Abstract
A randomized, controlled clinical trial was started in the pre-HAART era to compare the efficacy of zidovudine (AZT) and interferon-alpha (IFN-α) either alone or in combination to reduce HIV viremia, maintain CD4+ cell count, and decrease time to AIDS progression and death. The purpose of the current study was to compare the effects of AZT and IFN on HIV load using modern technology. One hundred and eighty patients with CD4+ counts above 500 cells/mm3 were randomized to receive AZT alone, IFN-α alone, or AZT and IFN-α in combination. CD4+ cell count and HIV load at baseline and at the last follow-up visit were compared, and time to AIDS or death was calculated by treatment group. At a mean follow-up of 45 weeks, the mean change in log HIV RNA was −0.06 for the AZT alone group, −0.47 for the AZT plus IFN-α group (P = 0.01 versus AZT group), and −0.35 for the IFN-α alone group (P = 0.02 versus AZT group). There was no significant difference among groups in change in total CD4+ count or in time to AIDS or death. Since treatment with IFN-α produces significant decreases in HIV load, additional studies of IFN-α as part of a combination regimen are warranted.
Introduction
Interferon-alpha (IFN-α) has antiviral properties that were recognized years before the beginning of the AIDS pandemic. In vitro studies have shown that IFN-α is capable of inhibiting HIV replication in both acutely and chronically infected cells by interfering with the assembly and budding of new HIV virions, reducing levels of reverse transcriptase activity, and down-regulating gene expression of the CCR5 and CXCR4 chemokine receptors, thus inhibiting viral entry into the host cell (Pitha 1994; Brassard and others 2002; Stylianou and others 2002). The first in vivo studies of IFN-α in immunosuppressed HIV-infected patients with Kaposi's sarcoma (KS) demonstrated that IFN-α is capable of inducing tumor regression in KS lesions, and these early studies also demonstrated evidence of anti-HIV activity as reflected in a drop in p24 antigen levels (Krown and others 1983; Kovacs and others 1989; Shepherd and others 1998). These effects were most pronounced in patients with high CD4+ T-lymphocyte counts.
In 1988, a randomized controlled study of 180 patients with CD4+ T-lymphocyte counts of at least 500 cells/μL was undertaken to compare the effects of zidovudine (AZT) and IFN-α either alone or in combination with HIV load, CD4+ T-lymphocyte count, and clinical disease progression. At the initiation of the trial, HIV load was determined using a commercially available p24 antigen assay. The purpose of this current study was to compare the effects of IFN-α on HIV load in prospectively collected serum samples using the advanced virologic quantitation methods that are now available.
Materials and Methods
The study was conducted at the National Institute of Allergy and Infectious Diseases (NIAID) HIV Outpatient Clinic, Warren Grant Magnuson Clinical Center, National Institutes of Health (NIH), Bethesda, Maryland. The NIAID Institutional Review Board reviewed and approved the protocol and all participants provided written consent prior to participation.
Study population
Patients were at least 18 years of age with CD4+ T-lymphocyte counts of at least 500 cells/mm3 and no clinically significant findings on hematology and chemistry profiles, physical exam, urinalysis, chest X-ray, electrocardiogram, and echocardiogram. Although HCV testing was not available when this study was launched, patients had no clinically significant elevations in liver enzymes at study entry. HIV infection was documented by a positive enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot. Exclusion criteria included a current or prior history of an AIDS-defining opportunistic infection, malignancy other than KS, or any history of significant depression.
Intervention
Study participants were randomized to 1 of 3 treatment arms: AZT (Retrovir™; Glaxo Wellcome, Research Triangle Park, NC) 200 mg orally every 4 h; recombinant IFN-α-2b (IFN-α; Intron-A, Schering-Plough, Kenilworth, NJ) at a starting dose of 1 MIU subcutaneously daily, or AZT in combination with IFN-α-2b. As more information became available on AZT dosing, patients were offered the option of alternative dosing schedules. IFN-dose escalations were permitted in increments of 2.5 MIU every 2 weeks to a maximum dose of 35 MIU. IFN-dose reductions were permitted for laboratory or clinical toxicity. Study participants were initially monitored weekly to attain a stable, tolerable dosing regimen of IFN-α. Thereafter, participants were followed every 2 weeks through week 52. In the long-term follow-up phase of the study, participants were seen every 6 to 12 months. Clinical and laboratory monitoring included interim medical history and physical examinations, hematology and chemistry profiles, urinalysis, chest X-ray, electrocardiography, and echocardiography.
CD4+ cell and HIV load evaluations
Serum samples collected at weeks 0, 4, 8, 16, 24, and 48 were tested for HIV RNA levels [Bayer branched DNA (bDNA) Quantiplex assay version 3.0; lower limit of detection = 50 copies/mL; an HIV RNA level of 49 copies/mL was used for values below this limit]. Baseline viral load was defined as the last available viral load measure before starting treatment. The last viral load visit was defined as the last visit within 52 weeks after initiation of treatment at which a serum sample was tested. Samples collected within the first 52 weeks of study participation were used to determine absolute counts and percentage of CD4+ T-lymphocyte counts. Baseline CD4+ T-lymphocyte count was defined as the last available measure before starting treatment. The last CD4+ T-lymphocyte count visit was defined as the last visit within the first 52 weeks of study participation at which time a sample was tested. Information on AIDS progression and death was collected through January 2009.
Phenotypic analysis of AZT resistance
AZT resistance was evaluated using a phenotypic assay using a subset of study participants that had samples available. Virus was isolated from 14 participants receiving AZT alone, 12 participants receiving AZT+IFN-α, and 5 patients receiving IFN-α alone at 60 weeks of follow-up. MT-2 cells (4 × 106) were infected with 2,500 TCID50 of HIV-1 for 2 h, washed twice, and resuspended in RPMI 1640 to a density of 4 × 105/mL, and 200-mL aliquots were plated in each well in 96-well plates. Each virus was evaluated at each of 8 concentrations of AZT in quadruplicate. The infected cells were cultured at 37°C for 7 days; virus replication was assessed by measuring the amount of p24 in the culture supernatant; and the 90% inhibitory concentrations (IC90) was determined.
Safety assessments
To assess side effects of the study interventions, interim medical histories, physical exams, hematology and chemistry profiles, and urinalyses were performed at weeks 0, 4, 8, 16, 24, and 48.
Results
One hundred and eighty participants were enrolled in the study between October 1988 and August 1991. Ninety-five percent of the cohort was male, 84% was white, and the mean age was 33. Forty-seven percent of the cohort had asymptomatic HIV infection.
Virologic outcomes
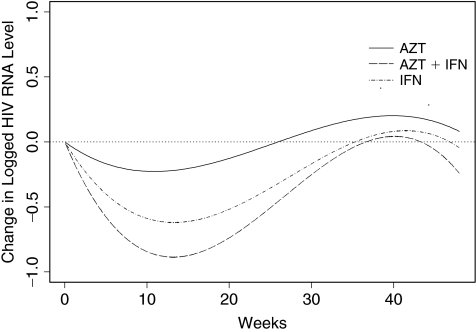
Among the 174 patients with baseline viral load measurements, 162 had at least one follow-up viral load value. Table 1 summarizes the HIV RNA levels at baseline and the changes from baseline at the last follow-up visit by treatment group. The difference among groups in mean duration of follow-up was not statistically significant. Levels of HIV RNA declined 0.06 log in the AZT group, 0.35 log in the IFN-α group, and 0.47 log in the combination group (P = 0.01 for comparison among the 3 groups). Pairwise comparisons demonstrate that the reduction in log HIV RNA was significantly smaller in the AZT group compared to the IFN-α alone group and the combined treatment group (P = 0.02 and 0.01, respectively). The reduction from baseline in HIV RNA level was highly significant in the IFN-α group (P = 0.002) and in AZT+IFN-α therapy group (P = 0.004), but not in the AZT group (P = 0.33). The degree of HIV RNA decrease in the IFN-α monotherapy and the AZT+IFN-α combination groups were not significantly different (P = 0.5). After adjusting for baseline log HIV RNA, baseline absolute CD4+ T-lymphocyte count, and baseline CD4+ T-lymphocyte percentage in a regression model, the reduction in HIV RNA in the AZT monotherapy group was smaller than the reduction in the IFN-α monotherapy group (P = 0.02) or in the AZT+IFN-α combination group (P = 0.003). Figure 1 shows the model-based mean change in log HIV RNA level by treatment, using a random effects model with a cubic function of time, treatment indicators, and their interactions.
Table 1.
Change in HIV RNA and CD4+ T-Lymphocyte Count
| AZT (N = 58) | IFN-α (N = 54)a | AZT+IFN-α (N = 54)a | P valueb | |
|---|---|---|---|---|
| Virologic | ||||
| Baseline log viral load (copies/mL) | 3.51 ± 0.09 | 3.57 ± 0.11 | 3.66 ± 0.11 | 0.56 |
| Time to last viral load visit (weeks) | 47.5 ± 0.5 | 44.9 ± 1.7 | 45.2 ± 1.3 | 0.20 |
| Difference in log HIV RNA (copies/mL) | −0.06 ± 0.06 | −0.35 ± 0.09 | −0.47 ± 0.12 | 0.01 |
| Standardized area under curve for log HIV RNA | −0.02 ± 0.01 | −0.27 ± 0.02 | −0.41 ± 0.02 | 0.0001 |
| % Baseline HIV RNA <50 (copies/mL) | 1.7% | 3.9% | 1.9% | 0.75 |
| % Last HIV RNA <50 (copies/mL) | 3.5% | 13.5% | 13.5% | 0.09 |
| Immunologic | ||||
| Baseline CD4 count | ||||
| Absolute | 744 ± 27 | 686 ± 23 | 790 ± 35 | 0.08 |
| Percentage | 37.3 ± 1.0 | 37.1 ± 1.3 | 38.1 ± 1.2 | 0.46 |
| Time to last CD4 count (weeks) | 47.3 ± 0.5 | 44.4 ± 1.5 | 45.6 ± 1.1 | 0.77 |
| Change in CD4 count | ||||
| Absolute | −40 ± 22 | −102 ± 24 | −97 ± 28 | 0.13 |
| Percentage | 0.6 ± 0.6 | −1.5 ± 1.0 | −1.1 ± 0.7 | 0.18 |
Values for continuous variables are expressed as mean ± standard error, and are expressed as percentages for binary variables.
N = 52 in viral load analysis.
P values for comparing continuous variables are derived by using the Kruskal–Wallis test and P values for comparing binary variables are derived by using the χ2-test; P values <0.05 represent a significant difference among the 3 groups.
Abbreviations: AZT, zidovudine; IFN-α, interferon-α.
FIG. 1.
Model-based mean change in log HIV RNA. Abbreviations: AZT, zidovudine; IFN-α, interferon-α.
Immunologic outcomes
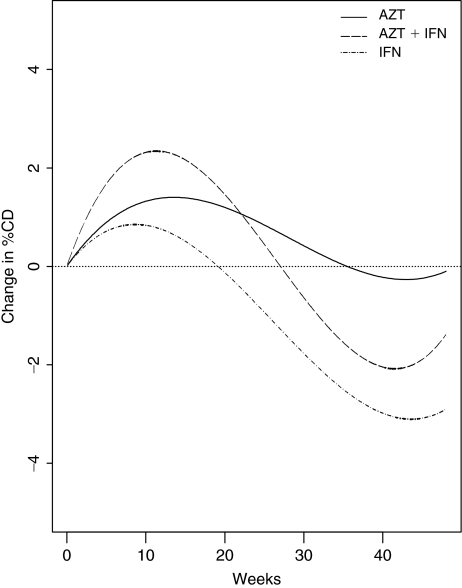
Table 1 summarizes the absolute and percentage CD4+ T-lymphocyte count at baseline and the change from baseline at the last follow-up visit by treatment group. There was no significant difference among groups in change in absolute or percentage CD4+ T-lymphocyte count. Figure 2 shows the model-based mean change in percentage CD4+ T-lymphocyte count by treatment using a linear mixed effects model with a cubic function of time, treatment indicators, and their interactions. As reported in other studies, treatment with IFN-α is associated with a transient rise in CD4+ cell percentage.
FIG. 2.
Model-based mean change in percentage CD4+ T-lymphocyte count. Abbreviations: AZT, zidovudine; IFN-α, interferon-α.
Phenotypic AZT resistance
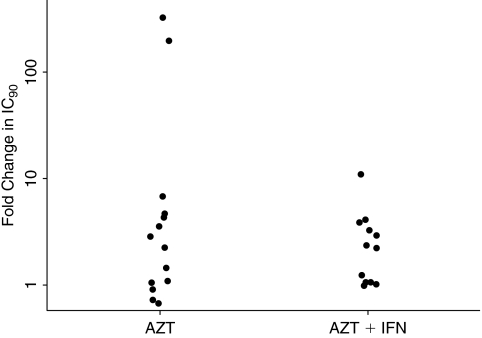
Figure 3 summarizes the IC90s of AZT for the viruses isolated in patients from the AZT alone and AZT+IFN-α combination group. For the AZT group, the average fold increase from baseline IC90 was 40, while the average fold increase in the AZT+IFN-α group was 3. The difference was due to 2 participants in the AZT group who developed high-level AZT resistance (203- and 327-fold increase in IC90). The median fold change, 2.5 and 2.6 for the AZT and AZT+IFN-α groups, respectively, was not statistically significant (P = 0.72).
FIG. 3.
AZT resistance: fold change in IC90 at week 60. *Fold change in IC90 = the ratio of IC90 at week 60 and IC90 at week 0. Abbreviations: AZT, zidovudine; IFN-α, interferon-α.
Safety and clinical outcomes
Side effects profiles for AZT and IFN-α within the first 12 months of follow-up were consistent with those previously cited in the literature. In the AZT alone group, 12 episodes of moderate to severe anemia, neutropenia, fatigue, nausea, or anorexia occurred. In the IFN-α alone arm, 20 episodes of moderate to severe fatigue, anorexia, nausea, elevated SGOT, or depression were reported. The AZT+IFN-α group had the most moderate to severe toxicities with 34 episodes of neutropenia, anemia, anorexia, or fatigue occurring within the first 12 months of follow-up (P = 0.01 for comparison among the 3 groups). At 15 years of follow-up, the observed event rates were not significantly different: 52% of patients in the AZT group, 50% in the IFN-α group, and 38% in the combination group demonstrated progression to AIDS or death (P = 0.26).
Discussion
Although IFN-α has been studied extensively in the setting of HIV infection—either as a potential treatment for HIV or as therapy for hepatitis C in co-infected individuals—there are few published data from controlled trials evaluating the effects of IFN-α on HIV load using modern methods of HIV quantitation. This randomized controlled trial demonstrates that IFN-α significantly decreases HIV level in a cohort with early HIV infection. Another randomized controlled trial used modern HIV quantitation techniques to study the effects of IFN-α-n1 (human lymphoblastoid interferon; Wellferon, Wellcome Laboratories, Beckenham, UK) in HIV-infected patients with CD4+ T-lymphocyte counts between 300 and 500 cells/μL (Haas and others 2000). That trial demonstrated that therapy with IFN-α-n1 in combination with AZT and zalcitabine (ddC) produced greater reductions in HIV load compared to AZT and ddC alone. IFN-α-n1 resembles human leukocyte IFN in that it is a mixture of IFN-α subtypes but differs in the proportions of these subtypes. IFN-α-n1 also differs from recombinant IFN-α preparations, such as the IFN-α-2b used in this study, which contain only a single subtype (Wellferon monograph 2009). Since IFN-α-n1 is no longer available in the United States while IFN-α-2b has broad approval and availability throughout the world for treating chronic hepatitis C infection, the data from the current study are particularly relevant.
Phenotypic resistance testing in a subset of study participants in the AZT and AZT+IFN-α groups revealed no statistically significant difference between groups in median change in IC90 at week 60. This analysis is limited by the small sample size, and resistance testing performed earlier in the course of the study may have shown a difference between groups. Of note, the 2 patients who developed high-level AZT resistance were both in the AZT alone group. Although caution must be taken in interpreting that finding, these data do suggest that more studies to determine the role of IFN-α in lowering viral load and impacting the emergence of viral resistance should be performed.
One of the major concerns with the use of IFN-α in the setting of HIV infection is the possibility that it may lower CD4+ T-lymphocyte counts and further compromise immunity (Yabrov 2000). This trial demonstrates that IFN-α significantly decreases HIV levels without lowering the percentage of CD4+ T-lymphocytes. Thus, although IFN-α may decrease total lymphocyte counts by means of antiproliferative activity on all lymphocytes, IFN-α does not appear to possess antiproliferative activity specific to CD4+ T lymphocytes.
Interest in IFN-α as a treatment for HIV infection has decreased because of the availability of potent, convenient antiretroviral drug regimens and because of IFN-α's well-known side-effect profile that includes flu-like symptoms, neutropenia, and depression. Nonetheless, viral mutations can render resistance to all classes of currently available antiretroviral drugs. Given its antiviral activity, additional studies of IFN-α as a component of a highly active antiretroviral regimen are warranted, particularly in patients with virological failure, higher CD4+ cells counts and limited or no options for optimal HIV suppression.
Acknowledgment
The authors are grateful to the study participants who made this clinical trial possible, and to the staff of the NIAID HIV Outpatient Clinic, Warren Grant Magnuson Clinical Center for their dedication and persistence.
Author Disclosure Statement
No competing financial interests exist.
References
- Brassard DL. Grace MJ. Bordens RW. Interferon-a as an immunotherapeutic protein. J Leukoc Biol. 2002;71:565–581. [PubMed] [Google Scholar]
- Haas DW. Lavelle J. Nadler JP. Greenberg SB. Frame P. Mustafa N. St Clair M. McKinnis R. Dix L. Elkins M. Rooney J. A randomized trial of interferon alpha therapy for HIV type 1 infection. AIDS Res Human Retro. 2000;16(3):183–190. doi: 10.1089/088922200309278. [DOI] [PubMed] [Google Scholar]
- Kovacs JA. Deyton L. Davey R. Falloon J. Zunich K. Lee D. Metcalf JA. Bigley JW. Sawyer LA. Zoon KC. Lane HC. Combined zidovudine and interferon-a therapy in patients with Kaposi sarcoma and the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1989;111(4):280–287. doi: 10.7326/0003-4819-111-4-280. [DOI] [PubMed] [Google Scholar]
- Krown SE. Real FX. Cunningham-Rundles S. Myskowski PL. Koziner B. Fein S. Mittelman A. Oettgen HF. Safai B. Preliminary observations on the effect of recombinant leukocyte A interferon in homosexual men with Kaposi's sarcoma. N Engl J Med. 1983;308:1071–1076. doi: 10.1056/NEJM198305053081806. [DOI] [PubMed] [Google Scholar]
- Pitha PM. Multiple effects of interferon on the replication of human immunodeficiency virus type 1. Antiviral Res. 1994;24(2–3):205–219. doi: 10.1016/0166-3542(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Shepherd FA. Beaulieu R. Gelmon K. Thuot CA. Sawka C. Read S. Singer J. Prospective randomized trial of two dose levels of interferon-alpha with zidovudine for the treatment of Kaposi's sarcoma associated with human immunodeficiency virus infection: a Canadian HIV Clinical Trials Network Study. J Clin Oncol. 1998;16:1736–1742. doi: 10.1200/JCO.1998.16.5.1736. [DOI] [PubMed] [Google Scholar]
- Stylianou E. Yndestad A. Sikkeland LI. Berkeli V. Damas JK. Haug T. Eiken HG. Aukrust P. Froland SS. Effects of interferon-alpha on gene expression of chemokines and members of the tumour necrosis factor superfamily in HIV-infected patients. Clin Exp Immunol. 2002;130:279–285. doi: 10.1046/j.1365-2249.2002.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellferonmonograph. 2009. www.rxmed.com/b.main/b2.pharma ceutical/b2.1.monographs/CPS-%20Monographs/CPS-%20(General%20Monographs-%20W)/WELLFERON.html www.rxmed.com/b.main/b2.pharma ceutical/b2.1.monographs/CPS-%20Monographs/CPS-%20(General%20Monographs-%20W)/WELLFERON.html
- Yabrov A. Is it hazardous to treat HIV patients with interferon-alpha? Med Hypotheses. 2000;54:131–136. doi: 10.1054/mehy.1998.0820. [DOI] [PubMed] [Google Scholar]