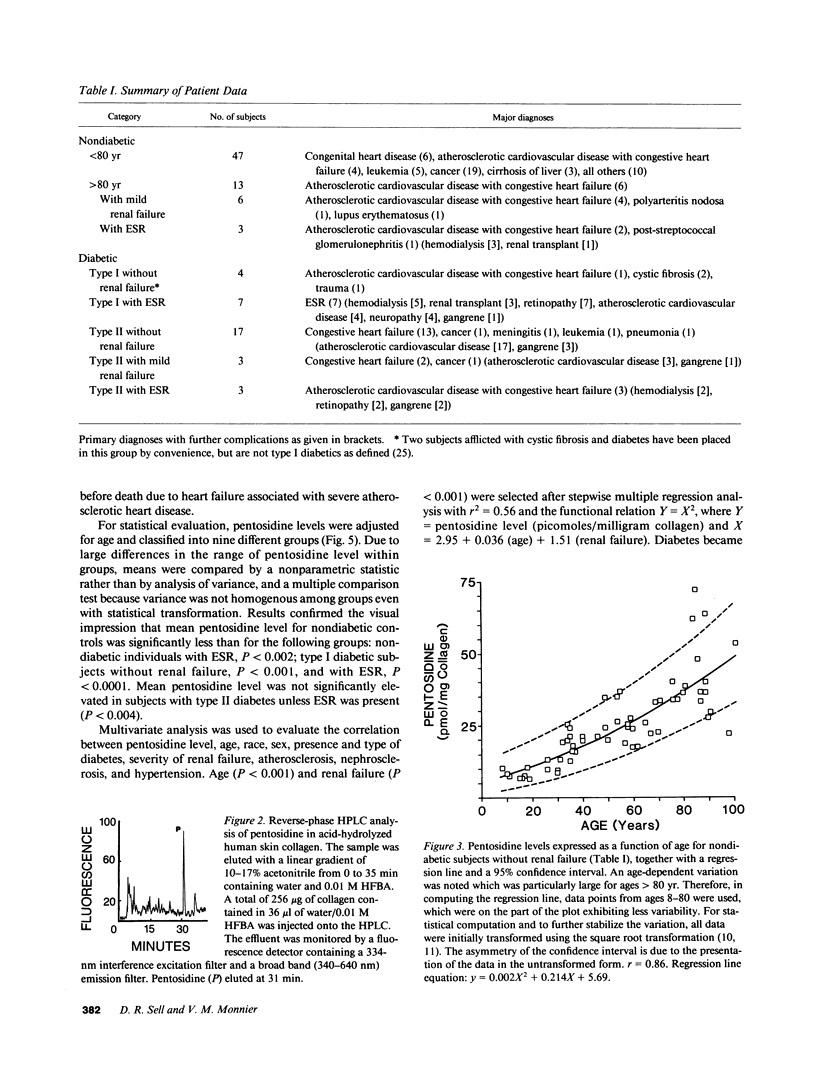
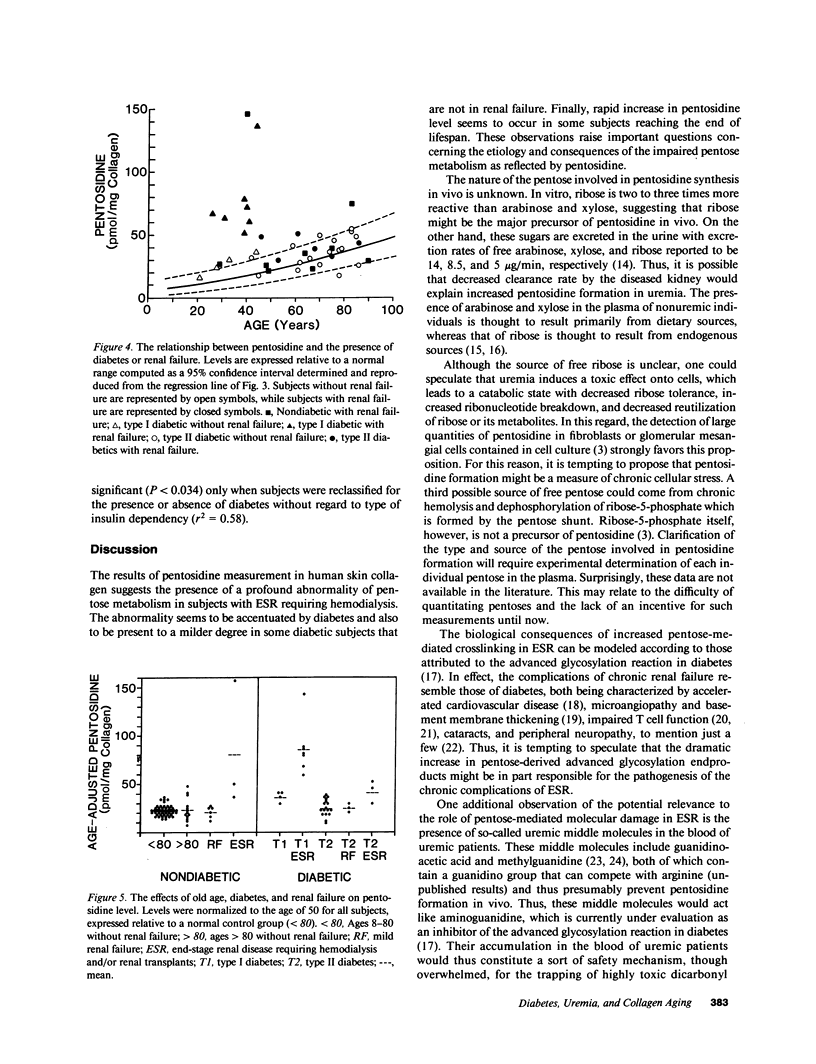
Abstract
Structure elucidation of a specific fluorophore from the aging extracellular matrix revealed the presence of a protein crosslink formed through nonenzymatic glycosylation of lysine and arginine residues. The unexpected finding that a pentose instead of a hexose is involved in the crosslinking process suggested that the crosslink, named pentosidine, might provide insight into abnormalities of pentose metabolism in aging and disease. This hypothesis was investigated by quantitating pentosidine in hydrolysates of 103 human skin specimens obtained randomly at autopsy. Pentosidine level was found to increase exponentially from 5 to 75 pmol/mg collagen over lifespan (r = 0.86, P less than 0.001). A three- to tenfold increase was noted in insulin-dependent diabetic and nondiabetic subjects with severe end-stage renal disease requiring hemodialysis (P less than 0.001). Moderately elevated levels were also noted in some very old subjects, some subjects with non-insulin dependent diabetes, and two subjects with cystic fibrosis and diabetes. The cause of the abnormal pentose metabolism in these conditions is unknown but may relate to hemolysis, impaired pentose excretion, cellular stress, and accelerated breakdown of ribonucleotides. Thus, pentosidine emerges as a useful tool for assessment of previously unrecognized disorders of pentose metabolism in aging and disease. Its presence in red blood cells and plasma proteins suggests that it might be used as a measure of integrated pentosemia in analogy to glycohemoglobin for the assessment of cumulative glycemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell D. J., Talukder M. Q. Rates of urinary excretion of free aldopentoses and fucose by fasting healthy adults: effects of ingested pentose-containing foods. Clin Chim Acta. 1972 Aug;40(1):13–20. doi: 10.1016/0009-8981(72)90245-8. [DOI] [PubMed] [Google Scholar]
- Bergström J., Fürst P. Uremic middle molecules. Clin Nephrol. 1976 Apr;5(4):143–152. [PubMed] [Google Scholar]
- Bergström J., Fürst P., Zimmerman L. Uremic middle molecules exist and are biologically active. Clin Nephrol. 1979 May;11(5):229–238. [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced products of nonenzymatic glycosylation and the pathogenesis of diabetic vascular disease. Diabetes Metab Rev. 1988 Aug;4(5):437–451. doi: 10.1002/dmr.5610040503. [DOI] [PubMed] [Google Scholar]
- DATE J. W. Quantitative determination of some carbohydrates in normal urine. Scand J Clin Lab Invest. 1958;10(2):155–162. [PubMed] [Google Scholar]
- Gilchrest B. A., Rowe J. W., Mihm M. C., Jr Clinical and histological skin changes in chronic renal failure: evidence for a dialysis-resistant, transplant-responsive microangiopathy. Lancet. 1980 Dec 13;2(8207):1271–1275. doi: 10.1016/s0140-6736(80)92337-5. [DOI] [PubMed] [Google Scholar]
- Hamlin C. R., Kohn R. R. Evidence for progressive, age-related structural changes in post-mature human collagen. Biochim Biophys Acta. 1971 May 25;236(2):458–467. doi: 10.1016/0005-2795(71)90226-1. [DOI] [PubMed] [Google Scholar]
- Heaf D. J., Davies J. I. The effect of RNA supplementation of rat diets on the composition of body fluids. Br J Nutr. 1976 Nov;36(3):381–402. doi: 10.1079/bjn19760094. [DOI] [PubMed] [Google Scholar]
- Jolley R. L., Warren K. S., Scott C. D., Jainchill J. L., Freeman M. L. Carbohydrates in normal urine and blood serum as determined by high-resolution column chromatography. Am J Clin Pathol. 1970 May;53(5):793–802. [PubMed] [Google Scholar]
- Monnier V. M., Vishwanath V., Frank K. E., Elmets C. A., Dauchot P., Kohn R. R. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986 Feb 13;314(7):403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- SEGAL S., FOLEY J. The metabolism of D-ribose in man. J Clin Invest. 1958 May;37(5):719–735. doi: 10.1172/JCI103658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Isolation, purification and partial characterization of novel fluorophores from aging human insoluble collagen-rich tissue. Connect Tissue Res. 1989;19(1):77–92. doi: 10.3109/03008208909016816. [DOI] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Touraine J. L., Touraine F., Revillard J. P., Brochier J., Traeger J. T-lymphocytes and serum inhibitors of cell-mediated immunity in renal insufficiency. Nephron. 1975;14(2):195–208. doi: 10.1159/000180448. [DOI] [PubMed] [Google Scholar]
- WYNGAARDEN J. B., SEGAL S., FOLEY J. B. Physiological disposition and metabolic fate of infused pentoses in man. J Clin Invest. 1957 Oct;36(10):1395–1407. doi: 10.1172/JCI103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zier K. S., Leo M. M., Spielman R. S., Baker L. Decreased synthesis of interleukin-2 (IL-2) in insulin-dependent diabetes mellitus. Diabetes. 1984 Jun;33(6):552–555. doi: 10.2337/diab.33.6.552. [DOI] [PubMed] [Google Scholar]



