Abstract
Type 1 regulatory (Tr1) cells have emerged as key players in the prevention of autoimmunity. They produce high levels of the immunosuppressive cytokine interleukin (IL)-10 and confer protection against a wide panel of autoimmune diseases. However, the molecular pathways leading to their generation have long remained elusive. We have recently identified IL-27, a member of the IL-12 cytokine family, as a novel cytokine that induces Tr1 cells. Further analysis of IL-27-driven Tr1 cells have identified a critical role of the transcription factor avian musculoaponeurotic fibrosarcoma v-maf and of IL-21 in the generation of IL-27-induced Tr1 cells. Importantly, IL-27 also induces Tr1 cells in humans, suggesting that IL-27 administration may dampen tissue inflammation in humans as well. Here, we review the role of IL-27 in the generation of Tr1 cells and discuss its potential to alleviate autoimmune diseases.
Introduction
Type 1 regulatory cells in autoimmune diseases
Regulatory T cells (Tregs) have a fundamental role in the establishment and maintenance of tolerance. Deficits in the numbers and/or function of different types of Tregs were shown to contribute to the development of autoimmunity, allergy, and graft rejection (Wing and Sakaguchi 2010). Conversely, an overabundance of Tregs can inhibit immune response to tumors and infections (Zou 2006). Two important classes of Tregs within the CD4+ subset have been identified: FoxP3+ Tregs and interleukin (IL)-10-producing type 1 regulatory (Tr1) cells. Despite their common role in the regulation of immune responses, these 2 Treg subsets feature major differences in their biology, including the cytokines that induce them and the mechanisms by which they mediate their suppressor function (Roncarolo and others 2006). Whereas both regulatory populations produce IL-10, Tr1 cells do not express the master Treg transcription factor Foxp3, a forkhead box family transcription factor associated with the generation of natural Tregs (Batten and others 2008).
Tr1 cells were first described in severe combined immunodeficient (SCID) patients who had developed long-term tolerance to stem cell allografts, suggesting that these cells suppressed immune responses in humans (Bacchetta and others 1994). The regulatory properties of Tr1 cells were further exemplified in another study that demonstrated that ex vivo activation of human or mouse CD4+ T cells with high doses of IL-10 induced T cell clones with a cytokine secretion profile distinct from that of T helper 1 (Th1) or Th2 cells but similar to that of host-reactive T cell clones isolated from successfully transplanted SCID patients (Groux and others 1997).
CD4+ Tr1 cells are characterized by their low proliferative capacity and their high levels of IL-10 secretion. The ability of Tr1 cells to downmodulate effector T cell responses has been ascribed to their high IL-10 production (Groux and others 1997). Although the suppressive activity of Tr1 cells can be reversed by the neutralization of IL-10, additional mechanisms such as the secretion of transforming growth factor (TGF)-β (Groux and others 1997) and cytotoxicity (Grossman and others 2004) also contribute to their regulatory function.
IL-10 has been known to exert an immunosuppressive activity for many years as it was first identified by its ability to inhibit T cell activation and effector functions in vitro (Moore and others 1990; de Waal Malefyt and others 1991). The importance of antiinflammatory properties of IL-10 was confirmed in IL-10-deficient animals. It was indeed shown that IL-10 deficient mice develop spontaneous colitis in their early age (Kuhn and others 1993). Similarly, immunization of IL-10 deficient mice with myelin antigens showed enhanced neuroinflammation with loss of recovery in experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis (MS) (Bettelli and others 1998). Altogether, these findings pointed to a key role of IL-10 in suppressing immune responses and maintaining tolerance. Many cell types have been described to produce IL-10 such as dendritic cells (DCs), macrophages, and Th1 and Th2 cells (Moore and others 2001). In contrast to Th2 cells, Tr1 cells produce TGF-β but very low levels of IL-2 and no IL-4 (Groux and others 1997). However, one striking feature of Tr1 cells is their ability to secrete particularly high levels of IL-10. It is because of their ability to produce overwhelming amounts of IL-10 that Tr1 cells have been shown to represent one of the main T-cell mediators of cytokine-dependent immune regulation in both mice and humans.
These pioneering studies provided impetus to study the potential of Tr1 cells to dampen tissue inflammation in vivo. Subsequent analysis performed using experimental models have demonstrated that manipulating the numbers and/or function of Tr1 cells alleviates pathology in a wide range of contexts, including transplantation and autoimmune diseases (Groux and others 1997; Battaglia and others 2006; Ahangarani and others 2009). Tr1 cells were indeed shown to be abundant in the intestine, and it has been proposed that their major function was to maintain immune homeostasis to the intestinal microbiota (Maynard and others 2007). The in vivo immunosuppressive activity of Tr1 cells in colitis was exemplified by studies from Groux et al., who showed that colitis induced in SCID mice by transfer of CD45RBhigh CD4+ T cells could be prevented by cotransfer of murine Tr1 clones derived from CD4+ T cells that expressed a transgenic T cell receptor specific for a peptide of ovalbumin (OVA). Importantly, the immune suppression relied on the antigen-specific activation of Tr1 cells in vivo as these cells inhibited colitis only in recipients that received specific antigen (OVA) in their drinking water. The regulatory properties of Tr1 cells have also been examined in human patients suffering from MS. In an elegant study, Astier and others (2006) showed that the generation of Tr1 cells from peripheral CD4+ T cells was greatly impaired in MS patients in comparison to healthy volunteers.
Given the strong immunosuppressive functions exhibited by Tr1 cells, several groups have attempted to generate large numbers of Tr1 in vitro, with the long-term goal to transfer Tr1 cells into humans suffering from autoimmune and inflammatory diseases. Tr1 cells have so far been generated in vitro by activating naïve CD4+ T cells in the presence of either IL-10 alone, IL-4 and IL-10 (Groux and others 1997), IL-10 and interferon (IFN)-α (Levings and others 2001), or 1,25 (OH) 2-vitamine D3 and dexamethasone (Barrat and others 2002; Roncarolo and others 2006). Moreover, tolerogenic DCs or DCs treated with IL-10 have also been shown to favor the generation/expansion of Tr1 cells (Mahnke and others 2003). Although these reports have increased our understanding on the biology of Tr1 cells, it has been difficult to expand Tr1 cells in vitro because of the immunosuppressive nature of IL-10 that prevents further proliferation of the differentiated Tr1 cells. We and others therefore investigated the role of other cytokines for their ability to promote the differentiation of naïve CD4+ T cells into Tr1 cells and found that IL-27, a cytokine of the IL-12 family, promotes the generation of Tr1 cells (Awasthi and others 2007; Fitzgerald and others 2007b; Stumhofer and others 2007).
The Biology of IL-27-Induced Tr1 Cells
IL-27 as an immunoregulatory cytokine
IL-27 is a heterodimeric cytokine that consists of Epstein-Barr virus–induced gene 3, an IL-12p40-related protein, and p28, a newly discovered IL-12p35-related polypeptide, which signals through a receptor containing the common IL-6 receptor chain gp130 and the unique IL-27 receptor α chain also called WSX-1 (Pflanz and others 2002, 2004). WSX-1 is expressed on T and B cells, natural killing cells, mast cells, monocytes/macrophages, DCs, and endothelial cells (Pflanz and others 2004). IL-27 was initially described as a proinflammatory cytokine. On the basis of the structural homology between IL-12 and IL-27, it was first proposed that IL-27 could sensitize lymphocytes to IL-12 and thus promote Th1 responses. Initial studies indeed showed that recombinant IL-27 could increase naïve CD4+ T cell proliferation and mediate IFN-γ production. Furthermore, this interpretation was consistent with the ability of IL-27 to induce the transcription factor T-bet, a master transcription factor whose target genes IL-12Rβ2 and IFN-γ are essential components of Th1 responses (Hibbert and others 2003; Lucas and others 2003; Takeda and others 2003). Consistent with this hypothesis, naïve WSX-1-deficient CD4+ T cells produced less IFN-γ than wild-type cells when cultured under nonpolarizing condition (Chen and others 2000; Yoshida and others 2001).
However, the aforementioned findings were later questioned by studies where WSX-1-deficient mice were challenged with intracellular pathogens. The analysis of WSX-1-deficient mice infected with Toxoplasma gondii unexpectedly revealed that, although the mice efficiently controlled parasitic inflammation, they developed severe immunopathology and died because of T-cell-mediated tissue inflammation (Villarino and others 2003). Similarly, infection of WSX-1-deficient mice with Leishmania donovani resulted in clearance of parasites with enhanced proinflammatory cytokines production and exaggerated T cell responses, resulting in severe immunopathology (Artis and others 2004; Rosas and others 2006), and infection with Trypanosom cruzi led to the development of immune-mediated liver necrosis (Hamano and others 2003). This phenotype was not only restricted to parasitic infections as WSX-1-deficient mice displayed more severe lung pathology after Mycobacterium tuberculosis and ultimately succumbed because of overwhelming inflammation (Pearl and others 2004; Holscher and others 2005).
These studies highlighted the important regulatory functions of IL-27, and this was further strengthened by the direct effects that IL-27 has on the development of other effector T cells like Th17 cells. Th17 cells that produce cytokine IL-17A, IL-17F, IL-21, and IL-22 are highly proinflammatory effector T cells that play an important role in the development of tissue inflammation and autoimmunity. In this regard, WSX-1-deficient mice showed enhanced central nervous system (CNS) inflammation in mice infected with Toxoplasma Gondii (Stumhofer and others 2006) and were highly susceptible to development of EAE with enhanced Th17 responses (Batten and others 2006). Further, administration of recombinant IL-27 was able to reduce the frequency of proinflammatory Th17 cells, resulting in a decreased severity in autoimmunity (Fitzgerald and others 2007b). These results led to hypothesis that IL-27 may not be necessary for the generation of proinflammatory T cells but may instead play a crucial role in regulating pro-inflammatory T cell responses.
The identification of IL-27 as an inhibitor of Th1 and Th17 responses suggested that IL-27 may have a broader role in controlling T cell responses, raising the possibility that IL-27 may induce regulatory T cells that control effector T cell responses and prevent development of tissue inflammation.
IL-27 in the promotion of Tr1 cells
IL-27 has recently been identified as a differentiation factor for the generation of IL-10-producing Tr1 cells that lack Foxp3 expression and regulate T cell functions in an IL-10-dependent manner (Awasthi and others 2007; Fitzgerald and others 2007b; Stumhofer and others 2007). DCs primed in vivo by induced Foxp3+ Tregs have been reported to secrete IL-27 and suppress immune responses. These Tregs-modified DCs express plasmacytoid-like markers, similar to what has been previously described for tolerogenic DCs (Ochando and others 2006), which in turn induced generation of IL-10-producing Tr1-like cells by an IL-27-dependent mechanism (Awasthi and others 2007). These findings were recapitulated in vitro as the addition of IL-27 to naïve T cells was able to drive the expansion of Tr1 cells, thus underscoring that IL-27 is indeed an essential differentiation/growth factor for Tr1 cell generation both in vitro and in vivo (Awasthi and others 2007). These findings provided a means by which Tr1 cells could be grown in large numbers in vitro and facilitated their functional analysis. However, the molecular mechanism by which IL-27 induces generation and/or expansion of Tr1 cells is just beginning to be understood (see below).
IL-21 is essential for the IL-27-induced expansion of Tr1 cells
Although IL-10 secretion is crucial for the effector functions of Tr1 cells, early studies have ruled out IL-10 as a potential growth factor for these cells. In an attempt to identify essential factors for Tr1 cell expansion, we have analyzed different cytokines secreted during the course of Tr1 cell differentiation and made the unexpected finding that IL-27-driven Tr1 cells secrete IL-21. IL-21, a member of IL-2 cytokine family, was initially identified as a soluble molecule produced by activated human T cells (Parrish-Novak and others 2000). IL-21 signals through a heterodimeric receptor containing IL-21R and the common cytokine receptor γ chain, which is also shared by other receptors like IL-2, IL-4, IL-7, IL-9, and IL-15 (Asao and others 2001). IL-21 has been reported to enhance humoral responses and to synergize with IL-7 or IL-15 to promote CD8+ T-cell expansion. On the other hand, IL-21 has been reported to dampen innate and adaptive immune responses by inducing B cell apoptosis and inhibiting DC maturation and function, thus suggesting that the effects of IL-21 may vary depending on the biological context (Leonard and Spolski 2005). In a recent report using BXSB-Yaa mice with systemic lupus erythematosus, Spolski and colleagues reported that IL-21 overexpression decreases specific antibody production after immunization in an IL-10-dependent fashion and that IL-21 signaling is required for maximal induction of IL-10 by IL-6 or IL-27 (Spolski and others 2009). This report therefore suggested that IL-21 may exert its immunosuppressive activity by inducing IL-10 production.
Recent studies have shown that Th1, Th2, and Th17 CD4+ T cell subsets also secrete IL-21. IL-21 has been proposed as a growth factor for Th17 cells (Korn and others 2007a; Nurieva and others 2007; Wei and others 2007). Given the strong links with the expression of IL-21 and the promotion of IL-10 secretion in vivo, we hypothesized that IL-27-driven IL-21 production from T cells may similarly act as an autocrine growth factor for the generation of Tr1 cells. To test this, we first isolated naïve T cells and differentiated them with IL-27 and assessed their ability to secrete IL-21. We found that IL-27-induced Tr1 cells, like Th17 cells, secrete substantial amounts of IL-21 (Pot and others 2009). To test the relevance of IL-21 secretion on the biology of IL-27-induced Tr1 cells, we then generated Tr1 cells with IL-27 but in the presence of a neutralizing IL-21 antibody. Consistent with our hypothesis, blockade of IL-21 significantly reduced the frequency of IL-10-producing T cells. We further confirmed the critical role of IL-21 in the expansion of Tr1 cells using naïve CD4+ T cells from IL-21R-deficient mice as the loss of IL-21 signaling also abrogated the generation of Tr1 cells even in the presence of IL-27. In contrast to recent studies using total CD4 splenocytes (Spolski and others 2009), it was noteworthy that the addition of recombinant IL-21 alone (without IL-27) on cell-sorted naïve CD4+ T cells was unable to generate Tr1 cells. This shows that IL-21 is necessary but not sufficient to induce the generation of Tr1 cells and suggested that additional molecular signals triggered by IL-27 are required for Tr1 differentiation.
Transcriptional Regulation of Tr1 Cells Generated with IL-27
Avian musculoaponeurotic fibrosarcoma v-maf
To identify the molecular signals that coordinately act together with IL-21 for the differentiation of Tr1 cells, we performed a microarray analysis of naïve T cells generated in the presence of IL-27 and found that the transcription factor avian musculoaponeurotic fibrosarcoma v-maf (c-Maf ) was induced by IL-27 in Tr1 cells. c-Maf belongs to a growing family of basic leucine zipper transcription factors (Blank and others 1997) and was first proposed to be essential for IL-4 production and differentiation of Th2 cells (Ho and others 1998; Hwang and others 2002). Subsequent studies have shown that the engagement of the Stat3 pathway by IL-6 leads to increased c-Maf expression as Stat3 transactivates the c-Maf promoter (Yang and others 2005). Our previous work had revealed that c-Maf was strongly upregulated in naïve T-cells differentiated into Th17 cells with TGF-β and IL-6 (Bauquet and others 2009). The role of c-Maf in different population of CD4+ T cells has been recently reassessed, and it has been proposed that c-Maf expression, in addition to inducing IL-4 in Th2 cells, was necessary for IL-10 expression in multiple CD4+ T helper subsets (Saraiva and others 2009). These results are supported by the study of Xu and others (2009), who showed that c-Maf directly transactivates the il10 promoter through binding to a conserved binding site (half Maf recognition element [MARE] located 500 bp upstream of the transcription initiation site in the il10 promoter).
Tr1 cells differentiated for 4 days in the presence of IL-27 had 500-fold higher levels of c-Maf expression than Th1, Th2, or Th0 cells reaching similar levels to Th17 cells. Upon monitoring the kinetics of c-Maf expression during the differentiation of Tr1 cells with IL-27, we noted that c-Maf expression could be detected as early as 12 h after the initiation of the differentiation. To investigate the role of c-Maf in the induction of Tr1 cells, we differentiated either wild-type or c-Maf-deficient naïve CD4+ T cells into Tr1 cells with IL-27. Strikingly, we noted that the absence of c-Maf greatly impaired the generation of IL-10-producing Tr1 cells, suggesting that c-Maf is critically involved in the generation of Tr1 cells (Pot and others 2009).
Given that we had previously shown that c-Maf is controlling IL-21 expression in Th17 cells, we hypothesized that c-Maf might induce Tr1 cells by transactivating il21 promoter. This contention was further supported by our observations, indicating that c-Maf and IL-21 were concomitantly expressed during Tr1 cell differentiation, suggesting that c-Maf could be a transcription factor for inducing IL-21 gene transcription. We thus investigated whether c-Maf could possibly bind to the il21 promoter and we identified 4 putative conserved binding sites located 1070 bp (half MARE), 370 bp (v-MARE), 260 bp (half MARE), and 200 bp (v-MARE) upstream of the transcriptional start site (where MARE is Maf recognition element and v-MARE is v-Maf recognition element). The functional relevance of those sites for the binding of c-Maf was then verified by cotransfecting of HEK 293T cells with a c-Maf expression plasmid and an il21 promoter-luciferase reporter. In this setting, we demonstrated that c-Maf could transactivate the il21 promoter-luciferase in a dose-dependent manner, supporting the hypothesis that c-Maf expands Tr1 cells by inducing IL-21 production. We finally showed that while c-Maf-deficient T cells activated in the presence of IL-27 featured a sustained production of IFNγ, the cells were unable to secrete IL-21 and IL-10. Collectively, these results established that c-Maf, by transactivating il21 and il10 promoters, is dictating the generation of IL-27-induced IL-10-producing Tr1 cells.
Inducible T-cell Costimulatory Molecule
The inducible costimulatory molecule (ICOS), a member of the CD28/CTLA4 family, was initially reported to be a costimulatory molecule responsible for the induction of IL-10 (Hutloff and others 1999). However, later ICOS was found to be expressed on activated T cells and has been shown to play an essential role in the expansion of all T cells in that T cell activation, growth, and survival are defective in the absence of ICOS. In addition, the finding that ICOS-deficient mice showed greatly enhanced susceptibility to EAE indicated that ICOS has a protective role in inflammatory autoimmune diseases (Dong and others 2001). Expression of ICOS in vivo by CD4+ T cells has been associated to their high secretion of IL-10 (Lohning and others 2003).
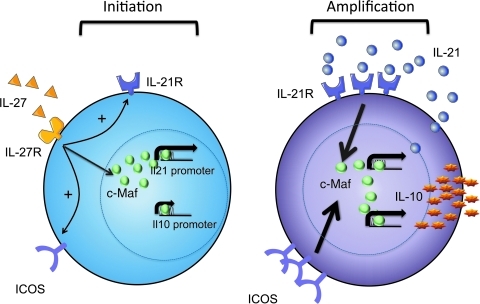
We have recently identified a role for ICOS in generation of Th17 cells. While ICOS was not essential for the differentiation of Th17 cells, we showed that ICOS-deficient mice demonstrated a defect in sustained expansion of Th17 cells after IL-23 stimulation. We could ascribe this defect as an essential function of ICOS to maintain a sustained level of c-Maf and thus continued IL-21 expression during Th17 differentiation (Bauquet and others 2009). We later found that ICOS also played an important role in stabilizing the phenotype of Tr1 cells. We first noticed that IL-27 upregulated ICOS expression in Tr1 cells (Pot and others 2009). Using ICOS-deficient T cells, we showed that the generation of IL-10-producing T cells was impaired at late stages of differentiation compared to wild-type T cells. Altogether, these findings led us to propose that ICOS, like in Th17 cells, in fact maintains c-Maf expression and promotes Tr1 cell differentiation. Our results would therefore suggest that ICOS signaling stabilizes IL-10 and IL-21 expression by maintaining c-Maf expression independently of IL-27 signaling. On the basis of our data we propose a model of self-sustainable generation of Tr1 cells in which an innate cytokine like IL-27 secreted by DCs first induces differentiation of naïve T cells into Tr1 cells. Subsequent expression of ICOS and IL-21 by differentiated Tr1 cells enables them to maintain their phenotype in the absence of IL-27. Overall, the differentiation of IL-27-induced Tr1 cells is therefore dependent on the concerted action of three different molecules, c-Maf, ICOS, and IL-21 (Pot and others 2009) (Fig. 1).
FIG. 1.
Molecular events leading to the generation of interleukin (IL)-27-induced type 1 regulatory (Tr1) cells. Treatment of naïve CD4+ T cells with IL-27 enhances expression of the transcription factor avian musculoaponeurotic fibrosarcoma v-maf (c-Maf ), IL-21 receptor, and inducible costimulatory molecule (ICOS), which are all essential for the differentiation of Tr1 cells. Upon induction of Tr1 cell differentiation (initiation phase), c-Maf transactivates the il21 and il10 promoters (left panel). During Tr1 cell expansion (amplification phase), IL-10 and IL-21 expression become independent of IL-27/IL-27R signaling as ICOS and IL-21 signaling maintain c-Maf expression in differentiating Tr1 cells (right panel).
Clinical Implications of IL-27- and IL-27-Induced Tr1 Cells
Four single-nucleotide polymorphisms within the human IL-27 gene have been associated with an enhanced susceptibility to asthma (Chae and others 2007) and inflammatory bowel disease (Li and others 2009), suggesting an important role of IL-27 in human autoimmune diseases. However, the in vivo role of IL-27 in humans is poorly understood. In a recent study, Murugaiyan and others (2009) showed that IL-27 can induce IL-10-producing Tr1 cells from human's PBMC that can inhibit the proliferation of CD4+ T cells in an IL-10-dependent manner. Similar to murine T cells, IL-27 was shown to regulate various molecules associated with the function and maintenance of Th17 cells. Indeed, IL-27 1) substantially reduced expression of the Th17 lineage-specific transcription factor Rorc; 2) decreased the production of the signature Th17 cytokines IL-17A, IL-17F, and IL-22; 3) reduced expression of IL-23R, which is responsible for the maintenance of Th17 lineage; and 4) modulated expression of the chemokine CCL20 and its receptor CCR6. IL-27 was also shown to inhibit IL-1β, IL-6, and IL-23 secretion from DCs and thereby impair the generation of Th17 cells (Murugaiyan and others 2009). These results confirm previous findings obtained in rodents that indicate that IL-27 not only induces Tr1 cells but also decreases the pathogenicity of Th17 cells (Fitzgerald and others 2007a). In conclusion, IL-27 appears to be a very potent suppressive cytokine that inhibits generation of Th17 cells and concomitantly induce Tr1 cells both in humans and in rodents.
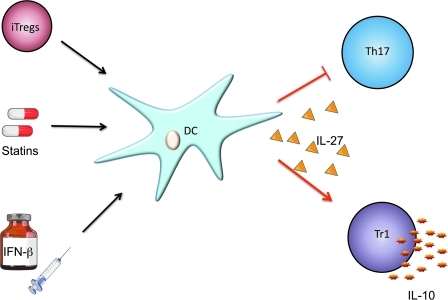
Another interesting aspect of IL-27 lies in the ability of this cytokine to be produced directly at the site of inflammation. Li and others (2005) reported that IL-27 is secreted by antigen-presenting cells (APCs) at the inflammatory interface between pathogenic cells and vulnerable neurons in the CNS of mice undergoing EAE. Further, microglia cells, resident brain APCs, have also been shown to secrete IL-27 (Sonobe and others 2005). While FoxP3+ Tregs fail to control autoimmune inflammation in the brain (Korn and others 2007b), this observation raises the possibility that IL-27 may be produced at the inflammation site and inhibit tissue damage by concomitantly dampening the proinflammatory functions of Th17 cells and promoting in situ differentiation of Tr1 cells (Fig. 2). This is further supported by the work from our laboratory that showed that tolerogenic, IL-27-secreting DCs could be induced by FoxP3+ Tregs. FoxP3+ Tregs are not able to readily suppress the activated effector cells from the brain during CNS inflammation, but the effector T cells eventually decrease with commensurate increase in FoxP3− IL-10-producing T cells that are phenotypically similar to Tr1 cells, resulting in resolution of inflammation. Tr1 cells may therefore play a crucial role in resolving inflammation and inducing recovery in an autoimmune disease.
FIG. 2.
Immunomodulation of in vivo inflammatory responses by interleukin (IL)-27. Induced Foxp3+ Tregs, interferon (IFN)-β, or statins induce the secretion of IL-27 from dendritic cells (DCs) that will in turn inhibit proinflammatory T helper 17 (Th17) cells and enhance the development of type 1 regulatory (Tr1) cells, resulting in the resolution of autoimmune inflammation.
The induction of IL-27 from DC is further exemplified in humans where IFN-β, a therapy approved for MS, increases induction of IL-27 from human DCs (Fig. 2) (Zhang and others 2009). This phenomenon has also been observed with other MS treatments such as statins, which are extensively used as cholesterol-lowering agents and were recently attributed with antiinflammatory properties (Fig. 2). Indeed, Zhang and others (2008) have shown that one of the statins, simvastatin, has the ability to increase IL-27 secretion from human monocytes of MS patients, suggesting that IL-27 may play a key role in mediating the antiinflammatory effects after this treatment. This raises the possibility that IL-27 may be an effective target that could be exploited for treatment of many immune-mediated diseases.
Conclusions and Perspectives
IL-27 was initially characterized in 2002 as a cytokine that induces the proliferation of naïve CD4+ cells. Although IL-27 was initially categorized as a proinflammatory cytokine that induces Th1 differentiation, pioneering work from Chris Hunter's laboratory later established that this cytokine in fact suppresses excessive immune responses. Over the last 3 years, mechanisms by which IL-27 mediates immunosuppression have been identified. First, IL-27 is a critical mediator secreted by tolerogenic DC that suppresses effector Th17 cells and supports the in vivo generation of regulatory IL-10-producing Tr1 cells. Second, molecular studies of IL-27-induced Tr1 cells revealed that the transcription factor c-Maf, ICOS, and IL-21 are critical for the generation of Tr1 cells in vitro and in vivo.
The lineage-specific transcription factors T-bet, Gata-3, Rorc, and Foxp3 have been associated with differentiation of Th1, Th2, Th17, and Treg cells, respectively. However, to the best of our knowledge, no lineage-specification transcription factor has been identified for Tr1 cells. Therefore, in contrast to FoxP3+ Tregs, which can be induced by TGF-β and then characterized by expression of the FoxP3 protein, the analysis of molecular mechanisms of Tr1 cells is hampered by the numerous variations between the experimental procedures and inability to produce Tr1 cells in large quantities. In this regard, the finding that IL-27 induces IL-10-producing Tr1 cells provides a simple method to obtain Tr1 cells for both molecular and functional analysis. This procedure would be advantageous over currently used conditions that involve the addition of feeder cells and multiple cytokines to generate Tr1 cells (Brun and others 2009). In conclusion, there is a substantial amount of evidence showing that cytokine-mediated therapy has an important place in the treatment of autoimmune diseases, including MS, and we believe that IL-27 may be exploited in the treatment of many autoimmune disorders based on its strong antiinflammatory properties. IL-27 can thus be considered as an attractive target, but the short in vivo half-life of this cytokine might hinder its clinical application. Therefore, a detailed understanding of the different IL-27-induced pathways will permit the discovery of new potential therapeutic targets downstream of IL-27.
Acknowledgments
This work was supported by grants from the National Institutes of Health (P01NS038037, R37NS030843, P01AI045757, P01AI056299). C.P. is supported by the Swiss National Science Foundation (FSBMB) and the Novartis Foundation, and L.A. by the European Molecular Biology Organization.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahangarani RR. Janssens W. VanderElst L. Carlier V. VandenDriessche T. Chuah M. Weynand B. Vanoirbeek JA. Jacquemin M. Saint-Remy JM. In vivo induction of type 1-like regulatory T cells using genetically modified B cells confers long-term IL-10-dependent antigen-specific unresponsiveness. J Immunol. 2009;183(12):8232–8243. doi: 10.4049/jimmunol.0901777. [DOI] [PubMed] [Google Scholar]
- Artis D. Johnson LM. Joyce K. Saris C. Villarino A. Hunter CA. Scott P. Cutting edge: Early IL-4 production governs the requirement for IL-27-WSX-1 signaling in the development of protective Th1 cytokine responses following Leishmania major infection. J Immunol. 2004;172(8):4672–4675. doi: 10.4049/jimmunol.172.8.4672. [DOI] [PubMed] [Google Scholar]
- Asao H. Okuyama C. Kumaki S. Ishii N. Tsuchiya S. Foster D. Sugamura K. Cutting edge: The common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167(1):1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- Astier AL. Meiffren G. Freeman S. Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116(12):3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A. Carrier Y. Peron JP. Bettelli E. Kamanaka M. Flavell RA. Kuchroo VK. Oukka M. Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8(12):1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Bacchetta R. Bigler M. Touraine JL. Parkman R. Tovo PA. Abrams J. de Waal Malefyt R. de Vries JE. Roncarolo MG. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179(2):493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ. Cua DJ. Boonstra A. Richards DF. Crain C. Savelkoul HF. de Waal-Malefyt R. Coffman RL. Hawrylowicz CM. O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195(5):603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M. Stabilini A. Draghici E. Gregori S. Mocchetti C. Bonifacio E. Roncarolo MG. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55(1):40–49. [PubMed] [Google Scholar]
- Batten M. Kljavin NM. Li J. Walter MJ. de Sauvage FJ. Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180(5):2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- Batten M. Li J. Yi S. Kljavin NM. Danilenko DM. Lucas S. Lee J. de Sauvage FJ. Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7(9):929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- Bauquet AT. Jin H. Paterson AM. Mitsdoerffer M. Ho IC. Sharpe AH. Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10(2):167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E. Das MP. Howard ED. Weiner HL. Sobel RA. Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161(7):3299–3306. [PubMed] [Google Scholar]
- Blank V. Kim MJ. Andrews NC. Human MafG is a functional partner for p45 NF-E2 in activating globin gene expression. Blood. 1997;89(11):3925–3935. [PubMed] [Google Scholar]
- Brun V. Bastian H. Neveu V. Foussat A. Clinical grade production of IL-10 producing regulatory Tr1 lymphocytes for cell therapy of chronic inflammatory diseases. Int Immunopharmacol. 2009;9(5):609–613. doi: 10.1016/j.intimp.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Chae SC. Li CS. Kim KM. Yang JY. Zhang Q. Lee YC. Yang YS. Chung HT. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52(4):355–361. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- Chen Q. Ghilardi N. Wang H. Baker T. Xie MH. Gurney A. Grewal IS. de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407(6806):916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R. Haanen J. Spits H. Roncarolo MG. te Velde A. Figdor C. Johnson K. Kastelein R. Yssel H. de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. Juedes AE. Temann UA. Shresta S. Allison JP. Ruddle NH. Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409(6816):97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC. Ciric B. Touil T. Harle H. Grammatikopolou J. Das Sarma J. Gran B. Zhang GX. Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007a;179(5):3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC. Zhang GX. El-Behi M. Fonseca-Kelly Z. Li H. Yu S. Saris CJ. Gran B. Ciric B. Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007b;8(12):1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- Grossman WJ. Verbsky JW. Tollefsen BL. Kemper C. Atkinson JP. Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104(9):2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- Groux H. O'Garra A. Bigler M. Rouleau M. Antonenko S. de Vries JE. Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Hamano S. Himeno K. Miyazaki Y. Ishii K. Yamanaka A. Takeda A. Zhang M. Hisaeda H. Mak TW. Yoshimura A. Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19(5):657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- Hibbert L. Pflanz S. De Waal Malefyt R. Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23(9):513–22. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- Ho IC. Lo D. Glimcher LH. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J Exp Med. 1998;188(10):1859–1866. doi: 10.1084/jem.188.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C. Holscher A. Ruckerl D. Yoshimoto T. Yoshida H. Mak T. Saris C. Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174(6):3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- Hutloff A. Dittrich AM. Beier KC. Eljaschewitsch B. Kraft R. Anagnostopoulos I. Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Hwang ES. White IA. Ho IC. An IL-4-independent and CD25-mediated function of c-maf in promoting the production of Th2 cytokines. Proc Natl Acad Sci USA. 2002;99(20):13026–13030. doi: 10.1073/pnas.202474499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T. Bettelli E. Gao W. Awasthi A. Jager A. Strom TB. Oukka M. Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007a;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T. Reddy J. Gao W. Bettelli E. Awasthi A. Petersen TR. Backstrom BT. Sobel RA. Wucherpfennig KW. Strom TB. Oukka M. Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007b;13(4):423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R. Lohler J. Rennick D. Rajewsky K. Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Leonard WJ. Spolski R. Interleukin-21: A modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5(9):688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- Levings MK. Sangregorio R. Galbiati F. Squadrone S. de Waal Malefyt R. Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166(9):5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- Li CS. Zhang Q. Lee KJ. Cho SW. Lee KM. Hahm KB. Choi SC. Yun KJ. Chung HT. Chae SC. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J Gastroenterol Hepatol. 2009;24(10):1692–1696. doi: 10.1111/j.1440-1746.2009.05901.x. [DOI] [PubMed] [Google Scholar]
- Li J. Gran B. Zhang GX. Rostami A. Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;232(1–2):3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Lohning M. Hutloff A. Kallinich T. Mages HW. Bonhagen K. Radbruch A. Hamelmann E. Kroczek RA. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197(2):181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S. Ghilardi N. Li J. de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100(25):15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke K. Qian Y. Knop J. Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101(12):4862–4869. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- Maynard CL. Harrington LE. Janowski KM. Oliver JR. Zindl CL. Rudensky AY. Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8(9):931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- Moore KW. de Waal Malefyt R. Coffman RL. O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Moore KW. Vieira P. Fiorentino DF. Trounstine ML. Khan TA. Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Murugaiyan G. Mittal A. Lopez-Diego R. Maier LM. Anderson DE. Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183(4):2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R. Yang XO. Martinez G. Zhang Y. Panopoulos AD. Ma L. Schluns K. Tian Q. Watowich SS. Jetten AM. Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Ochando JC. Homma C. Yang Y. Hidalgo A. Garin A. Tacke F. Angeli V. Li Y. Boros P. Ding Y. Jessberger R. Trinchieri G. Lira SA. Randolph GJ. Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- Parrish-Novak J. Dillon SR. Nelson A. Hammond A. Sprecher C. Gross JA. Johnston J. Madden K. Xu W. West J. Schrader S. Burkhead S. Heipel M. Brandt C. Kuijper JL. Kramer J. Conklin D. Presnell SR. Berry J. Shiota F. Bort S. Hambly K. Mudri S. Clegg C. Moore M. Grant FJ. Lofton-Day C. Gilbert T. Rayond F. Ching A. Yao L. Smith D. Webster P. Whitmore T. Maurer M. Kaushansky K. Holly RD. Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408(6808):57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Pearl JE. Khader SA. Solache A. Gilmartin L. Ghilardi N. deSauvage F. Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173(12):7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- Pflanz S. Hibbert L. Mattson J. Rosales R. Vaisberg E. Bazan JF. Phillips JH. McClanahan TK. de Waal Malefyt R. Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172(4):2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- Pflanz S. Timans JC. Cheung J. Rosales R. Kanzler H. Gilbert J. Hibbert L. Churakova T. Travis M. Vaisberg E. Blumenschein WM. Mattson JD. Wagner JL. To W. Zurawski S. McClanahan TK. Gorman DM. Bazan JF. de Waal Malefyt R. Rennick D. Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Pot C. Jin H. Awasthi A. Liu SM. Lai CY. Madan R. Sharpe AH. Karp CL. Miaw SC. Ho IC. Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183(2):797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG. Gregori S. Battaglia M. Bacchetta R. Fleischhauer K. Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Rosas LE. Satoskar AA. Roth KM. Keiser TL. Barbi J. Hunter C. de Sauvage FJ. Satoskar AR. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168(1):158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M. Christensen JR. Veldhoen M. Murphy TL. Murphy KM. O'Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31(2):209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonobe Y. Yawata I. Kawanokuchi J. Takeuchi H. Mizuno T. Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040(1–2):202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- Spolski R. Kim HP. Zhu W. Levy DE. Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182(5):2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer JS. Laurence A. Wilson EH. Huang E. Tato CM. Johnson LM. Villarino AV. Huang Q. Yoshimura A. Sehy D. Saris CJ. O'Shea JJ. Hennighausen L. Ernst M. Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS. Silver JS. Laurence A. Porrett PM. Harris TH. Turka LA. Ernst M. Saris CJ. O'Shea JJ. Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8(12):1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Takeda A. Hamano S. Yamanaka A. Hanada T. Ishibashi T. Mak TW. Yoshimura A. Yoshida H. Cutting edge: Role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170(10):4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- Villarino A. Hibbert L. Lieberman L. Wilson E. Mak T. Yoshida H. Kastelein RA. Saris C. Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19(5):645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Wei L. Laurence A. Elias KM. O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282(48):34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K. Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11(1):7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- Xu J. Yang Y. Qiu G. Lal G. Wu Z. Levy DE. Ochando JC. Bromberg JS. Ding Y. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182(10):6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Ochando J. Yopp A. Bromberg JS. Ding Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J Immunol. 2005;174(5):2720–2729. doi: 10.4049/jimmunol.174.5.2720. [DOI] [PubMed] [Google Scholar]
- Yoshida H. Hamano S. Senaldi G. Covey T. Faggioni R. Mu S. Xia M. Wakeham AC. Nishina H. Potter J. Saris CJ. Mak TW. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15(4):569–78. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- Zhang X. Jin J. Peng X. Ramgolam VS. Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180(10):6988–6996. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- Zhang X. Jin J. Tang Y. Speer D. Sujkowska D. Markovic-Plese S. IFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol. 2009;182(6):3928–3936. doi: 10.4049/jimmunol.0802226. [DOI] [PubMed] [Google Scholar]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]