Abstract
Interleukin (IL)-27 is a member of IL-12 family cytokine. We have previously reported that IL-27 inhibits human immunodeficiency virus type-1 (HIV-1) replication in CD4+ T cells and monocyte-derived macrophages, even though IL-12 enhances HIV-1 replication in primary CD4+ T cells. Further study demonstrates that IL-27 induces antiviral genes including RNA-dependent protein kinase, oligoadenylate synthetase, and myxovirus protein in the same manner as interferon (IFN)-α. Neutralization assay using anti-IFN antibodies, real-time RT-PCR, and enzyme-linked immunosorbent assay demonstrated that IL-27 induces the antiviral genes without the induction of IFNs. IFN-α has been administered to hepatitis C virus (HCV)-infected patients as well as HCV/HIV-1 co-infected patients. Despite the improved immunotherapy, some patients are still failed to respond to the treatment. Since IL-27 induces IFN-α-like responses including the induction of antiviral genes, it was speculated that IL-27 may impact the replication of HCV. In this study, we evaluated the role of IL-27 on HCV replication using Huh7.5, an HCV permissive cell line. IL-27 induces STAT-1 and −3 in the cell line, and dose-dependently inhibited HCV. These data suggest that IL-27 may play a role in the development of a novel immunotherapeutic strategy for HCV and HCV/HIV co-infection.
Interleukin (IL)-27 is a member of the IL-12 family cytokines that consists of p28 and Epstein-Barr virus-induced gene 3 (EBI3) (Pflanz and others 2002; Kastelein and others 2007). The p28 chain is related to a subunit of IL-12 (IL-12p35) and has a classical cytokine structure, while the EBI3 is related with IL-12p40 and structurally resembles the soluble IL-6 receptor α chain. IL-27 binds to its receptor (IL-27R), which is composed of ligand-specific chain, IL-27 receptor α chain (IL-27Rα), and of gp130, a signal-transducing molecule shared with other cytokines, IL-6, IL-11, oncostatin M, and leukemia inhibitory factor (Pflanz and others 2004; Kastelein and others 2007). IL-27 is capable of binding to IL-27Rα in the absence of gp130; however, the co-expression of both receptor subunits is required to induce signal (Pflanz and others 2004). Most of studies using IL-27 have been conducted on T cells, B cells, monocytes, and natural killer cells and, upon ligand binding, phosphorylation of the signal transducers and activators of transcription protein (STAT)-1, −2, −3, −4, or −5 occurs (Lucas and others 2003; Kamiya and others 2004; Batten and Ghilardi 2007; Kastelein and others 2007). We and other group report that IL-27 inhibits human immunodeficiency virus type-1 (HIV-1) replication in CD4+ T cells and macrophages (Fakuruddin and others 2007; Imamichi and others 2008; Greenwell-Wild and others 2009) as interferon (IFN)-α does, although IL-12 enhances HIV-1 replication in CD4+ T cells (Foli and others 1995).
The mechanism of antiviral effect by IFN-α has been well investigated (Pestka and others 1987, 2004; Samuel 2001; Langer and others 2004; Galligan and others 2006). IFN-α is the only cytokine that suppresses HIV-1 replication in vivo (Lane 1991; Poli and others 1994; Brassard and others 2002) and it has been used in clinical therapy for hepatitis C virus (HCV) mono-infected and HCV/HIV co-infected patients (Carrat and others 2004; Chung and others 2004; Laguno and others 2004; Torriani and others 2004; Kottilil and others 2009), and co-infection with HCV is present in one-third of all HIV-infected individuals in the United States and is associated with rapid progression of liver fibrosis and poor response to IFN and ribavirin (Benhamou and others 1999; Alter 2006). An aberrant type-IIFN response seen exclusively in HIV-infected individuals could be responsible for the poor therapeutic response experienced by HCV/HIV co-infected individuals receiving IFN-α-based current standard of care, necessitating of the development of novel immunotherapeutic strategies (Lempicki and others 2006).
A recent study demonstrates that IL-27 displays anti-avian influenza virus properties in hepatoma cell line, HepG2 (Bender and others 2009). IL-27 induced phosphorylation of STAT-1 and −3 in these cells, and FACS analysis demonstrated that not only HepG2 but also a human hepatocyte cell line, PH5CH8 express IL-27 receptor on the cell surface. These data suggest that IL-27 may affect on HCV replication in hepatocytes. In this study, we evaluated the impact of IL-27 on HCV replication using Huh7.5 cell, an HCV permissive cell line (Blight and others 2002).
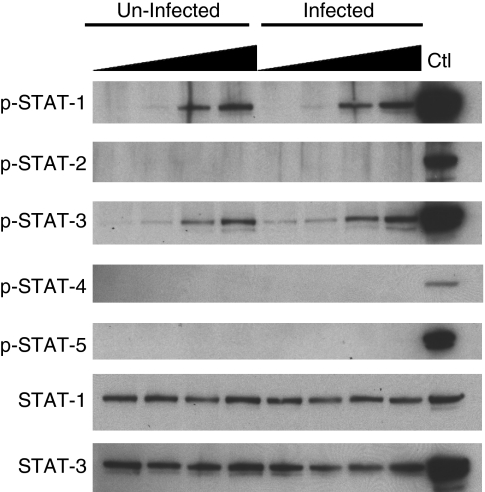
Since it has not been shown whether the Huh7.5 cell responds to IL-27, we analyzed a profile of STAT phosphorylation using HCV-uninfected and -infected Huh7.5 cell line. The HCV infection system including Huh7.5 cell line and the plasmid encoding full-length of infectious HCV J6/JFH1 gene was provided by Apath LLC (St. Louis, MO) (Lindenbach and others 2005; Wakita and others 2005). Cells were cultured in DMEM medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Hyclone, Logan UT) and 100 U/mL penicillin/100 μg/mL streptomycin (Invitrogen) at 37°C in a 5% CO2 incubator. In vitro transcription of HCV RNA using the HCV plasmid as a template and the HCV RNA transfection into Huh7.5 cells were performed following the protocol provided by the Apath LLC. Culture supernatants from the HCV RNA-transfected Huh7.5 cells were used as an HCV virus stock, HCV titters of the stock were determined by HCV RNA copy number in it using quantitative real-time RT-PCR. To obtain HCV-infected Huh7.5 cell, fresh Huh7.5 cells were infected with 2.3 million copies of HCV RNA per milliliter for 4 h. During the infection, >90% cells were infected with HCV (data not shown). The HCV-infected Huh7.5 cells and uninfected Huh7.5 cells were stimulated with different concentrations (0, 1, 10, or 100 ng/mL) of IL-27 (R&D systems, Minneapolis, MN) for 15 min at 37°C. After stimulation, reaction was stopped by using cold PBS and then whole cell lysate was prepared with radio-immunoprecipitation assay buffer containing phosphatase inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA) and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) as previously described (Imamichi and others 2008). Protein concentration was measured using BCA protein assay (Thermo Fisher Scientific) and total of 30 μg cellular proteins were separated on 4%–12% NuPAGE 4%–12% gel (Invitrogen), and then Western blotting analyses were performed using antibody to phosphorylated signal transducers and activators of transcription (STAT)-1, −2, −3, or −5 (Cell Signaling Technology, Danvers, MA) and phosphorylated STAT-4 (Invitrogen). Signals on the membranes were detected using HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) with the ECL-Plus kit (GE-Healthcare, Piscataway, NJ). For verification of the same protein expression level, antibodies were stripped from blots, and then the blots were re-probed with antibody to total STAT-1 or −3. As positive controls for phosphorylated STAT-2 and −4, PHA-stimulated CD4+ T cells were stimulated with 1,000 IU/mL of IFN-α (R&D Systems) and 40 ng/mL of IL-12 (R&D Systems), respectively (Kamiya and others 2004), and whole cell lysate was obtained from the cells as described above. The Western blot analysis revealed that IL-27 dose-dependently induced activation of STAT-1 and −3, but not STAT-2, −4, or −5 in Huh7.5 cells (Fig. 1), indicating that consistent with the STAT activation profile in HepG2 cell (Bender and others 2009), Huh7.5 cell maintains a functional IL-27 receptor and upon stimulation, it induces phosphorylation of STATs. Even though near 90% of cells were infected with HCV during infection, the viral infection indicated no significant impact on the STAT activation.
FIG. 1.
Interleukin (IL)-27 induces phosphorylation of signal transducers and activators of transcription protein (STAT)-1 and −3 in Huh7.5 cell. Whole cell lysate was prepared from hepatitis C virus (HCV)-infected and -uninfected Huh7.5 cells stimulated with different concentrations of IL-27 (0, 1, 10, and 100 ng/mL) for 15 min. As positive control for Western blot of STAT-1, −2, −3, and −5, whole cell lysate from 1,000 U/mL of interferon (IFN)-stimulated CD4+ T cells was used. A positive control of the activation of STAT-4, cell lysate from 40 ng/mL IL-12-stimulated T cells was used. Western blotting analyses were performed using antibody to phosphorylated STAT-1, −2, −3, −4, or −5. For verification of the same protein expression level, antibodies were stripped from the blots, and then the blots were re-probed with antibody to total STAT-1 or −3.
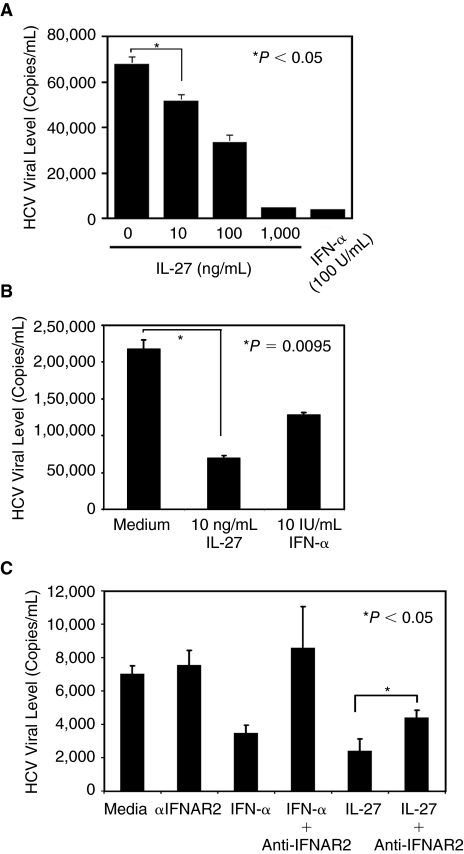
To evaluate the impact of IL-27 on HCV replication, HCV-infected Huh7.5 cells were cultured for 2 days in the presence of various concentrations of IL-27. As a positive control for HCV inhibition, the infected cells were cultured in the presence of 100 IU/mL of IFN-α. HCV replication was determined by quantitation of HCV RNA copy numbers in culture supernatants using real-time RT-PCR. The assay revealed that IL-27 dose-dependently suppressed HCV replication (Fig. 2A) and 10 ng/mL of IL-27 inhibited HCV replication by 25%–67% (Fig. 2A and 2B).
FIG. 2.
IL-27 inhibits hepatitis C virus (HCV) replication. (A and B) Huh7.5 cells were infected with infectious HCV particle (2.3 million copies of HCV RNA per milliliter) for 4 h, and then the infected cells were incubated for 2 days in the absence or presence of Interleukin (IL)-27 or interferon (IFN)-α. HCV replication in the cell culture was determined by detecting HCV RNA copy number in culture supernatants by real-time RT-PCR. (C) HCV-infected Huh7.5 cells were pretreated with 5 μg/mL of anti-IFNAR2 antibody (R&D systems) for 30 min, and then cultured for 2 days in the presence of 100 ng/mL of IL-27 or 10 U/mL of IFN-α. HCV copy numbers in culture supernatants were determined by quantitative real-time RT-PCR. Each assay was performed triplicate at least in 3 independent studies, and data indicate means ± SD. P value was assessed using t-test.
It is reported that the antiviral activity of IL-27 is direct and not mediated by IFN because neutralizing type-I IFN antibodies did not block IL-27-induced antiviral state in cells (Imamichi and others 2008; Bender and others 2009). In contrast, a recent report attributes the antiviral action of IL-27 to the rapid induction of type-I IFNs in response to IL-27: neutralizing antibody against IFN-α receptor 2 (IFNAR2) completely inhibited the antiviral effect (Greenwell-Wild and others 2009). To define whether IFN intermediates in the IL-27-mediated anti-HCV effect, HCV-infected Huh7.5 cells were pretreated with the IFNAR2 antibody, and then antiviral effect by IL-27 was assessed. As a positive control for the neutralization, the HCV-infected cells were also treated with IFN-α. The IFN-α-mediated anti-HCV effect was completely blocked by the neutralizing antibody; however, the IL-27-mediated HCV effect was partially suppressed by the antibody (Fig. 2C).
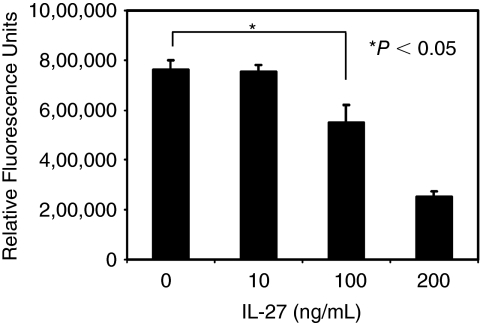
To determine a cytotoxic effect by IL-27, Calcein-Acetoxymethyl-Cytotoxic Assay (Biotium, Inc., Hayward, CA) was performed on HCV-infected Huh cell using 0, 10, 100, and 200 ng/mL of IL-27. Even though 10 ng/mL of IL-27 had no significant impact on cell viability (P = 0.76), 100 ng/mL of IL-27 partially reduced cell viability to 73 ± 13% (P < 0.05) (Fig. 3).
FIG. 3.
High concentrations of IL-27 induce cytotoxic effect in hepatitis C virus (HCV)-infected cells. To determine the cytotoxic effect by IL-27, the Calcein-AM-Cytotoxic Assay was performed following a protocol provided by the vender. HCV-infected Huh7.5 cells were cultured for 2 days in the presence of 0, 10, 100, and 200 ng/mL of IL-27 in a 96-well tissue culture plate. After incubation, cells were incubated with a final 4 μM of Calcein-AM for 30 min followed by quantitation of fluorescence intensity using fluorescence plate reader. P value was assessed using t-test.
IFN-α inhibits influenza virus and HCV replication via the induction of genes encoding antiviral proteins, such as double-stranded RNA-dependent kinase, 2′,5′ oligoadenylate synthetase and myxovirus protein (MxA), and the mechanism of antiviral property has been well investigated (Pestka and others 1987, 2004; Samuel 2001; Langer and others 2004; Galligan and others 2006). We have previously demonstrated that IL-27 induces the activation of those antiviral genes in M-CSF-induced macrophages without induction of IFNs (Imamichi and others 2008). A recent study indicates that IL-27 inhibits avian virus replication in HepG2 cell with the induction of MxA (Bender and others 2009). The induction of MxA was not associated with the activation of IFNs in the cell. Thus, it is predicted that the mechanism by which IL-27 inhibits HCV in the hepatoma cell line is similar to that by IFN. IL-27 at 10 ng/mL induced the activation of STAT-1 and −3 in HCV-infected cells and significantly inhibited HCV replication without any impact on cell viability, while IL-27 at 100 ng/mL inhibited HCV with reduction of cell viability. The result from the neutralization assay illustrated that the mechanism by which IL-27 inhibits HCV replication may involve IFN-dependent and -independent pathway, and IL-27 may induce the production of IFN from hepatocytes. Taken together, IL-27 may exert antiviral cytokine against HIV-1, HCV, avian virus, and other viral infections.
In conclusion, we demonstrated that IL-27 is capable to inhibit replication of HCV. Since IL-27 inhibits replication of HIV-1 and HCV, achieving a better understanding of the role of IL-27 in regulation of gene activation and mechanism of the antiviral effect may aid in the development of a novel immunotherapeutic strategy for HCV and HCV/HIV co-infection as well as other infectious diseases.
Acknowledgments
Authors thank H.C. Lane for guidance and support, and R. Dewar for a critical reading. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institute Health under contact No. HHSN261200800001E. The content of this publication does not necessarily reflect the view or policies of the department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. This research was supported by the National Institute of Allergy and Infectious Disease.
Author Disclosure Statement
No competing financial interests exist.
References
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(1):S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Batten M. Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med. 2007;85(7):661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- Bender H. Wiesinger MY. Nordhoff C. Schoenherr C. Haan C. Ludwig S. Weiskirchen R. Kato N. Heinrich PC. Haan S. Interleukin-27 displays interferon-gamma-like functions in human hepatoma cells and hepatocytes. Hepatology. 2009;50(2):358–360. doi: 10.1002/hep.22988. [DOI] [PubMed] [Google Scholar]
- Benhamou Y. Bochet M. Di Martino V. Charlotte F. Azria F. Coutellier A. Vidaud M. Bricaire F. Opolon P. Katlama C. Poynard T. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- Blight KJ. McKeating JA. Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76(24):13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassard DL. Grace MJ. Bordens RW. Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol. 2002;71(4):565–581. [PubMed] [Google Scholar]
- Carrat F. Bani-Sadr F. Pol S. Rosenthal E. Lunel-Fabiani F. Benzekri A. Morand P. Goujard C. Pialoux G. Piroth L. Salmon-Céron D. Degott C. Cacoub P. Perronne C ANRS HCO2 RIBAVIC Study Team. Pegylated interferon alfa-2b vs. standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292(23):2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- Chung RT. Andersen J. Volberding P. Robbins GK. Liu T. Sherman KE. Peters MG. Koziel MJ. Bhan AK. Alston B. Colquhoun D. Nevin T. Harb G. van der Horst C AIDS Clinical Trials Group A5071 Study Team. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351(5):451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakruddin JM. Lempicki RA. Gorelick RJ. Yang J. Adelsberger JW. Garcia-Pineres AJ. Pinto LA. Lane HC. Imamichi T. Noninfectious papilloma virus-like particles inhibit HIV-1 replication: implications for immune control of HIV-1 infection by IL-27. Blood. 2007;109(5):1841–1849. doi: 10.1182/blood-2006-02-001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foli A. Saville MW. Baseler MW. Yarchoan R. Effects of the Th1 and Th2 stimulatory cytokines interleukin-12 and interleukin-4 on human immunodeficiency virus replication. Blood. 1995;85(8):2114–2123. [PubMed] [Google Scholar]
- Galligan CL. Murooka TT. Rahbar R. Baig E. Majchrzak-Kita B. Fish EN. Interferons and viruses: signaling for supremacy. Immunol Res. 2006;35(1–2):27–40. doi: 10.1385/IR:35:1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell-Wild T. Vazquez N. Jin W. Rangel Z. Munson P. Wahl SM. IL-27 inhibition of HIV-1 involves an intermediate induction of type I IFN. Blood. 2009;114(9):1864–1874. doi: 10.1182/blood-2009-03-211540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamichi T. Yang J. Huang DW. Brann TW. Fullmer BA. Adelsberger JW. Lempicki RA. Baseler MW. Lane HC. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS. 2008;22(1):39–45. doi: 10.1097/QAD.0b013e3282f3356c. [DOI] [PubMed] [Google Scholar]
- Kamiya S. Owaki T. Morishima N. Fukai F. Mizuguchi J. Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173(6):3871–3877. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- Kastelein RA. Hunter CA. Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- Kottilil S. Yan MY. Reitano KN. Zhang X. Lempicki R. Roby G. Daucher M. Yang J. Cortez KJ. Ghany M. Polis MA. Fauci AS. Human immunodeficiency virus and hepatitis C infections induce distinct immunologic imprints in peripheral mononuclear cells. Hepatology. 2009;50(1):34–45. doi: 10.1002/hep.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguno M. Murillas J. Blanco JL. Martínez E. Miquel R. Sánchez-Tapias JM. Bargallo X. García-Criado A. de Lazzari E. Larrousse M. León A. Loncá M. Milinkovic A. Gatell JM. Mallolas J. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18(13):F27–F36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- Lane HC. The role of alpha-interferon in patients with human immunodeficiency virus infection. Semin Oncol. 1991;18(5):46–52. [PubMed] [Google Scholar]
- Langer JA. Cutrone EC. Kotenko S. The class II cytokine receptor (CRF2) family: overview and patterns of receptor–ligand interactions. Cytokine Growth Factor Rev. 2004;15(1):33–48. doi: 10.1016/j.cytogfr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Lempicki RA. Polis MA. Yang J. McLaughlin M. Koratich C. Huang DW. Fullmer B. Wu L. Rehm CA. Masur H. Lane HC. Sherman KE. Fauci AS. Kottilil S. Gene expression profiles in hepatitis C virus (HCV) and HIV coinfection: class prediction analyses before treatment predict the outcome of anti-HCV therapy among HIV-coinfected persons. J Infect Dis. 2006;193(8):1172–1177. doi: 10.1086/501365. [DOI] [PubMed] [Google Scholar]
- Lindenbach BD. Evans MJ. Syder AJ. Wölk B. Tellinghuisen TL. Liu CC. Maruyama T. Hynes RO. Burton DR. McKeating JA. Rice CM. Complete replication of hepatitis C virus in cell culture. Science. 2005;309(5734):623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- Lucas S. Ghilardi N. Li J. de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100(25):15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Krause CD. Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Pestka S. Langer JA. Zoon KC. Samuel CE. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Pflanz S. Hibbert L. Mattson J. Rosales R. Vaisberg E. Bazan JF. Phillips JH. McClanahan TK. de Waal Malefyt R. Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172(4):2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- Pflanz S. Timans JC. Cheung J. Rosales R. Kanzler H. Gilbert J. Hibbert L. Churakova T. Travis M. Vaisberg E. Blumenschein WM. Mattson JD. Wagner JL. To W. Zurawski S. McClanahan TK. Gorman DM. Bazan JF. de Waal Malefyt R. Rennick D. Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(16):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Poli G. Biswas P. Fauci AS. Interferons in the pathogenesis and treatment of human immunodeficiency virus infection. Antiviral Res. 1994;24(2–3):221–233. doi: 10.1016/0166-3542(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriani FJ. Rodriguez-Torres M. Rockstroh JK. Lissen E. Gonzalez-García J. Lazzarin A. Carosi G. Sasadeusz J. Katlama C. Montaner J. Sette H., Jr Passe S. De Pamphilis J. Duff F. Schrenk UM. Dieterich DT APRICOT Study Group. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351(5):438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- Wakita T. Pietschmann T. Kato T. Date T. Miyamoto M. Zhao Z. Murthy K. Habermann A. Kräusslich HG. Mizokami M. Bartenschlager R. Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11(7):791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]