that Grx2 could re-activate or protect complex I activity loss resulted from glutathionylation. Even the cytosolic isoform Grx1 (thioltransferase) was shown to be an essential protective enzyme for brain mitochondrial complex 1 dysfunction in mice when subjected to neurotoxin MPTP to induce Parkinson’s disease-like symptoms [25].
In our current study, we also found that H2O2 stress could inhibit complex I activity in a dose-dependent manner in the HLE-B3 cells. Although we have not determined whether complex I has been glutathionylated, however, the fact that complex I inhibition could be partially eradicated in cells that were enriched with Grx2, and enhanced in cells that were depleted Grx2 through gene siRNA indicate that H2O2 at the concentration we used likely caused glutathionylation of complex I [28]. Johansson et al. [29] have shown that Grx 2, similar to Grx1, possesses dethiolase activity to reduce protein-SS-glutathione in many cell types. We also found that Grx2 was capable of deglutathionylation in HLE-B3 cells [30]. Furthermore, we have found that Grx2 possesses peroxidase activity by accepting electron from either GSH or thioredoxin reductase [10]. Therefore it is likely that the anti-apoptotic property of Grx2 shown in this study may contribute from its ability to preserve complex I activity, thus the function of mitochondria, either directly through deglutathionylation of the oxidized and inactivated complex I, or indirectly by detoxifying H2O2 via its peroxidase activity. Further study is needed in this area.
Our results also showed that the Grx2 protective ability for complex 1 appeared to be compromised when the cells were pretreated with rotenone, a specific inhibitor that is known to bind to the hydrophobic arm and interrupt coenzyme Q binding for completion of the electron transport [31-33]. It is likely that rotenone binding may have altered the conformation of complex I so much so that the specific and critical glutathionylated sites in the subunits of complex I were no longer accessible by Grx2 and whose protective function could not be delivered. Finally we have provided evidence with immunoprecipitation studies that Grx2 can interact with the 75 kDa subunit of complex I, further strengthen the potential protective role of Grx2 for the integrity of complex 1.
In summary, the results of current studies indicate that the protective mechanism of Grx2 in HLE cells is likely through its ability to interact and to prevent complex I activity loss from oxidation-induce glutathionylation, thus preserving the biological function of mitochondria.
Acknowledgements
The authors appreciate Vadim Gladyshev of the University of Nebraska-Lincoln (UNL) for providing human Grx2 plasmid, and Joel Lechner (also UNL) for reading of this manuscript. This work has been supported by National Institute of Health.
Footnotes
Part of the work was presented at the Annual meeting for the Association of Research in Vision and Ophthalmology, May 2-7, 2009, Fort Lauderdale, FL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- [2].Banerjee R, Becker D, Dickman M, Gladyshev VN, Ragsdale S. Redox Biochemistry. Wiley & Sons; Hoboken: 2008. [Google Scholar]
- [3].Holmgren A. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- [4].Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV, Lou MF. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J. Biol. Chem. 2001;276:30374–30380. doi: 10.1074/jbc.M100020200. [DOI] [PubMed] [Google Scholar]
- [5].Lundberg M, Johansson C, Chandra J, Enoksson M, Jacobsson G, Ljung J, Johansson M, Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J. Biol. Chem. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- [6].Daily D, Vlamis-Gardikas A, Offen D, Mittelman L, Melamed E, Holmgren A, Barzilai A. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by dual activation of the ras-phosphoinositide 3-kinase and jun n-terminal kinase pathways. J. Biol. Chem. 2001;276:21618–21626. doi: 10.1074/jbc.M101400200. [DOI] [PubMed] [Google Scholar]
- [7].Enoksson M, Fernandes AP, Prast S, Lillig CH, Holmgren A, Orrenius S. Overexpression of glutaredoxin 2 attenuates apoptosis by preventing cytochrome c release. Biochem. Biophys. Res. Commun. 2005;327:774–779. doi: 10.1016/j.bbrc.2004.12.067. [DOI] [PubMed] [Google Scholar]
- [8].Diotte NM, Xiong Y, Gao J, Chua BH, Ho YS. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim. Biophys. Acta. 2009;1793:427–438. doi: 10.1016/j.bbamcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- [9].Lillig CH, Lonn ME, Enoksson M, Fernandes AP, Holmgren A. Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells toward doxorubicin and phenylarsine oxide. Proc. Natl. Acad. Sci. USA. 2004;101:13227–13232. doi: 10.1073/pnas.0401896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fernando MR, Lechner JM, Lofgren S, Gladyshev VN, Lou MF. Mitochondrial thioltransferase (glutaredoxin 2) has GSH-dependent and thioredoxin reductase-dependent peroxidase activities in vitro and in lens epithelial cells. FASEB J. 2006;20:2645–2647. doi: 10.1096/fj.06-5919fje. [DOI] [PubMed] [Google Scholar]
- [11].Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- [12].Lenaz G, Genova ML. Structural and functional organization of the mitochondrial respiratory chain: a dynamic super-assembly. Int. J. Biochem. Cell Biol. 2009;41:1750–1772. doi: 10.1016/j.biocel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- [13].Kira Y, Sato EF, Inoue M. Association of Cu,Zn-type superoxide dismutase with mitochondria and peroxisomes. Arch. Biochem. Biophys. 2002;399:96–102. doi: 10.1006/abbi.2001.2738. [DOI] [PubMed] [Google Scholar]
- [14].Augusteyn RC. Mechanisms of Cataract Formation in the Human Lens. Academic Press; New York: 1981. [Google Scholar]
- [15].Lou MF. Redox regulation in the lens. Prog. Retina Eye Res. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- [16].Lou MF. Oxidants and antioxidants in Eye Lens Damage. Marcel Dekker; NewYork: 2003. [Google Scholar]
- [17].Li WC, Kuszak JR, Dunn K, Wang RR, Ma W, Wang GM, Spector A, Leib M, Cotliar AM, Weiss M, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J. Cell Biol. 1995;130:169–181. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li DW, Spector A. Hydrogen peroxide-induced expression of the proto-oncogenes, c-jun, c-fos and c-myc in rabbit lens epithelial cells. Mol. Cell Biochem. 1997;173:59–69. doi: 10.1023/a:1006828402225. [DOI] [PubMed] [Google Scholar]
- [19].Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- [20].Janssen AJ, Trijbels FJ, Sengers RC, Smeitink JA, van den Heuvel LP, Wintjes LT, Stoltenborg-Hogenkamp BJ, Rodenburg RJ. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin. Chem. 2007;53:729–734. doi: 10.1373/clinchem.2006.078873. [DOI] [PubMed] [Google Scholar]
- [21].Yuan J, Horvitz HR. A first insight into the molecular mechanisms of apoptosis. Cell. 2004;116:S53–56. doi: 10.1016/s0092-8674(04)00028-5. 51 p following S59. [DOI] [PubMed] [Google Scholar]
- [22].Brandt U. Energy converting NADH: quinone oxidoreductase (complex I) Annu. Rev. Biochem. 2006;75:69–92. doi: 10.1146/annurev.biochem.75.103004.142539. [DOI] [PubMed] [Google Scholar]
- [23].Hurd TR, Requejo R, Filipovska A, Brown S, Prime TA, Robinson AJ, Fearnley IM, Murphy MP. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J. Biol. Chem. 2008;283:24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jacotot E, Costantini P, Laboureau E, Zamzami N, Susin SA, Kroemer G. Mitochondrial membrane permeabilization during the apoptotic process. Ann. N. Y. Acad. Sci. 1999;887:18–30. doi: 10.1111/j.1749-6632.1999.tb07919.x. [DOI] [PubMed] [Google Scholar]
- [25].Kenchappa RS, Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: studies with MPTP. FASEB J. 2003;17:717–719. doi: 10.1096/fj.02-0771fje. [DOI] [PubMed] [Google Scholar]
- [26].Carroll J, Fearnley IM, Skehel JM, Shannon RJ, Hirst J, Walker JE. Bovine complex I is a complex of 45 different subunits. J. Biol. Chem. 2006;281:32724–32727. doi: 10.1074/jbc.M607135200. [DOI] [PubMed] [Google Scholar]
- [27].Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KB, Zweier JL, Chen YR. Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry. 2007;46:5754–5765. doi: 10.1021/bi602580c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant defense. J. Biol. Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- [29].Johansson C, Lillig CH, Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J. Biol. Chem. 2004;279:7537–7543. doi: 10.1074/jbc.M312719200. [DOI] [PubMed] [Google Scholar]
- [30].Fernando RL, Lou MF. Unpublished results.
- [31].Okun JG, Lummen P, Brandt U. Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:ubiquinone oxidoreductase) J. Biol. Chem. 1999;274:2625–2630. doi: 10.1074/jbc.274.5.2625. [DOI] [PubMed] [Google Scholar]
- [32].Fendel U, Tocilescu MA, Kerscher S, Brandt U. Exploring the inhibitor binding pocket of respiratory complex I. Biochim. Biophys. Acta. 2008;1777:660–665. doi: 10.1016/j.bbabio.2008.04.033. [DOI] [PubMed] [Google Scholar]
- [33].Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]