Abstract
We report a new bioorthogonal ligation reaction between p-nitrodiphenylazirine and dimethyl fumarate. This photoinduced azirine-alkene cycloaddition provides a rapid (~ 2 min) and highly selective route to protein conjugation at neutral pH and room temperature in biological medium.
Bioorthogonal chemistry has provided a multiplexed covalent strategy to study biomolecular dynamics and function in their native environment.1 A central challenge in this emerging field is the development of new bioorthogonal reactant pairs that show high, yet selective, reactivity toward each other in biological media.2 To this end, we have recently reported a photoinduced tetrazole-alkene cycloaddition reaction (“photoclick chemistry”) for labeling of proteins in both biological media3 and live cells.4 A key attribute of this reaction is that the reactive nitrile imine dipoles are generated in situ in the native biological environment only upon photoirradiation, thereby providing a spatiotemporal control over the reaction potentially useful in the study of spatial cell biology.5
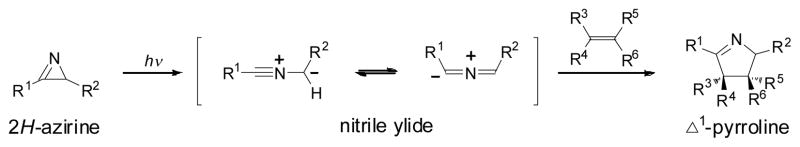
In our efforts to exploit the reactivity of the photogenerated reactive intermediates for bioorthogonal reaction development, we were intrigued by the earlier reports in which highly reactive nitrile ylides are generated photochemically via ring opening of 2H-azirines6 with essentially monophotonic efficiencies.7 The nitrile ylides react spontaneously with a wide variety of alkene dipolarophiles to produce Δ1-pyrrolines (Scheme 1).8 Computational studies suggested that nitrile ylide has a lower activation barrier during the cycloaddition reaction with ethylene compared to nitrile imine and thus is a more reactive dipole.9 By harnessing the latent high reactivity of azirine, herein we report the development of a new bioorthogonal reaction based on the photoinduced azirine-alkene cycloaddition, termed “azirine ligation”, and its application for fast and selective protein conjugation with a polyethylene glycol in biological medium.
Scheme 1.
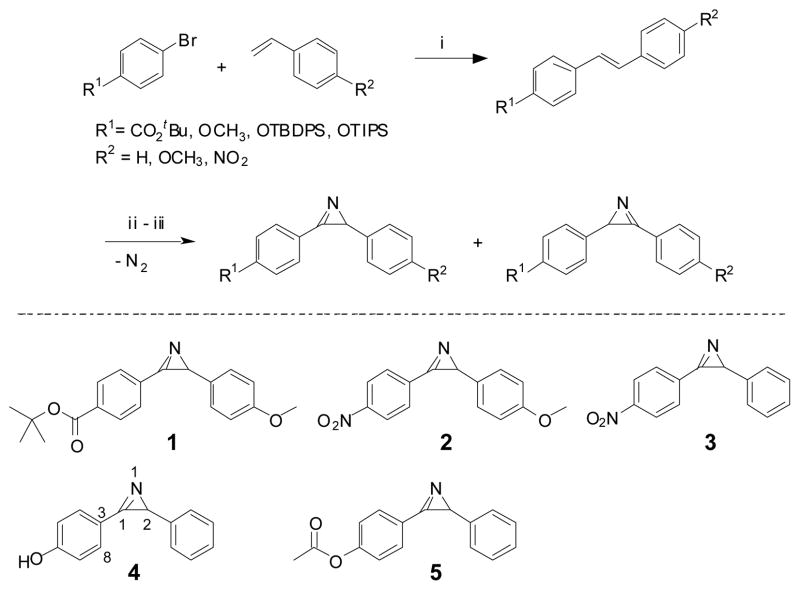
To examine whether the in situ photogenerated nitrile ylides undergo selective reactions with alkenes in aqueous medium, we first prepared a series of 2H-diarylazirines (Scheme 2) carrying either electron-withdrawing or electron-donating groups on the phenyl rings by following the published procedures.10 Briefly, 2H-diarylazirines were readily accessed through a three-step procedure: (i) the Mizoroki-Heck reaction to generate the substituted stilbenes, (ii) the iodoazidation of stilbenes by treatment with sodium iodide and iodine monochloride, and (iii) the elimination of HI with potassium tert-butoxide to yield initially vinyl azide, which upon standing at room temperature releases N2 and cyclizes to form azirines. The kinetic azirines were the major products in the mixture and were isolated and used in the subsequent studies (see Table S1 in the Electronic Supplemental Information for yields). The azirine structures were assigned based on 1H- and HMBC-NMR data (Figure S1). The structure of azirine 4 was also confirmed by X-ray crystallography (Figure S2). As expected, the C=N bond of the azirine ring is perfectly co-planar with respect to the neighboring benzene ring with the dihedral angle of N1-C1-C3-C8 equal to −4.4(12)°, suggesting that azirine π system is in excellent conjugation with the benzene ring. Furthermore, the bond angles of N1-C1-C2 and C1-C2-N1 were found to be 70.5(5)° and 48.0(4)°, respectively, both of which deviate dramatically from typical values for the sp2 (120°) and sp3 (109.5°) hybridized carbons and thus provide the basis for the inherent high reactivity of azirines.11
Scheme 2.
Synthesis of 2H-diarylazirinesa
a Conditions: i) 5 mol% Pd(OAc)2, triethanolamine, 100 °C; ii) NaN3, ICl, acetonitrile, 0 °C to r.t.; iii) KOtBu, Et2O, 0°C to r.t., then r.t., 72 hr.
b TBDPS was removed by treatment with TBAF in THF to give compound 4. Compound 5 was derived from 4 through acetylation. See Electronic Supplemental Information for yields and synthetic details.
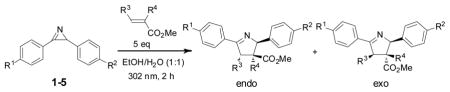
To test the compatibility of the azirine-mediated cycloaddition reaction with protic solvents including water, a solution of azirine 1 and 5 equiv of methyl methacrylate in benzene, ethyl acetate, ethanol, or ethanol/water (1:1) mixture was irradiated with a handheld UV lamp (UVM-57, 302 nm, 115V, 0.16 Amps) at room temperature for 2 h. We found that the cycloaddition reactions were highly regioselective: only two diastereomers were observed in the 1H-NMR spectra with the endo/exo ratios of roughly 1:1. The yields ranged from 100% in benzene and ethanol, to 87% in ethyl acetate, to 56% in ethanol/water (1:1) mixture (Table S2). This partial tolerability to water prompted us to examine the reactivity of other 2H-diarylazirines in ethanol/water mixture, and the results are summarized in Table 1. The nitro group-containing azirines (2, 3) afforded the cycloadducts in greater than 95% yields and essentially 1:1 endo:exo ratios (entries 2–3 in Table 1). The azirines containing the electron-donating groups (4, 5) did not yield any cycloadducts (entries 4–5 in Table 1), indicating that the electron-withdrawing groups are crucial for the stabilization of nitrile ylides in aqueous medium.12 Presumably, by delocalizing the negative charge on carbon into the neighboring phenyl rings, nitro group facilitates the nitrile ylides to adopt the linear geometries (shown in Scheme 1) which have been postulated to accelerate the cycloaddition reaction.13 When dimethyl fumarate was used as dipolarophile, the reactions showed higher conversions and endo selectivity compared to those with methyl methacrylate (entries 6–10 in Table 1), indicating the notion that higher dipolarophile reactivity leads to faster nitrile ylide-mediated cycloaddition14 also operates in aqueous medium.
Table 1.
Photoinduced 1,3-Dipolar Cycloaddition of Azirines with Dipolarophiles in Ethanol/Water (1:1) Mixturea
 | ||||
|---|---|---|---|---|
| entry | azirine | dipolarophile | conversionb | endo:exo b |
| 1 | 1 | 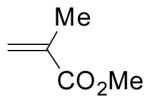 |
56% | 50:50 |
| 2 | 2 | 96% | 52:48 | |
| 3 | 3 | 95% | 55:45 | |
| 4 | 4 | 0%c | - | |
| 5 | 5 | 0%c | - | |
| 6 | 1 | 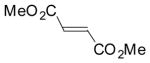 |
84% | 70:30 |
| 7 | 2 | 97% | 70:30 | |
| 8 | 3 | 99% | 70:30 | |
| 9 | 4 | 54% | 65:35 | |
| 10 | 5 | 93% | 70:30 | |
Reactions were conducted by irradiating 5 mg of azirines 1–5 and 5 equiv of either methyl methacrylate or dimethylfumarate in 2 mL ethanol/water (1:1) mixture in quartz test tubes for 2 h.
Based on 1H-NMR of the crude products after removal of solvent and excess reagent.
Ethanol addition products were observed in 1H-NMR.
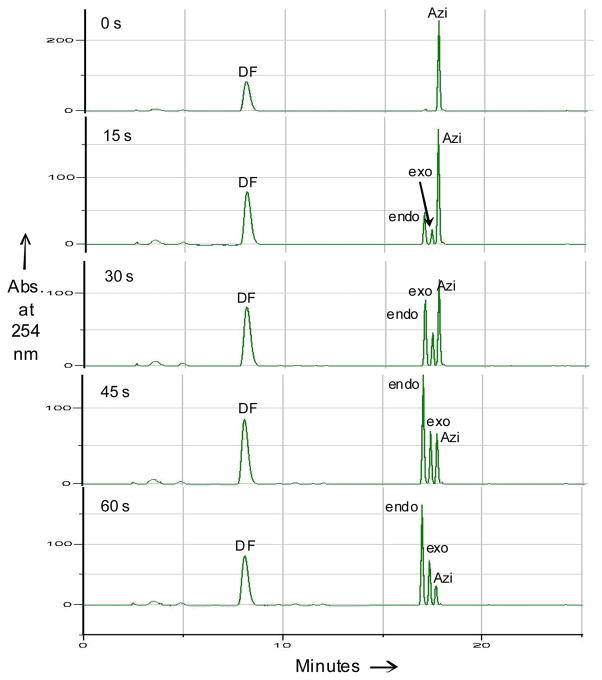
To simulate how the cycloaddition reaction proceeds in a biological medium, we chose the water-tolerant azirine 2 and performed an HPLC-based kinetic analysis of the cycloaddition reaction between 2 and 20 equiv of dimethyl fumarate in an acetonitrile/PBS buffer (1:1) mixture (Figure 1).4b We found that azirine 2 disappeared in about 100 sec, with concurrent appearance of two pyrroline cycloadducts (endo: exo ≈ 7:3). No nitrile ylide intermediate was detected in HPLC, suggesting that the nitrile-ylide mediated cycloaddition is very fast and that the azirine ring-opening reaction is likely the rate-determining step. By fitting the data to an exponential decay equation, the first-order rate constant for the azirine ring-opening was determined to be 0.0379 s1 (Figure S3).
Fig. 1.
HPLC traces of the mixtures of the azirine 2-fumarate cycloaddition reactions in acetonitrile/PBS buffer (1:1) at intervals of 0, 15, 30, 45, and 60 sec. DF = dimethylfumarate; Azi = azirine 2.
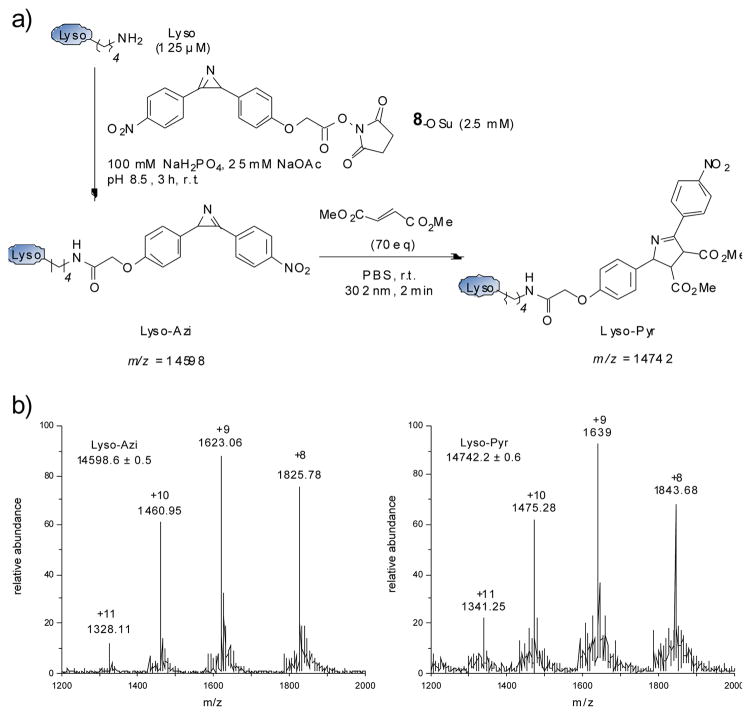
To assess whether the azirine-alkene cycloaddition is suitable for protein ligation, we prepared the azirine-containing lysozyme by acylating lysozyme surface lysines with azirine 8 succinimide ester (Figure 2a). LC-MS analysis of the reaction mixture revealed the following composition: 44% monoacylated (Lyso-Azi; expected mass 14598 Da, found 14598.6 ± 0.5 Da; Figure 2b left panel), 23% diacylated, 7% triacylated and 26% unreacted lysozyme. After removal of the excess small-molecule reagent, 70 equiv of dimethyl fumarate was added and the mixture was irradiated at 302 nm for 2 min before the LC-MS analysis. The mass of the pyrroline cycloadduct (Lyso-Pyr) was identified in the reaction mixture (expected 14742 Da, found 14742.2 ± 0.6 Da; Figure 2b right panel); the conversion from Lyso-Azi to Lyso-Pyr was estimated to be 80% based on the ion counts (Figure S4). It is noteworthy that the Michael addition products derived from lysozyme lysine addition to dimethyl fumarate were not detected in the reaction mixture during the LC-MS analysis.
Fig. 2.
Selective modification of the azirine-containing lysozyme by dimethyl fumarate: a) reaction scheme; b) ESI-MS of the azirine-modified lysozyme (Lyso-Azi, left) and pyrroline cycloadduct (Lyso-Pyr, right) showing the charge ladder and the calculated intact mass.
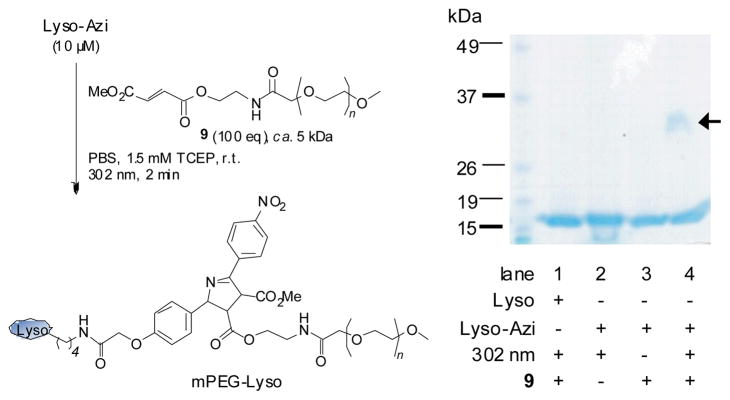
To further establish the yield and selectivity of the azirine ligation, the azirine-containing lysozyme mixture was incubated with 100 equiv of dimethyl fumarate covalently linked to a monodisperse polyethylene glycol (mPEG-fumarate 9, MW ≈ 5 kDa) in PBS buffer, and the mixture was irradiated at 302 nm at room temperature for 2 min. As shown in Figure 3, a distinct higher molecular weight band corresponding to the PEGylated lysozyme was observed only for the azirine-containing lysozyme and only after photoirradiation (compare lane 4 to lanes 1–3).15 Importantly, the Michael addition adducts were not observed (lanes 1, 3), indicating that fumarate serves as selective reaction partner for the nitro-substituted azirine 8. The yield as measured by densitometry was about 41% after the total amount of azirine-modified lysozymesin the mixture (74%) was considered as the starting materials.
Fig. 3.
Selective PEGylation of the azirine-containing lysozyme via azirine ligation: left, reaction scheme; right, SDS-PAGE analysis of the reaction mixtures after photoirradiation at 302 nm for 2 min in the presence or absence of mPEG-fumarate 9. The gel was stained with Coomassie blue. The mPEG-lysozyme adduct was indicated with an arrow.
In summary, we have developed a new bioorthogonal reactant pair, namely, p-nitrodiphenylazirine and dimethyl fumarate. This photoinduced azirine-alkene cycloaddition provided a fast (~2 min) and highly selective route to protein conjugation at neutral pH and room temperature in biological media. Compared to photogenerated nitrile imine which was detectable during the cycloaddition reaction in aqueous buffer,4b the in situ generated nitrile ylide intermediate appears to be more reactive and thus require highly electron-deficient dipolarophiles such as fumarate for efficient reactions in aqueous buffer. At present, 302-nm UV light was used for photoactivation of the azirine-fumarate cycloaddition reaction, which may cause photodamage to living cells. However, it should be possible to tune photoactivation to the long-wavelength region, e.g., 365 nm, by placing auxochromic groups on the phenyl rings,16 thereby affording two-photon 700-nm photoactivatable azirines.17 Nevertheless, this azirine ligation reaction could be particularly valuable in the future for spatially controlled protein functionalization both in vitro and in vivo.
Supplementary Material
Acknowledgments
R. L. thanks Dr. Z. Yu for providing mPEG-fumarate reagent and William Brennessel at University of Rochester for X-ray structural determination. We gratefully acknowledge the National Institutes of Health (GM 085092) for financial support.
Footnotes
Electronic supplemental information (ESI) available: Full experimental details and compound characterization data.
Notes and references
- 1.(a) Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Martin BR, Cravatt BF. Nat Methods. 2009;6:135. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tsukiji S, Miyagawa M, Takaoka Y, Tamura T, Hamachi I. Nat Chem Biol. 2009;5:341. doi: 10.1038/nchembio.157. [DOI] [PubMed] [Google Scholar]
- 2.(a) Sletten EM, Bertozzi CR. Angew Chem Int Ed. 2009;48:6974. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lim RK, Lin Q. Chem Comm. 2010;46:1589. doi: 10.1039/b925931g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song W, Wang Y, Qu J, Madden MM, Lin Q. Angew Chem Int Ed. 2008;47:2832. doi: 10.1002/anie.200705805. [DOI] [PubMed] [Google Scholar]
- 4.(a) Song W, Wang Y, Qu J, Lin Q. J Am Chem Soc. 2008;130:9654. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Song W, Hu W, Lin Q. Angew Chem Int Ed. 2009;48:5330. doi: 10.1002/anie.200901220. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Song W, Yu Z, Madden MM, Lin Q. Mol Biosyst. 2010;6:1576. doi: 10.1039/c003470c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Scott JD, Pawson T. Science. 2009;326:1220. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shapiro L, McAdams HH, Losick R. Science. 2009;326:1225. doi: 10.1126/science.1175685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padwa A, Smolanoff J. J Am Chem Soc. 1971;93:548. [Google Scholar]
- 7.Albrecht E, Mattay J, Steenken S. J Am Chem Soc. 1997;119:11605. [Google Scholar]
- 8.Padwa A. Acc Chem Res. 1976;9:371. [Google Scholar]
- 9.Su M-D, Liao H-Y, Chung W-S, Chu S-Y. J Org Chem. 1999;64:6710. doi: 10.1021/jo990504j. [DOI] [PubMed] [Google Scholar]
- 10.(a) Fowler FW, Hassner A, Levy LA. J Am Chem Soc. 1967;89:2077. [Google Scholar]; (b) Li HJ, Wang L. Eur J Org Chem. 2006:5099. [Google Scholar]; (c) Singh PND, Carter CL, Gudmundsdottir AD. Tetrahedron Lett. 2003;44:6763. [Google Scholar]
- 11.See Tables S3-S6 in the Supporting Information for details of the crystal structure.
- 12.Hegarty AF, Eustace SJ, Tynan NM, Pham-Tran NN, Nguyen MT. J Chem Soc, Perkin Trans. 2001;2:1239. [Google Scholar]
- 13.Fergus S, Eustace SJ, Hegarty AF. J Org Chem. 2004;69:4663. doi: 10.1021/jo049748g. [DOI] [PubMed] [Google Scholar]
- 14.Padwa A, Dharan M, Smolanoff J, Wetmore SI., Jr Pure Appl Chem. 1973;33:269. [Google Scholar]
- 15.The greater than 5 kDa increase in apparent MW is typically observed in electrophoresis of the PEGylated protein adducts; also see ref. 4b.
- 16.Wang, Hu WJ, Song W, Lim RK, Lin Q. Org Lett. 2008;10:3725. doi: 10.1021/ol801350r. [DOI] [PubMed] [Google Scholar]
- 17.Song W, Wang Y, Yu Z, Rivera Vera CI, Qu J, Lin Q. ACS Chem Biol. 2010;5 doi: 10.1021/cb100193h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.