Abstract
Background
The most common type of ovarian germ cell tumor is the teratoma. Thyroid tissue, both benign and malignant, may be a component of an ovarian teratoma. Here we review this topic and illustrate major features by presenting multimodal management of a patient with BRAF-positive disseminated follicular thyroid cancer arising in an ovarian teratoma.
Summary
Malignant thyroid tissue is often difficult to distinguish from benign thyroid tissue arising in ovarian teratomas. Preoperatively, an elevated thyroglobulin (Tg) level, laboratory or clinical evidence of hyperthyroidism, or ultrasonography appearance of “struma pearl” should prompt referral to oncologist for surgical management of a possibly malignant ovarian teratoma. Postoperatively, tumor tissue should be referred to pathologists experienced with differentiating benign from malignant struma ovarii. Once diagnosed, treatment of this rare condition should be handled by a team of specialists with combined treatment modalities. We cared for woman with disseminated thyroid cancer arising in an ovarian teratoma whose history illustrates the complexity of managing ovarian teratomas with malignant thyroid tissue. At age 33 she had an intraoperative rupture of an ovarian cyst, thought to be struma ovarii. During her next pregnancy, pelvic masses were noted; biopsies revealed well-differentiated papillary thyroid carcinoma, follicular variant. She was euthyroid, but had elevated serum Tg levels. Surgical staging demonstrated widely metastatic intraabdominal dissemination. A thyroidectomy revealed no malignancy. A post-131I treatment scan revealed diffuse uptake throughout the abdomen. She then developed abdominal pain and, on computed tomography, was found to have multiple intraabdominal foci of disease. Serum Tg was 264 ng/mL while on L-thyroxine for hypothyroidism and to obtain thyrotropin suppression. A 18 fluorodeoxyglucose positron emission tomography scan showed no pathological uptake. The tumor was found to be BRAF mutation positive (K601E). She underwent extensive secondary debulking and a second course of 131I with lithium pretreatment. Posttreatment scan revealed diffuse abdominal uptake. Six months posttherapy, the patient is asymptomatic with a serum Tg of 18.1 ng/mL.
Conclusions
Aggressive multimodal management appears to be the most promising approach for malignant thyroid tissue arising in ovarian teratomas.
Introduction
Many types of both benign and malignant masses can arise from the ovaries. Common benign cysts are hemorrhagic cysts (often associated with ovulation), serous cysts, endometriomas, and teratomas. Ovarian germ-cell tumors can be benign or malignant, composed of mature tissue types or immature tissues, and be from a single germ line origin or all three. The most common type of ovarian germ cell tumor is the teratoma. Ninety-five percent of teratomas, also known as dermoid cysts, are benign structures made from adult tissue types. These are usually cystic. They are derived from ectodermal tissue such as skin, hair, and sebaceous material. They also may contain mesodermal tissue (bone, muscle, heart, lymph cells, and spleen), and tissue of endodermal origin (digestive tract, pancreas, liver, and thyroid). Teratomas are bilateral in 10%–12% of cases and appear most commonly in the second and third decades of life. Rupture of these cysts into the abdomen either spontaneously or during surgery can cause a chemical peritonitis. Approximately 1% of mature teratomas can undergo malignant degeneration of any of the underlying tissue types. Squamous cell carcinoma is the most common sub type (1), occurring more frequently than basal cell carcinoma, melanoma, adenocarcinoma, sarcoma, neuroectodermal tumor, and thyroid cancer. Solid teratomas are most often composed of immature tissue elements from all three germ layers and are called immature teratomas or malignant teratomas.
Struma Ovarii
Occasionally ovarian teratomas can be comprised of a single cell type, a monodermal teratoma, which are usually struma ovarii or less frequently carcinoid tumors. There is controversy regarding the nomenclature and clinicopathologic criteria of struma ovarii. While up to 20% of ovarian teratomas contain thyroid tissue, <5% of teratomas are struma ovarii, defined as the tumor being more than half thyroid tissue. Women with struma ovarii usually present with a unilateral adnexal mass (58%). Patients may experience acute abdominal pain (12%) (2), but may be asymptomatic in as many as 40% of cases (3). Peritoneal strumosis or stromatosis, a term used when thyroid tissue is spread in a miliary fashion over the serosal surfaces of the abdominal cavity, has also been referred to as malignant struma ovarii with peritoneal dissemination (4,5).
It is difficult to distinguish peritoneal spread of a benign ruptured struma ovarii from a highly differentiated cancer, highlighting the importance of referral of the original surgical specimens of teratomas with thyroid tissue to experienced pathologists (6). It is impossible to distinguish benign peritoneal strumosis from metastatic spread of a follicular variant of papillary thyroid cancer (PTC) arising from the ovary, that is, minimal deviation follicular thyroid type carcinoma based on histology (7–9). Only if this entity exhibits overtly metastatic behavior by spreading to lymph nodes or distant sites can the definitive diagnosis of malignancy be made. Roth and Talerman have proposed that this distinction may only be made retrospectively, by following a patient for 5 years with progression to establish the diagnosis of strumosis (7). However, distant recurrences have been reported beyond this time frame with the range of detection of metastatic disease between 5 months to 26 years (6).
Given the difficulty of definitive diagnosis even on histology, it is easy to imagine the difficulty in diagnosing this rare condition via radiographic imaging. Savelli et al. reported a summary of ultrasonography findings in patients with struma ovarii (3). In this series, all struma ovarii were cystic ovarian masses. The most specific ultrasonographic feature of struma ovarii is the struma pearl, which is a smooth round solid ball within a fluid-filled cyst and similar to the round white ball consisting of hair and sebum described in the setting of dermoid ovarian cysts. The struma pearl itself may contain areas of cystic degeneration, appearing as hypoechoic spots in the otherwise hyperechoic struma pearl. These struma pearls are often vascularized areas of ovarian cysts demonstrated on Doppler flow that represent the thyroid tissue components on histology of the ovarian mass.
Thyroid Cancer Arising in the Ovary
The word “struma” is derived from the Latin word for goiter, a benign condition. Therefore, the terminology “malignant struma ovarii” or “disseminated struma ovarii” is somewhat of a misnomer when referring to a malignant process. We prefer a histologic description for the diagnosis, for example, PTC in an ovarian teratoma or follicular thyroid carcinoma in an ovarian teratoma. Thyroid cancer (i.e., malignant degeneration) is found in ∼5% of cases of struma ovarii (10). The average age for presentation with PTC arising in an ovarian teratoma is in the fifth decade (2). Abdominal ascites and pleural effusions (Meigs syndrome) are most often associated with benign struma ovarii (11–14), but can rarely be associated with malignant PTC of the ovary (i.e., malignant struma ovary) (15,16).
Metastatic spread of PTC in an ovarian teratoma is most common to the peritoneum, mesentery, and omentum, reflecting the proximity of this intraabdominal location of thyroid cancer compared to eutopic thyroid cancer. Less common sites of distant metastases, in descending order of prevalence, are liver, contralateral ovary, bone, and lung, which mimic the pattern of metastases in eutopic thyroid cancer (6). Recurrences remote from presentation reflect the indolent nature of this disease.
BRAF and Other Mutations in Thyroid Cancer Arising from Ovarian Teratomas
Multiple genetic abnormalities have been described in thyroid cancers arising in ovarian teratomas, all of which have been described in thyroid cancer originating from the neck. Many of these alterations are associated with perturbations in the mitogen-activated protein kinase pathway. Point mutations in BRAF (in hotspot region, often V600E or K601E) (17,18), point mutations in HRAS (19), NRAS (20), loss of heterozygosity in the PTEN region (21), and ret/PTC rearrangements (tyrosine kinase receptor RET activated by fusion of the 3′ portion of the RET gene to the 5′ portion of various genes) (22) have been described in thyroid cancers arising in teratomas.
Prognosis
Survival data have been reported by Devaney et al. in a small case series collected between 1950 and 1983 (2). Length of follow-up ranged from 3 to 16 (avg. 8.7) years in 22 patients with proliferative struma ovarii and 2 to 18 (avg. 7.3) years in 8 patients with malignant struma ovarii. All patients were alive at last contact except one patient with proliferative struma, who succumbed to congestive heart failure. Forty-four percent of patients with thyroid cancer arising in the ovary were alive with no reported recurrence or disease, whereas 22% were alive with persistent disease. Fifteen percent of patients died from their metastatic disease. A full 17% of patients in this series, however, lacked follow-up (23).
Case Study
A 33-year-old Gravida 3 Para 2 woman was found to have a 10-cm right adnexal mass at the time of a spontaneous first trimester miscarriage initially treated with a completion dilatation and curettage. The patient was later taken to the operating room for a laparoscopic ovarian cystectomy, which was complicated by intraoperative cyst rupture with spill into the abdomen. The provisional diagnosis was ovarian teratoma with evidence of struma ovarii. The patient was released without further follow-up.
Two years later she became pregnant again, and during this pregnancy, new adnexal masses were discovered. Magnetic resonance imaging revealed multiple bilateral pelvic masses, separate from the uterus and the ovary. The largest left adnexal mass was 4 cm and the largest right adnexal mass was ∼2.4 cm.
The patient had a normal vaginal delivery, and then underwent a laparoscopic tubal ligation with biopsy of a right anterior peritoneal mass. These specimens were initially thought to be similar to the benign process (struma ovarii without atypia), but further investigation revealed that the specimen was positive for keratins AE1/AE3/CK7, thyroglobulin (Tg), and thyroid transcription factor, and negative for estrogen receptor, progesterone receptor, carcinoembryonic antigen, and cytokeratin 20, favoring metastatic follicular carcinoma of the thyroid.
This prompted an investigation of the thyroid, which revealed a 6-mm hyperechoic nodule in the left superior thyroid lobe. The patient was euthyroid (Fig. 1). The serum Tg was 49,000 ng/mL (normal range, 1.6–59.9 ng/mL); however, her CA-125 was 99 units/mL (normal range, 0–35 ng/mL).
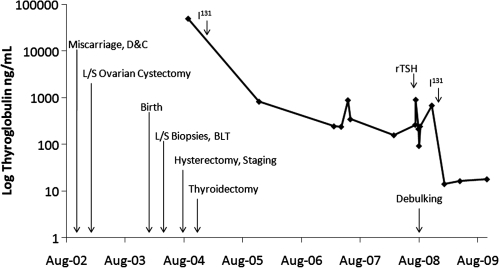
FIG. 1.
Graphical representation of thyroglobulin levels (ng/mL) and associated clinical events from right to left: initial presentation during a miscarriage requiring dilatation & curettage (D&C), laparoscopic cystectomy with intraoperative rupture, normal spontaneous vaginal delivery, laparoscopic biopsies (L/S) and bilateral tubal ligation (BLT), total abdominal hysterectomy with bilateral salpingo-oophorectomy, and lymph node dissection/partial omentectomy, total thyroidectomy, 253 mCi of radioactive 131iodine, recombinant thyrotropin (rTSH) injection for thyrogen-stimulated positron emission tomography/computed tomography, secondary surgical debulking of multiple implants/completion of omentectomy, and 308 mCi of radioactive 131iodine.
To address the malignant intraabdominal process, the patient underwent exploratory laparotomy, where widely metastatic intraabdominal dissemination of her disease was found. A gynecological staging procedure was performed: total abdominal hysterectomy, bilateral salpingo-oophorectomy, partial omentectomy, and peritoneal washings, where all residual tumor implants were <1 cm in their greatest dimension, that is, optimal cytoreduction or optimal debulking. After extensive review of the hysterectomy and staging specimens, it was concluded that the patient's intraabdominal disease was most likely due to disseminated struma ovarii.
Given the concern for metastatic thyroid cancer in the setting of a thyroid nodule, and in view of the planned radioablation, a total thyroidectomy was performed. Surgical pathology of the thyroidectomy specimen revealed no malignancy. The patient was then treated with 253 mCi of 131I, and posttreatment scan revealed diffuse uptake throughout the abdomen. During the following 4 years the patient was treated with thyrotropin (TSH) suppressive doses of thyroid hormone.
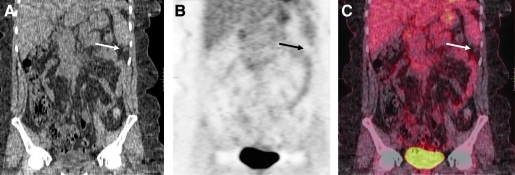
The patient was referred to our institution for evaluation because of persistent abdominal pain. At the time of first encounter at the National Institutes of Health (NIH), her serum TSH was 0.3 mcIU/mL with negative Tg antibodies. A serum Tg while on suppressive doses of thyroxine was 264 ng/mL and multiple intraabdominal foci of disease were observed by computed tomography (Fig. 2). No intrahepatic dissemination was noted. A recombinant human TSH-stimulated 18 fluorodeoxyglucose positron emission tomography scan showed no pathological uptake (Fig. 2).
FIG. 2.
Coronal body view of recombinant human thyrotropin-stimulated functional positron emission tomography/computed tomography with 15.2 mCi of 18fluorine-flourodeoxyglucose, which revealed multiple subcentimeter nodules (the most visible lesion is indicated by the arrow) without pathologic uptake of 18fluorine-flourodeoxyglucose. (A) CT image; (B) PET scan; (C) reconstruction image.
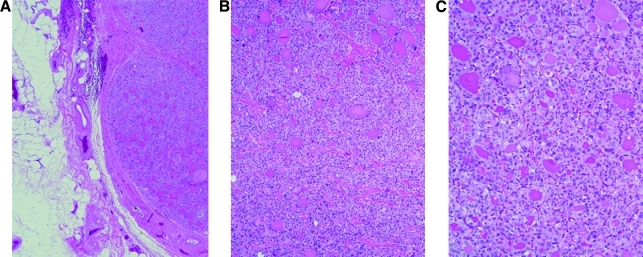
The patient underwent extensive secondary surgical debulking of her metastatic abdominal disease, including exploratory laparotomy, lysis of adhesions, greater and lesser omentectomy, mobilization of the splenic flexure, diaphragmatic stripping, pelvic peritonectomy, and resection of cecal and jejunal serosal nodules. Multiple small lesions, <1 cm, were removed from both hemidiaphragms. Larger, almost 2 cm, masses were resected form the greater omentum toward the spleen. A nodule tucked behind the spleen was also removed. The pelvis revealed studding of the peritoneum as well as the pelvic sidewalls. There were lesions in the lateral sigmoid in the tinea epiploica. Histopathology examination of the lesions revealed follicular variant of PTC throughout the abdomen. This was similar in appearance to the samples obtained from the initial surgical staging procedure (Fig. 3). Two weeks after surgery her serum Tg was 93.2 ng/mL.
FIG. 3.
Omental implant of metastatic papillary thyroid from secondary debulking surgery. (A) Papillary thyroid carcinoma, follicular variant, arising from the ovary, low power (B) and high power (C).
Eight weeks postoperatively, the patient underwent thyroid hormone withdrawal and 10 days treatment with lithium 300 mg three times daily by mouth, achieving a serum lithium level of 0.9 mEq/L (0.6–1.2). One week after starting lithium she received a second course of 308 mCi of 131I, which was calculated by dosimetry. At the time of radioactive iodine (RAI) therapy her serum TSH was 59.7 mcIU/mL and her Tg was 682 ng/mL. Posttreatment scan revealed diffuse uptake throughout the abdomen without distant metastases (Fig. 4). Six months after this therapy the patient was asymptomatic with a serum Tg of 18.1 ng/mL and a serum TSH of 0.02 mcIU/mL while on thyroid hormone.
FIG. 4.
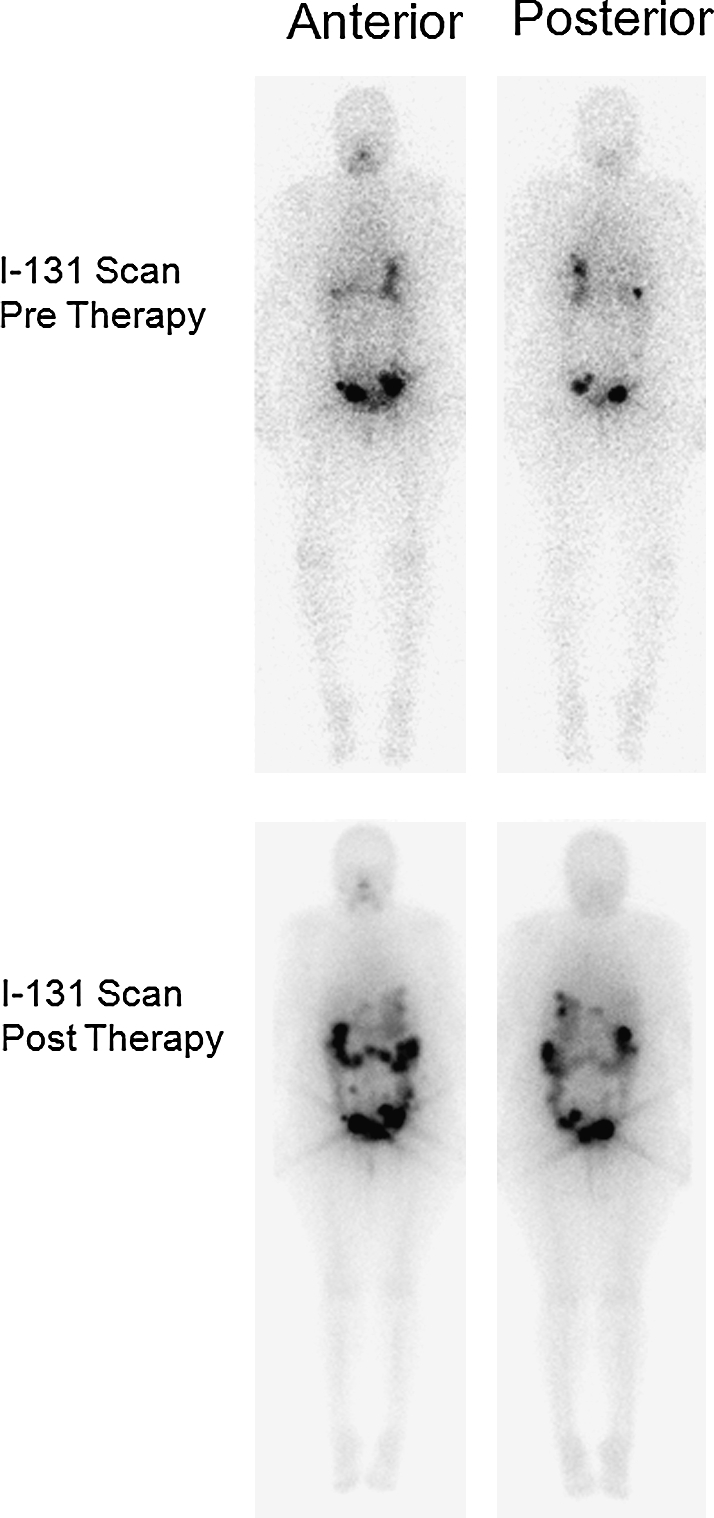
Top panel shows diagnostic 131I scan before therapeutic doses radioactive iodine, anterior (left) and posterior (right) views. The bottom panel shows posttreatment (308 mCi of 131I) scan in anterior (left) and posterior (right) views. The abdominal uptake is attributed to metastatic disease, with the exception of some residual uptake in the large colon.
Molecular analysis
To evaluate the tumor for oncogene expression, tumor tissue was collected from pathology specimens. Formalin-fixed paraffin-embedded slides, 3–4.5 mm cut section thick, were deparaffinized, rehydrated, and stained with hematoxylin and eosin. Selected cells of the tumor were procured by microdissection with a 30-gauge needle under microscopic control. The procured tissue was resuspended in a lysis buffer containing 0.5 mg/mL proteinase K, 0.5% Tween 20, 1 mM EDTA (pH 8), and 50 mM Tris-HCl (pH 8.5), and incubated at 55°C for 48 hours to ensure a complete digestion. Then, samples were heated at 95°C for 5 minutes to inactivate the proteinase K and stored at −20°C until use. Normal thyroid tissue was used as negative control. Genomic DNA was amplified by polymerase chain reaction (touch down starting at 61°C minus 0.5°C each cycle, 72°C for 1:30 for 8 cycles, followed by 35 cycles of 95°C 60 seconds, 57°C for 60 seconds, and 72°C for 60 seconds, and extension at 72°C for 15 minutes). The following primers were used: K-Ras, 5′-ggcctgctgaaaatgactgaa-3′ sense and 5′-ggtcctgcaccagtaatatgc-3′ antisense; BRAF, 5′-tgtaaaacgacggccagtcataatgcttgctctgatagga-3′ sense and 5′-agcggataacaatttcacacaggccaaaaatttaatcagtgga-3′ antisense. Polymerase chain reaction products were observed on 2% agarose gel with ethidium bromide and sequenced on both strands by BigDye®terminator version 3.1 using an ABI PRISM 3730XL Analyzer (Applied Biosystems, Foster City, CA). Sequences were compared to human genome using BLAST.
Although no KRAS mutations were found in the tumor sample, an A/G transition was detected at exon 601 of the BRAF gene, resulting in a lysine to glutamine missense mutation (K601E) previously associated with follicular variant of PTC (24).
Management of Thyroid Cancer Arising in Ovarian Teratomas
On the basis of our experience with this and other patients and the literature, we propose that work-up for suspected ovarian teratomas include laboratory assessment of Tg levels and thyroid function tests. As in our patient, PTC of an ovarian teratoma (i.e., malignant struma ovarii) can be associated with elevated Tg levels (25). When elevated Tg levels (above the normal range) are encountered in the setting of a suspected ovarian teratoma, we propose preoperative referral to a gynecology oncologist for operative management of teratoma due to the increased risk of underlying ovarian malignancy. However, an elevated Tg level could also be associated with benign struma ovarii. Tg levels have been shown to correlate with the burden of disease, particularly in the follicular variant of thyroid cancer (26). Conversely, if evidence of hyperthyroidism is encountered on laboratory assessment in the setting of an ovarian teratoma, it is more likely to be a benign hyperfunctioning ovarian struma ovarii, which occurs in ∼5%–15%. In contrast, hyperthyroidism associated with PTC arising from an ovarian teratoma is rare (27). When a majority of thyroid tissue is encountered in an ovarian teratoma, we urge referral to a pathologist experienced with struma ovarii and PTC arising in the ovary. As many as 34% of cases of thyroid cancer arising in the ovary were initially misdiagnosed as benign struma ovarii, reflecting the underlying difficulty in distinguishing follicular thyroid cancers from benign processes and highlighting the importance of an experienced pathologist (6). Molecular genetics can also aid in differentiating benign from malignant tumors, as is demonstrated with the case reported here.
We also stress the importance of combining gynecologic and endocrine approaches to management of thyroid cancer arising in ovarian teratomas. Surgical resection of as much malignant cancer as possible has emerged as paradigm of ovarian cancer management, which has been show to improve survival in patients with Stage IIIC disease (28), the most common stage at diagnosis. Cytoreductive surgical management has become the standard of care for recurrent ovarian cancer to decrease the bulk and density of malignancy and optimize the effects of chemotherapy. We agree with the proposal (23) for management by combining approaches typical for ovarian cancer and for thyroid cancer. Given our experience with the case reported here we would also like to stress the importance of primary surgical management with total abdominal hysterectomy, bilateral salpingo-oophorectomy, and surgical tumor debulking (gynecologic oncology or general surgery) to cytoreduce the total burden of intraabdominal disease in this condition as it mimics borderline ovarian malignancies. Chemotherapeutic approaches should be tailored more to a metastatic thyroid cancer rather than ovarian cancer; patients with thyroid cancer arising in the ovary should therefore undergo total thyroidectomy by endocrine surgeons to allow for treatment with 131I ablation (6,15). The presence of local or distant spread should also prompt the use of dosimetry to minimize the toxicity associated with this modality (29). Notably, to optimize the chances for response to 131I therapy in the patient reported here, we opted for pretreatment with lithium as it may increase 131I retention in neoplastic tissue (30). We also used a strict low-iodine diet (31,32).
Fertility
Because thyroid cancers arising in teratomas are generally tumors with indolent courses, fertility sparing surgery (e.g., unilateral oophorectomy) should be considered in reproductive aged patients with minimal disease burden. RAI ablation for thyroid cancer has been associated with temporary ovarian failure and early menopause (33), or transient amenorrhea with elevated gonadotropins (34). Pretreatment with GnRH agonists to induce a more quiescent (and less susceptible) state in the presence of gonadotoxic agents is generally a very safe option to protect ovarian function, but the utility of this therapy is controversial (35,36). GnRH antagonists have been suggested as an alternative to GnRH agonists as GnRH agonists are associated with an initial (undesirable) flare in ovarian stimulation before (protective) downregulation eventually occurs. GnRH antagonists, however, have not been rigorously tested in clinical trials (37).
While RAI has modest lasting effects on fertility on patients treated for eutopic thyroid cancer, thyroid cancer arising in the ovary presents a very different situation. First, surgical removal of the primary tumor requires removing the gonad, which is thought to hasten diminishing ovarian reserve due to incessant ovulatory demand from a single ovary instead of from two. Second, even if fertility sparing surgery is employed and a gonad is spared, RAI will concentrate much closer to the remaining gonad in the case of thyroid cancer arising in a teratoma than eutopic thyroid cancer. Radioactive damage will concentrate near the site of the remaining gonad and reproductive damage will be far greater. For patients with advanced disease, a consideration of fertility options should be discussed before definitive surgery, as is becoming standard for many cancers striking women of reproductive age like breast, gynecologic, and hematologic malignancies (38). Experimental approaches included ovarian cryopreservation, ovarian cortex harvest and cryopreservation, and whole ovary cryopreservation, but are not yet standard of care. Patients should be referred to Institutional Review Board–approved protocols when feasible.
In summary, struma ovarii is a rare tumor that is difficult to accurately characterize preoperatively and sometimes difficult to distinguish from thyroid cancer arising in an ovarian teratoma histologically. The nomenclature used in the literature for these conditions adds to the difficulty of managing these conditions. It is preferable that patients with these disorders be handled by an experienced team of specialists.
Acknowledgments
This work was supported by the Intramural Research Program of the NIDDK, project number Z01-DK047057-02. The authors gratefully acknowledge the comments and suggestions of Dr. E. Kebebew (NCI-NIH).
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Dos Santos L. Mok E. Iasonos A. Park K. Soslow R. Aghajanian C. Alektiar K. Barakat R. Abu-Rustum N. Squamous cell carcinoma arising in mature cystic teratoma of the ovary: a case series and review of the literature. Gynecol Oncol. 2007;105:321–324. doi: 10.1016/j.ygyno.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Devaney K. Snyder R. Norris H. Tavassoli F. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int J Gynecol Pathol. 1993;12:333–343. doi: 10.1097/00004347-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Savelli L. Testa A. Timmerman D. Paladini D. Ljungberg O. Valentin L. Imaging of gynecological disease (4): clinical and ultrasound characteristics of struma ovarii. Ultrasound Obstet Gynecol. 2008;32:210–219. doi: 10.1002/uog.5396. [DOI] [PubMed] [Google Scholar]
- 4.Karseladze A. Kulinitch S. Peritoneal strumosis. Pathol Res Pract. 1994;190:1082–1085. doi: 10.1016/S0344-0338(11)80905-2. discussion 1086–1088. [DOI] [PubMed] [Google Scholar]
- 5.Tennvall J. Ljungberg O. Högberg T. Malignant struma ovarii' with peritoneal dissemination. Histopathology. 1997;31:289–290. doi: 10.1046/j.1365-2559.1997.2130860.x. [DOI] [PubMed] [Google Scholar]
- 6.McGill J. Sturgeon C. Angelos P. Metastatic struma ovarii treated with total thyroidectomy and radioiodine ablation. Endocr Pract. 2009;15:167–173. doi: 10.4158/EP.15.2.167. [DOI] [PubMed] [Google Scholar]
- 7.Roth L. Talerman A. The enigma of struma ovarii. Pathology. 2007;39:139–146. doi: 10.1080/00313020601123979. [DOI] [PubMed] [Google Scholar]
- 8.Roth L. Karseladze A. Highly differentiated follicular carcinoma arising from struma ovarii: a report of 3 cases, a review of the literature, and a reassessment of so-called peritoneal strumosis. Int J Gynecol Pathol. 2008;27:213–222. doi: 10.1097/PGP.0b013e318158e958. [DOI] [PubMed] [Google Scholar]
- 9.Roth L. Miller AW., 3rd Talerman A. Typical thyroid-type carcinoma arising in struma ovarii: a report of 4 cases and review of the literature. Int J Gynecol Pathol. 2008;27:496–506. doi: 10.1097/PGP.0b013e31816a74c6. [DOI] [PubMed] [Google Scholar]
- 10.Ayhan A. Yanik F. Tuncer R. Tuncer Z. Ruacan S. Struma ovarii. Int J Gynaecol Obstet. 1993;42:143–146. doi: 10.1016/0020-7292(93)90628-a. [DOI] [PubMed] [Google Scholar]
- 11.Rim S. Kim S. Choi H. Struma ovarii showing clinical characteristics of ovarian malignancy. Int J Gynecol Cancer. 2005;15:1156–1159. doi: 10.1111/j.1525-1438.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 12.Huh J. Montz F. Bristow R. Struma ovarii associated with pseudo-Meigs' syndrome and elevated serum CA 125. Gynecol Oncol. 2002;86:231–234. doi: 10.1006/gyno.2002.6741. [DOI] [PubMed] [Google Scholar]
- 13.Loizzi V. Cormio G. Resta L. Fattizzi N. Vicino M. Selvaggi L. Pseudo-Meigs syndrome and elevated CA125 associated with struma ovarii. Gynecol Oncol. 2005;97:282–284. doi: 10.1016/j.ygyno.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 14.Bokhari A. Rosenfeld G. Cracchiolo B. Heller D. Cystic struma ovarii presenting with ascites and an elevated CA-125 level. A case report. J Reprod Med. 2003;48:52–56. [PubMed] [Google Scholar]
- 15.Amalaseelan J. Perera K. An unusual variant of malignant struma ovarii. Ceylon Med J. 2008;53:55–56. doi: 10.4038/cmj.v53i2.234. [DOI] [PubMed] [Google Scholar]
- 16.Zannoni G. Gallotta V. Legge F. Tarquini E. Scambia G. Ferrandina G. Pseudo-Meigs' syndrome associated with malignant struma ovarii: a case report. Gynecol Oncol. 2004;94:226–228. doi: 10.1016/j.ygyno.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 17.Flavin R. Smyth P. Crotty P. Finn S. Cahill S. Denning K. Li J. O'Regan E. O'Leary J. Sheils O. BRAF T1799A mutation occurring in a case of malignant struma ovarii. Int J Surg Pathol. 2007;15:116–120. doi: 10.1177/1066896906299131. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt J. Derr V. Heinrich M. Crum C. Fletcher J. Corless C. Nosé V. BRAF in papillary thyroid carcinoma of ovary (struma ovarii) Am J Surg Pathol. 2007;31:1337–1343. doi: 10.1097/PAS.0b013e31802f5404. [DOI] [PubMed] [Google Scholar]
- 19.Coyne C. Nikiforov Y. RAS mutation-positive follicular variant of papillary thyroid carcinoma arising in a struma ovarii. Endocr Pathol. 2010;21:144–147. doi: 10.1007/s12022-009-9097-8. [DOI] [PubMed] [Google Scholar]
- 20.Celestino R. Magalhães J. Castro P. Triller M. Vinagre J. Soares P. Sobrinho-Simões M. A follicular variant of papillary thyroid carcinoma in struma ovarii. Case report with unique molecular alterations. Histopathology. 2009;55:482–487. doi: 10.1111/j.1365-2559.2009.03401.x. [DOI] [PubMed] [Google Scholar]
- 21.Tate G. Tajiri T. Suzuki T. Mitsuya T. Mutations of the KIT gene and loss of heterozygosity of the PTEN region in a primary malignant melanoma arising from a mature cystic teratoma of the ovary. Cancer Genet Cytogenet. 2009;190:15–20. doi: 10.1016/j.cancergencyto.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Boutross-Tadross O. Saleh R. Asa S. Follicular variant papillary thyroid carcinoma arising in struma ovarii. Endocr Pathol. 2007;18:182–186. doi: 10.1007/s12022-007-0022-8. [DOI] [PubMed] [Google Scholar]
- 23.McGill J. Sturgeon C. Angelos P. Metastatic struma ovarii treated with total thyroidectomy and radioiodine ablation. Endocr Pract. 2009;15:167–173. doi: 10.4158/EP.15.2.167. [DOI] [PubMed] [Google Scholar]
- 24.Trovisco V. Soares P. Soares R. Magalhães J. Sá-Couto P. Sobrinho-Simões M. A new BRAF gene mutation detected in a case of a solid variant of papillary thyroid carcinoma. Hum Pathol. 2005;36:694–697. doi: 10.1016/j.humpath.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Elisei R. Romei C. Castagna M. Lisi S. Vivaldi A. Faviana P. Marinò M. Ceccarelli C. Pacini F. Pinchera A. RET/PTC3 rearrangement and thyroid differentiation gene analysis in a struma ovarii fortuitously revealed by elevated serum thyroglobulin concentration. Thyroid. 2005;15:1355–1361. doi: 10.1089/thy.2005.15.1355. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A. Sarda A. Chattopadhyay T. Kapur M. The role of estimation of the ratio of preoperative serum thyroglobulin to the thyroid mass in predicting the behaviour of well differentiated thyroid cancers. J Postgrad Med. 1996;42:39–42. [PubMed] [Google Scholar]
- 27.Matsuda K. Maehama T. Kanazawa K. Malignant struma ovarii with thyrotoxicosis. Gynecol Oncol. 2001;82:575–577. doi: 10.1006/gyno.2001.6315. [DOI] [PubMed] [Google Scholar]
- 28.Berek J. Preoperative prediction of optimal resectability in advanced ovarian cancer using CA-125. Gynecol Oncol. 2000;77:225–226. doi: 10.1006/gyno.2000.5808. [DOI] [PubMed] [Google Scholar]
- 29.Van Nostrand D. Atkins F. Moreau S. Aiken M. Kulkarni K. Wu J. Burman K. Wartofsky L. Utility of the radioiodine whole-body retention at 48 hours for modifying empiric activity of 131-iodine for the treatment of metastatic well-differentiated thyroid carcinoma. Thyroid. 2009;19:1093–1098. doi: 10.1089/thy.2008.0339. [DOI] [PubMed] [Google Scholar]
- 30.Koong S. Reynolds J. Movius E. Keenan A. Ain K. Lakshmanan M. Robbins J. Lithium as a potential adjuvant to 131I therapy of metastatic, well differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:912–916. doi: 10.1210/jcem.84.3.5527. [DOI] [PubMed] [Google Scholar]
- 31.Hinds SR., 2nd Stack A. Stocker D. Low-iodine diet revisited: importance in nuclear medicine imaging and management. Clin Nucl Med. 2008;33:247–250. doi: 10.1097/RLU.0b013e3181662f9e. [DOI] [PubMed] [Google Scholar]
- 32.Lakshmanan M. Schaffer A. Robbins J. Reynolds J. Norton J. A simplified low iodine diet in I-131 scanning and therapy of thyroid cancer. Clin Nucl Med. 1988;13:866–868. doi: 10.1097/00003072-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Ceccarelli C. Bencivelli W. Morciano D. Pinchera A. Pacini F. 131I therapy for differentiated thyroid cancer leads to an earlier onset of menopause: results of a retrospective study. J Clin Endocrinol Metab. 2001;86:3512–3515. doi: 10.1210/jcem.86.8.7719. [DOI] [PubMed] [Google Scholar]
- 34.Raymond J. Izembart M. Marliac V. Dagousset F. Merceron R. Vulpillat M. Vallée G. Temporary ovarian failure in thyroid cancer patients after thyroid remnant ablation with radioactive iodine. J Clin Endocrinol Metab. 1989;69:186–190. doi: 10.1210/jcem-69-1-186. [DOI] [PubMed] [Google Scholar]
- 35.Seli E. Tangir J. Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol. 2005;17:299–308. doi: 10.1097/01.gco.0000169108.15623.34. [DOI] [PubMed] [Google Scholar]
- 36.Georgescu E. Goldberg J. du Plessis S. Agarwal A. Present and future fertility preservation strategies for female cancer patients. Obstet Gynecol Surv. 2008;63:725–732. doi: 10.1097/OGX.0b013e318186aaea. [DOI] [PubMed] [Google Scholar]
- 37.Blumenfeld Z. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:2680–2681. author reply 2682–2683. [PubMed] [Google Scholar]
- 38.Salvatori M. Dambra D. D'Angelo G. Conte L. Locantore P. Zannoni G. Campo V. Campo S. A case of metastatic struma ovarii treated with 131I therapy: focus on preservation of fertility and selected review of the literature. Gynecol Endocrinol. 2008;24:312–319. doi: 10.1080/09513590802095787. [DOI] [PubMed] [Google Scholar]