Abstract
Background
There is growing evidence that inhalants are neurotoxic to white matter, yet limited work has been conducted to investigate the neurobiologic effects of long-term exposure among adolescent users, despite inhalant use being most prominent during this developmental period.
Methods
We used diffusion tensor imaging to examine white-matter integrity in 11 adolescents who used inhalants, 11 matched cannabis users and 8 drug-naive controls.
Results
Although both groups of drug users had white-matter abnormalities (i.e., lower fractional anisotropy), abnormalities were more pronounced in the inhalant group, particularly among early-onset users.
Limitations
The findings of this study should be considered in light of its small sample size, cross-sectional design and the complex psychosocial background of long-term inhalant users.
Conclusion
White-matter abnormalities may underpin long-term behavioural and mental health problems seen in individuals with long-term inhalant use.
Introduction
Inhalants, such as toluene, are neurotoxic to white matter and are one of the first substances to be abused by adolescents.1–4 Such use may negatively impact brain development, especially white-matter connectivity, which continues to develop throughout adolescence.4–6 We sought to examine axonal integrity in the white matter of adolescent users of inhalants. As a comparison group, we included cannabis-using adolescents matched on multiple variables to control for differences associated with potential psychosocial confounds (e.g., unstable and dysfunctional families and school absenteeism), as well as to test for the specificity of any identified white-matter differences.4,6,7
Methods
We recruited 30 adolescents (Table 1). The participants comprised long-term (daily or almost daily use for > 12 mo) inhalant users (n = 11), matched cannabis users (n = 11) and drug-naive controls (n = 8). Inhalant and cannabis users were recruited via the Department of Human Services from the Western and Northern regions of Melbourne, Australia. The controls were matched for years and quality of education, intelligence, sex and use of tobacco, alcohol and cannabis. Controls were recruited from the general community in the same geographic location as the cannabis and inhalant users.
Table 1.
Demographic, substance use and clinical characteristics of study participants
| Group; mean (SD)* |
|||||
|---|---|---|---|---|---|
| Characteristic | Inhalant use, n = 11 | Cannabis use, n = 11 | Controls, n = 8 | F, χ2 or t value | p value |
| Age, yr | 18.2 (1.6) | 19.4 (1.9) | 19.7 (2.7) | F2,27 = 1.4 | 0.27 |
| Education | 9.2 (1.4) | 9.2 (1.3) | 11.0 (1.6) | F2,27 = 4.63 | 0.019‡ |
| Sex, % male | 45.5 | 66.7 | 25.0 | χ22 = 3.3 | 0.19 |
| Intelligence quotient | 84.7 (16.2) | 82.2 (16.3) | 106.2 (18.4) | F2,27 = 9.68 | 0.001§ |
| Regular tobacco use, % of participants | 100 | 100 | 66.7 | χ22 = 6.97 | 0.031 |
| Age of initiation, yr | 12.44 (1.5) | 13.0 (2.6) | 16.50 (0.71) | F2,19 = 3.0 | 0.07 |
| No. of cigarettes in a typical day | 15.44 (10.1) | 16.8 (10.1) | 3.0 (2.8) | F2,19 = 1.69 | 0.21 |
| Regular alcohol use, % of participants | 70 | 81.8 | 37.5 | χ22 = 4.16 | 0.13 |
| Age of initiation, yr | 15.29 (1.6) | 14.2 (2.9) | 18.33 (0.58) | F2,16 = 3.55 | 0.05 |
| No. of standard drinks† in a typical day | 2.61 (6.1) | 1.7 (2.6) | 0.42 (0.38) | F2,20 = 0.490 | 0.62 |
| Regular cannabis use, % | 77.8 | 100 | — | χ21 = 0.563 | 0.45 |
| Age of initiation, yr | 14.0 (1.8) | 15.0 (1.6) | — | t16 = −1.22 | 0.24 |
| No. of grams in a typical day | 1.6 (2.5) | 0.8 (0.5) | — | t14 = 0.949 | 0.36 |
| Regular inhalant use, % | 100 | 22.2 | — | χ21 = 8.42 | 0.004 |
| Age of initiation, yr | 15.2 (1.8) | 12.7 (1.2) | — | t11 = 2.32 | 0.041 |
| No. of cans in a typical day | 1.61 (1.3) | — | — | — | — |
SD = standard deviation.
Unless otherwise indicated.
A standard drink was defined as containing 10 grams of alcohol.
Tukey post-hoc tests for education: inhalant v. cannabis = 1.0, inhalant v. controls = 0.035, cannabis v. controls = 0.029.
Tukey post-hoc tests for intelligence: inhalant v. cannabis = 0.930, inhalant v. controls = 0.003, cannabis v. controls = 0.001.
The Melbourne Health Research and Ethics Committee and the Department of Human Services approved this study, and consent was obtained from all participants as well as their parents or legal guardians if relevant.
Both the cannabis and inhalant users had been using drugs (i.e., inhalants or cannabis) at least weekly for 1 or more years. We excluded participants who were taking psychotropic medications; we also excluded those with a psychotic disorder or serious medical condition. Cannabis users were excluded if they had used inhalants for 6 months or more. Controls had no history of substance abuse or mental disorders. Although age was comparable across all 3 groups, both drug-using groups had fewer years of education and lower intelligence quotients than controls.
We acquired diffusion-tensor magnetic resonance images using a Siemens Trio 3.0-T scanner. The spin-echo echo-planar imaging sequence (echo time 106 ms, repetition time 6.1 s, field of view 220 × 220 mm2) consisted of 48 isotropically distributed gradient directions (b = 1000 s/mm2) acquired over a 128 × 128 image matrix at 38 consecutive axial slices, 3-mm thick, resulting in voxel dimensions of 1.72 × 1.72 × 3.0 mm.
We used fractional anisotropy (FA) to index white-matter integrity; we computed FA for each participant using the methods described by Basser and colleagues.8 We conducted voxel-based comparisons of FA within the tract-based spatial statistical analytic framework.9 The analysis was performed on a voxelwise basis within a white-matter skeleton to detect regions exhibiting statistically significant differences in FA between the 3 groups. Each voxel making up the white-matter skeleton was chosen based on the criterion that it exhibits the maximal value of FA in a local neighbourhood. This enables more accurate voxel alignment between participants at the expense of neglecting potential abnormalities confined to peripheral white matter.
We calculated t statistics for each skeleton voxel, and voxels with t < 2 were excluded. Adjacent skeleton voxels with a t statistic exceeding the suprathreshold were clustered, and the significance of cluster size was determined by comparing the volume of each cluster with the maximal cluster volume observed under the null hypothesis, sampled via permutation testing. Given the exploratory nature of this study and the relatively small sample sizes, we report all group differences satisfying a less stringent significance threshold of p < 0.2. We also explicitly reported the significance of each cluster satisfying this criterion to show clusters satisfying a more stringent significance threshold. We also conducted a post-hoc analysis whereby the average value of FA within a cluster was compared between users and controls using a 2-sample t test. We also computed effect sizes (Cohen d) for each cluster.
We tested for associations between regional FA, age and substance use parameters. Specifically, we tested for 7 associations with the average value of FA at each cluster: age, age at which cannabis use began, age at which inhalant use began, duration of regular inhalant use, duration of regular cannabis use, weekly cannabis dosage and weekly inhalant dosage. We also used the first 3 associations as regressors in the general linear model.
Results
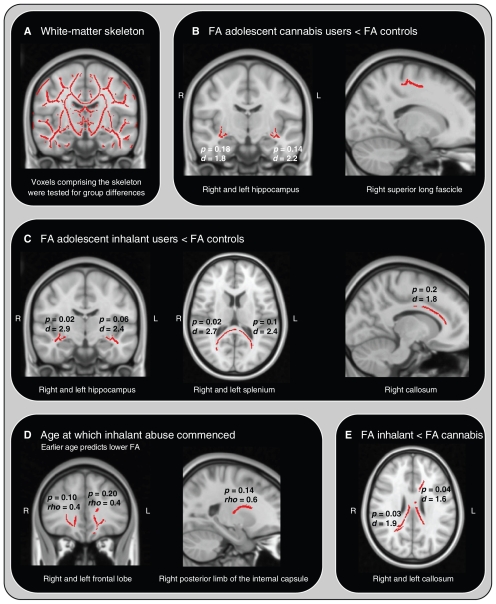
Relative to controls, inhalant users had lower FA in a portion of the fasciculus adjacent to the left hippocampus (possibly the fornix and stria terminalis) and the left and right limbs of the splenium of the corpus callosum (Table 2). Relative to controls, cannabis users had lower FA in the fasciculus adjacent to the right hippocampus. There was no evidence of higher FA in the drug-using groups compared with the control group. A direct comparison of the drug-using groups revealed lower FA in the left and right callosa in the inhalant users (Fig. 1). The only significant covariate was age of onset of regular inhalant use, which was positively associated with FA in the left and right frontal lobes (Fig. 1).
Table 2.
Significant clusters segregated according to group differences*
| Group difference; cluster | Mean (SD) |
p post-hoc | Cluster size, no. voxels | Group; mean (SD) |
Cohen d | MNI centre of mass |
|||
|---|---|---|---|---|---|---|---|---|---|
| p cluster | FA controls | FA users | x | y | z | ||||
| FA cannabis < FA controls, df = 17 | |||||||||
| Right hippocampus | 0.18 (0.01) | 0.001 | 498 | 0.40 (0.02) | 0.37 (0.02) | 1.8 | +31 | −11 | −5 |
| Left hippocampus | 0.14 (0.01) | 0.0002 | 588 | 0.50 (0.02) | 0.45 (0.02) | 2.2 | −38 | −19 | −9 |
| Right superior longitudinal fascicle | 0.23 (0.01) | 0.0009 | 407 | 0.57 (0.03) | 0.50 (0.03) | 1.8 | +15 | −17 | +54 |
| FA inhalant < FA controls, df = 17 | |||||||||
| Right hippocampus | 0.02 (0.004) | < 0.0001 | 1592 | 0.44 (0.02) | 0.39 (0.01) | 2.9 | +34 | −16 | −8 |
| Left hippocampus | 0.06 (0.007) | < 0.0001 | 954 | 0.47 (0.02) | 0.43 (0.02) | 2.4 | −36 | −16 | −10 |
| Right splenium | 0.02 (0.005) | < 0.0001 | 1513 | 0.59 (0.01) | 0.54 (0.03) | 2.7 | +21 | −53 | +21 |
| Left splenium | 0.1 (0.01) | 0.0002 | 675 | 0.71 (0.02) | 0.64 (0.03) | 2.4 | −21 | −49 | +12 |
| Right corpus callosum | 0.2 (0.01) | 0.001 | 434 | 0.47 (0.03) | 0.40 (0.03) | 1.8 | +23 | +31 | +13 |
| Age at which inhalant use began | |||||||||
| Right frontal lobe | 0.10 (0.01) | 0.14 | 706 | — | 0.48 (0.05) | 0.4† | +16 | +33 | −5 |
| Left frontal lobe | 0.20 (0.01) | 0.20 | 399 | — | 0.64 (0.05) | 0.4† | −15 | +33 | +6 |
| Right posterior limb of the internal capsule | 0.14 (0.01) | 0.02 | 560 | — | 0.50 (0.04) | 0.6† | +23 | −16 | +31 |
| FA inhalant < FA cannabis, df = 20 | |||||||||
| Right corpus callosum | 0.03 (0.005) | 0.0002 | 1375 | 0.60 (0.01) | 0.56 (0.03) | 1.9 | +15 | −32 | +26 |
| Left corpus callosum | 0.04 (0.007) | 0.001 | 1130 | 0.65 (0.02) | 0.60 (0.03) | 1.6 | −14 | −14 | +27 |
df = degrees of freedom; FA = fractional anisotropy; MNI = Montreal Neurological Institute; SD = standard deviation.
A post-hoc analysis was performed to test for a difference between users and controls in the average value of FA over a cluster. The p value derived from this post-hoc analysis (i.e., 2-sample t test) is reported in the third column.
Spearman rho.
Fig. 1.
(A) Tract-based spatial statistics skeleton used for fractional anisotropy analyses and (B) group differences for controls versus cannabis users, (C) inhalant users versus controls and (E) inhalent users versus cannabis users. Also shown are the (D) age-dependent effects on fractional anisotropy in the inhalant group. Some regions that were not statistically significant are shown here for display purposes.
Discussion
To our knowledge, this is the first diffusion tensor imaging study of long-term inhalant use among adolescents. Our findings indicate that both adolescent use of inhalants and cannabis are associated with white-matter abnormalities, particularly in the medial temporal and callosal pathways, with callosal abnormalities being more severe among inhalant users. In addition, younger age at the onset of inhalant use was associated with lower frontal white-matter integrity, suggesting that earlier use may result in greater injury. Our findings are consistent with those from animal studies showing neurobiologic sequelae of long-term toluene exposure during adolescence.1–3 Our findings are also consistent with the broader substance use literature suggesting that early use is associated with poorer outcomes.6
Limitations
We matched the comparison groups as closely as possible for relevant sociodemographic factors, but because of the small sample size and cross-sectional design, these findings should be replicated in larger cohorts before firm conclusions can be made. For example, despite not being statistically different, sex and alcohol and tobacco use may still be background confounding variables. Moreover, the typically complex presentation of long-term inhalant users (i.e., disadvantaged psychosocial background, comorbid psychopathology and poor motivation or engagement) is rarely considered when investigating associated neurobiologic impairments. Future research should carefully consider the role of such factors, given the evidence suggesting that they can considerably alter the association between inhalant use and neurobiologic impairments.2,4 Future studies using longitudinal designs will prove valuable in better understanding the toxic effects of inhalant use.
Conclusion
Long-term inhalant use, compared with the use of other drugs, may be more hazardous for the developing brain because inhalants are highly lipophilic and toxic, making them particularly dangerous to fatty tissue such as myelin. Indeed, the neuropsychologic deficits commonly reported among inhalant users are consistent with white-matter pathology.4 Such deficits may render individuals vulnerable to impaired decision-making (e.g., impulsivity, emotional dysregulation), disturbed behaviours (e.g., delinquency, aggression, amotivation, suicide) and future psychiatric problems.2,4 Delayed use of such substances would help minimize neuronal injury, while abstinence may assist in the recovery of any lost function.10
Acknowledgements
Dr. Yücel is supported by a National Health and Medical Research Council (NHMRC) Clinical Career Development Award (ID 509345). Dr. Zalesky is supported by an Australian Research Council research fellowship (ID DP0986320). Dr. Fornito is supported by an NHMRC C.J. Martin Fellowship (ID 454797). Dr. Lubman is supported by the Colonial Foundation
Footnotes
Previously published at www.jpn.ca
Competing interests: None declared.
Contributors: Drs. Yücel, Pantelis and Lubman designed the study. Drs. Yücel, Takagi, Fornito, Ditchfield and Pantelis acquired the data. Drs. Yücel, Zalesky, Takagi, Bora, Fornito, Egan and Lubman analyzed the data. Drs. Yücel, Zalesky, Takagi, Fornito and Lubman wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Filley CM, Halliday W, Kleinschmidt-DeMasters BK. The effects of toluene on the central nervous system. J Neuropathol Exp Neurol. 2004;63:1–12. doi: 10.1093/jnen/63.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Lubman DI, Yücel M, Lawrence AJ. Inhalant abuse among adolescents: neurobiological considerations. Br J Pharmacol. 2008;154:316–26. doi: 10.1038/bjp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg NL, Kleinschmidt-DeMasters BK, Davis KA, et al. Toluene abuse causes diffuse central nervous system white matter changes. Ann Neurol. 1988;23:611–4. doi: 10.1002/ana.410230614. [DOI] [PubMed] [Google Scholar]
- 4.Yücel M, Takagi M, Walterfang M, et al. Toluene misuse and long-term harms: a systematic review of the neuropsychological and neuroimaging literature. Neurosci Biobehav Rev. 2008;32:910–26. doi: 10.1016/j.neubiorev.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubman DI, Yücel M, Hall WD. Substance use and the adolescent brain: A toxic combination? J Psychopharmacol. 2007;21:792–4. doi: 10.1177/0269881107078309. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers EM. Volatile substance abuse. Med J Aust. 1991;154:269–74. doi: 10.5694/j.1326-5377.1991.tb121089.x. [DOI] [PubMed] [Google Scholar]
- 8.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–67. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Schiffer WK, Lee DE, Alexoff DL, et al. Metabolic correlates of toluene abuse: decline and recovery of function in adolescent animals. Psychopharmacology (Berl) 2006;186:159–67. doi: 10.1007/s00213-006-0359-6. [DOI] [PubMed] [Google Scholar]