Abstract
We investigated the development of an injectable, biodegradable hydrogel composite of oligo(poly(ethylene glycol) fumarate) (OPF) with encapsulated rabbit marrow mesenchymal stem cells (MSCs) and gelatin microparticles (MPs) loaded with transforming growth factor-β1 (TGF-β1) for cartilage tissue engineering applications. Rabbit MSCs and TGF-β1-loaded MPs were mixed with OPF, a poly(ethylene glycol)-diacrylate crosslinker and the radical initiators ammonium persulfate and N,N,N’,N’-tetramethylethylenediamine, and then crosslinked at 37°C for 8 min to form hydrogel composites. Three studies were conducted over 14 days in order to examine the effects of: 1) the composite formulation, 2) the MSC seeding density, and 3) the TGF-β1 concentration on the chondrogenic differentiation of encapsulated rabbit MSCs. Bioassay results showed no significant difference in DNA amount between groups, however, groups with MPs had a significant increase in glycosaminoglycan content per DNA starting at day 7 as compared to controls at day 0. Chondrocyte-specific gene expression of type II collagen and aggrecan were only evident in groups containing TGF-β1-loaded MPs and varied with TGF-β1 concentration in a dose dependent manner. Specifically, type II collagen gene expression exhibited a 161 ± 49 fold increase and aggrecan gene expression a 221 ± 151 fold increase after 14 days with the highest dose of TGF-β1 (16 ng/ml). These results indicate that encapsulated rabbit MSCs remained viable over the culture period and differentiated into chondrocyte-like cells, thus suggesting the potential of OPF composite hydrogels as part of a novel strategy for localized delivery of stem cells and bioactive molecules.
Keywords: Cartilage tissue engineering, marrow mesenchymal stem cells, gelatin microparticles, injectable hydrogels, TGF-β1
Introduction
Current treatments for cartilage lesions, including autografts and subchondral drilling, have significant complications such as secondary site morbidity and incomplete defect healing [1]. Over the past decade, tissue engineering approaches have been developed, which combine cells, bioactive molecules and scaffolding materials [2]. Particularly, during this time, synthetic, biodegradable hydrogels have been designed as cell and growth factor delivery systems in orthopedic tissue engineering. These materials can provide support during the healing process and are often used in an injectable form as a minimally invasive method to fill irregularly shaped defects [3–5].
Recently, a novel class of injectable hydrogels based on the oligomer oligo(poly(ethylene glycol)fumarate) (OPF) has been developed for the delivery of cells and growth factors to cartilage lesions [6–9]. The poly(ethylene glycol) (PEG) repeating unit imparts water solubility to this oligomer, while its repeating double bonds facilitate crosslinking to form a three dimensional gel structure. Biodegradation of OPF hydrogels is possible through the hydrolysis of the ester linkages in the fumarate group [10, 11]. Additionally, peptide sequences can be incorporated before OPF crosslinking to promote specific, interactions with cells [12, 13]. Thus, as an injectable, in situ crosslinkable, and biodegradable oligomer, OPF is a suitable candidate for minimally invasive, localized cell and drug delivery therapies.
In a previous study, it was demonstrated that rat marrow mesenchymal stem cells (MSCs) could be encapsulated in OPF and their differentiation induced over 28 days in vitro with the addition of osteogenic supplements to the culture medium [14, 15]. In another series of experiments, our laboratory has demonstrated that incorporating gelatin microparticles into OPF hydrogel scaffolds is a useable strategy for localized and sustained release of a growth factor [9]. Specifically, gelatin microparticles loaded with transforming growth factor-β1 (TGF-β1) were encapsulated into OPF hydrogels and the rate of TGF-β1 release could be controlled by altering OPF formulation and the crosslinking extent of gelatin microparticles [9]. Building on these studies, TGF-β1-loaded gelatin microspheres were then embedded with bovine chondrocytes in OPF hydrogels. The results demonstrated that the presence of loaded gelatin microparticles promoted cell proliferation while maintaining a chondrocytic phenotype [7].
The findings from these in vitro as well as in vivo studies suggest that OPF hydrogel composites possess distinct advantages for use in vivo [6]. In addition to holding cells at the defect site, the localized, sustained growth factor release should encourage cell proliferation, differentiation and matrix production in the hydrogel while preventing bolus release of TGF-β1, a known pro-inflammatory agent [16], into the joint space. However, recognizing the limited amount of donor tissue for autologous chondrocyte transplantation, we have chosen to focus our attention in the current study on the effects of this carrier system on chondrogenic differentiation of MSCs. Because of the availability of MSCs and the potential for expansion of these cells without affecting their differentiation capacity, this cell source could serve as a key component of future approaches using this hydrogel system to regenerate cartilage lesions.
Thus, the objectives of the present study were to examine the effects of the presence of gelatin microspheres on cell proliferation, gene expression, and matrix production by rabbit MSCs embedded in OPF hydrogels and to determine if chondrogenic differentiation of embedded MSCs could be directed by controlled release of low levels of TGF-β1. An additional objective was to investigate how the proliferation, gene expression and matrix production from encapsulated rabbit MSCs were affected by changes in the local hydrogel environment brought about by alterations in cell seeding density and dose of TGF-β1 released.
Materials and Methods
OPF synthesis and characterization
OPF was synthesized from fumaryl chloride and PEG of number average molecular weight 10 kDa according to a previously established method [11]. Oligomer molecular weight (n=3) was determined by gel permeation chromatography (GPC; Model 410; Waters, Milford, PA) using a refractive index detector. Purified OPF was stored at −20°C and sterilized prior to use by exposure to ethylene oxide for 16 h.
Gelatin microparticle preparation
Gelatin microparticles were fabricated from acidic gelatin (Nitta Gelatin Inc., Osaka, Japan), following established procedures [7]. Briefly, a gelatin solution was prepared by dissolving 5 g gelatin in 45 ml distilled, deionized water (ddH2O) at 60oC. Then, this solution was added dropwise to 250 ml chilled olive oil while stirring at 500 rpm. After 30 min, 100 ml chilled acetone (4°C) was added to the emulsion. After an additional 60 min, the microspheres were collected by filtration and washed with acetone. These microparticles were then crosslinked in 0.1 wt % Tween 80 (Sigma, St. Louis, MO) solution with 10 mM glutaraldehyde (GA) (Sigma, St. Louis, MO) while stirring at 500 rpm at 15°C. After 15 h, crosslinked microparticles were collected by filtration, washed with ddH2O, and then agitated in 25 mM glycine solution for 1 h to inactivate any unreacted GA. These microparticles were collected by filtration, washed with ddH2O, and then lyophilized overnight. Finally, dried microparticles were sieved to obtain particles of 50–100 μm in diameter and sterilized with ethylene oxide for 16 h.
Rabbit MSC isolation and pre-culture
Rabbit MSCs were isolated from the femur of 4 week old rabbits as previously described [17]. Briefly, after anesthesia, rabbit bone marrow was collected into 10 ml syringe containing 5000 U of heparin. The bone marrow was then filtered through a cell strainer (40 μm) and cultured in DMEM-LG supplemented medium containing 10% v/v fetal bovine serum (Gemini, Calabasas, CA), 250 μg fungizone/l, 100 mg ampicillin/l, and 50 mg gentamicin/l (Invitrogen) for 2 weeks. After cells became confluent, they were released from the flasks with 0.05% trypsin-EDTA (Invitrogen) and placed in medium containing 20% FBS and 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen prior to use. A pool of rabbit MSCs from 6 rabbits were used for all studies. For experiments, cryopreserved cells were thawed at 37°C, seeded in T-75 flasks, and expanded for 14 days of culture in DMEM supplemented medium containing 10% v/v fetal bovine serum (Gemini, Calabasas, CA), 250 μg fungizone/l, 100 mg ampicillin/l, and 50 mg gentamicin/l.
Microparticle and rabbit MSC encapsulation
Before encapsulation, OPF and gelatin microparticles were sterilized with ethylene oxide for 16h. Then, sterilized microparticles were loaded with TGF-β1 by immersing them in aqueous TGF-β1 solution at pH 7.4 and incubating them at 4°C for 15h as has previously been established [9]. At this pH, there is an ionic complex of gelatin microparticles and TGF-β1 due to the negative charge of the acidic gelatin (IEP of 5.0) and positive charge of TGF-β1 (IEP of 9.5) [18]. TGF-β1-free microparticles were also prepared for comparison.
Following this incubation period, isolated MSCs and gelatin microparticles were encapsulated in a network of OPF. First, OPF and the crosslinking agent poly(ethylene glycol) diacrylate (PEG-DA; Nektar Therapeutics, Huntsville, AL) were combined in 300 μl PBS using a 2:1 ratio of OPF to PEG-DA by weight, and mixed with microparticles partially swollen in 110 μl of PBS containing 3.6 ng TGF-β1/μl. Equal volumes (46.8 μl) of the thermal radical initiators, 25 mM ammonium persulfate (APS) and 25 mM N,N,N`,N`-tetramethylethylenediamine (TEMED) in PBS were then added. After this mixture was vortexed, a 168 μl PBS suspension containing 6.7 million MSCs was added to achieve a cell concentration of 10 million cells/ml in the final suspension. After gentle mixing, the suspension was quickly injected into Teflon molds (6 mm diameter, 0.5 mm thickness) followed by incubation at 37°C for 8 min. Final gel constructs were transferred into 12 well tissue culture plates. Each well contained one gel construct and 1.25 ml medium. The medium was changed every 3 days. At day 3, 7, and 14, samples were collected for biochemical assays (n=4), quantitative reverse transcriptase polymer chain reaction (n=4) and histological analysis (n=2). Samples at day 0 were collected immediately after encapsulation process and used as a control for all other groups. Cell-free, hydrogels (n=3) were also prepared following the same methods. These cell-free, hydrogels were analyzed with samples to establish any background contribution from the hydrogel to fluorescence and absorbance measurements in the biochemical assays.
To examine the effect of hydrogel formulation, the first study included OPF hydrogels containing embedded MSCs, OPF hydrogels containing embedded MSCs and unloaded microparticles, and OPF hydrogels containing embedded MSCs and TGF-β1-loaded microparticles. 10 million cells/ml of MSCs were encapsulated in each gel construct since a previous study with bovine chondrocytes showed that OPF hydrogels encapsulating a similar number of bovine chondrocytes (9 million cells/ml) showed significant increases in DNA and GAG production in the presence of the TGF-β1-loaded microparticles [7]. The second study for the effect of cell seeding density on differentiation of MSCs used two seeding densities (10 and 20 million cells/ml). In the third study to examine the effect of TGF-β1 concentration on rabbit MSC function, three different concentrations of TGF-β1 (3 ng/ml, 10 ng/ml, 16 ng/ml) have been loaded into microparticles.
Biochemical assays for cell proliferation and GAG production
At each time point, samples and cell-free hydrogels were removed from medium, rinsed in 2 ml PBS, homogenized with a pellet grinder (Fisher Scientific) and digested in 500 μl of a proteinase K solution (1 mg proteinase K/ml (Sigma-Aldrich), 10 μg pepstatin A/ml (Sigma-Aldrich), and 185 μg iodoacetamide/ml (Sigma-Aldrich)) in tris-EDTA solution (6.055 mg Tris(hydroxymethyl aminomethane)/ml (Sigma-Aldrich), 0.372 mg EDTA/ml (Sigma-Aldrich), pH 7.6 adjusted by HCl) at 60°C for 16 h. After collection and digestion of all samples and cell-free hydrogels, specimens were subjected to three repetitions of a freeze/thaw/sonication cycle (30 min at −80°C, 30 min at room temperature, 30 min of sonication) to facilitate the complete extraction of DNA from the cell cytoplasm. DNA and GAG assays were then run in triplicate for each experimental and control groups at each time point.
DNA content was calculated by measuring double-stranded DNA content using the PicoGreen assay (Molecular Probes, Eugene, OR) according to the manufacturer’s instructions. The fluorescence of negative, cell-free hydrogels was subtracted from the fluorescence values of experimental groups to account for fluorescence of the material alone.
Similarly, glycosaminoglycan content was also determined using a biochemical assay, the dimethylmethylene blue dye (DMMB) assay (Sigma-Aldrich), as previously described [19]. Upon DMMB binding to GAG, a pink color is produced, allowing for quantification of GAG by measuring absorbance at 520 nm. GAG content in hydrogels was calculated by comparison to a curve generated from standards of known amounts of chondroitin sulfate (Sigma-Aldrich). A microplate reader (BIO-TEK Instrument, Winooski, VT) was utilized for both the absorbance/fluorescence measurements.
Histology
Histology samples were prepared as previously described [7]. Briefly, samples from each time point were rinsed in 2 ml PBS for 1 h and then fixed in 10 % neutral buffered formalin (Sigma-Aldrich). After fixation, these samples were dehydrated by immersion in a series of ethanol solutions (70%, 80%, 85%, 90%, 95%, and 100%) and xylene solutions in ethanol (50% and 100%). Specimens were then embedded in paraffin and cross-sectioned to a thickness of 10 μm using a microtome (Microm, Walldorf, Germany). Sections from all groups were simultaneously stained with Safranin-O. Images were acquired with a light microscope (Eclipse E600; Nikon, Melvile, NY) equipped with a video camera (3CCD Color Video Camera DXC-950P; Sony, Park Ridge, NJ).
Real time PCR
Total RNA was extracted from hydrogel composites at each time point via the RNeasy Mini Kit (Qiagen). Briefly, hydrogels were transferred into RNA lysis buffer solution and homogenized by gentle pipetting. The homogenized solution was purified using a Qiagen shredder column and total RNA was extracted with the RNeasy Mini Kit. RNA samples were then reverse-transcribed to cDNA using Oligo dT primers (Promega) and superscript III transcriptase (Invitrogen). The final cDNA were then subjected to real time PCR (ABI Biomed 7300 Real-Time PCR System) to determine the expression of genes for type II collagen genes, aggrecan, and type I collagen. PCR reaction was carried out with specific primers and qPCR MasterMix Plus (Eurogentec) containing dNTPs, HotGoldStar DNA polymerase, MgCl2, Urasil-N-Clycosylase, SYBR Green I, and stabilizers as described in the manufacturer’s instruction. All gene expressions were normalized to expression of the house-keeping gene, glyceraldehyde-3-phosphatase dehydrogenase (GAPDH) and expressed as the fold ratio as compared with a control groups. For these studies, the control group contained MSCs embedded in OPF with unloaded microparticles that were analyzed just after encapsulation (day 0). The sequence of primers for GAPDH, type I collagen, type II collagen, and aggrecan were as follows: GAPDH: 5-TCACCATCTTCCAGGAGCGA-3, 5-CACAATGCCGAAGTGGTCGT-3; type I collagen gene: 5-CTTCTGGCCCTGCTGGAAAGGATG-3, 5-CCCGGATACAGGTTTCGCCAGTAG-3; type II collagen gene: 5′-AACACTGCCAACGTCCAGAT-3′, 5′-CTGCAGCACGGTATAGGTGA-3′; Aggrecan: 5′-GCTACGGAGACAAGGATGAGTTC-3′, 5′-CGTAAAAGACCTCACCCTCCAT-3′.
Statistical Analysis
DNA, GAG, and gene expression are reported as means ± standard deviation with n=4. Repetitive ANOVA and Tukey’s multiple comparison test were used to determine possible significant differences (p < 0.05) in the DNA contents, GAG contents, and gene expression for each group.
Results
The composition of each group compared in the study is provided in Table 1. All the rabbit MSCs were homogenously dispersed throughout hydrogels and observed to be intact and viable when encapsulated in OPF hydrogel composites (Figure 1). The cells were also shown to aggregate around gelatin microparticles at later time points (Figure 1B); cell aggregation was not observed in groups containing only OPF.
Table 1.
Experimental groups of hydrogel composites.
| Study 1: Effect of Formulation of Hydrogel Composites on MSC Differentiation | |||||
|---|---|---|---|---|---|
| Formulation | OPF | PEG-DA | Microparticles | TGF-β1 | MSCs |
| OPF | 0.1 g | 0.05 g | - | - | 10 million cells/ml |
| OPF + UP | 0.1 g | 0.05 g | 0.0219 g | - | 10 million cells/ml |
| OPF + LP | 0.1 g | 0.05 g | 0.0219 g | 10 ng/ml | 10 million cells/ml |
|
Study 2: Effect of MSC Seeding Density of Hydrogel Composites on MSC Differentiation | |||||
| Seeding density | OPF | PEG-DA | Microparticles | TGF-β1 | MSCs |
| Low cell density | 0.1 g | 0.05 g | 0.0219 g | 10 ng/ml | 10 million cells/ml |
| High cell density | 0.1 g | 0.05 g | 0.0219 g | 10 ng/ml | 20 million cells/ml |
|
Study 3: Effect of TGF-β1 concentration of Hydrogel Composites on MSC Differentiation | |||||
| Concentration | OPF | PEG-DA | Microparticles | TGF-β1 | MSCs |
| Low dose | 0.1 g | 0.05 g | 0.0219 g | 3.6 ng/ml | 10 million cells/ml |
| Middle dose | 0.1 g | 0.05 g | 0.0219 g | 10 ng/ml | 10 million cells/ml |
| High dose | 0.1 g | 0.05 g | 0.0219 g | 16 ng/ml | 10 million cells/ml |
Figure 1.
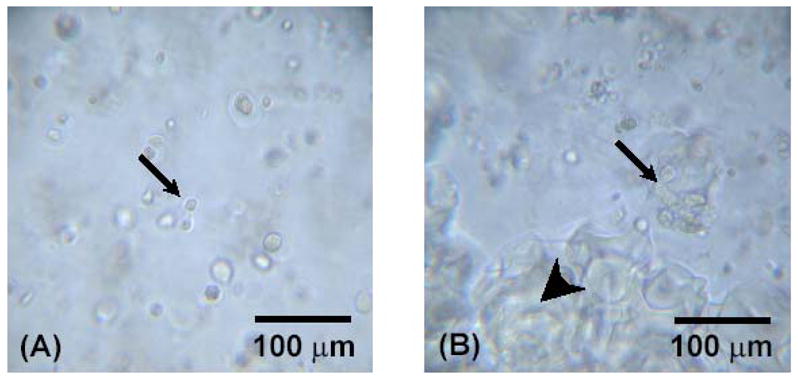
Light microscopy of OPF hydrogel composites containing rabbit MSCs. Small arrows indicate encapsulated rabbit MSCs, and big arrows indicate encapsulated microparticles. Figures (A) and (B) represent OPF hydrogel composites with only rabbit MSCs and with rabbit MSCs and TGF-β1-loaded microparticles at day 7, respectively.
Effect of carrier composition and TGF-β1 release on chondrogenic differentiation of encapsulated MSCs
Biochemical assays
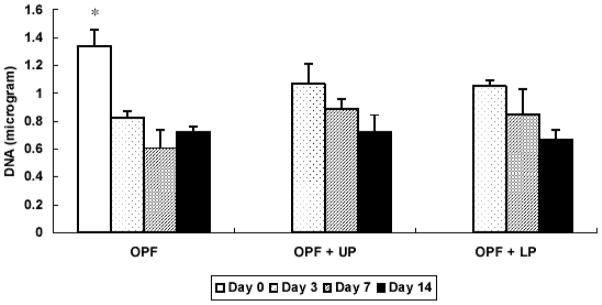
DNA content at each time point is provided in Figure 2. A significant decrease in DNA content was observed during the first 3 days. However, the DNA content remained statistically unchanged for days 3–14 for all sample types.
Figure 2.
DNA content of OPF hydrogel composites encapsulating rabbit MSCs, rabbit MSCs and unloaded microparticles, or rabbit MSCs and TGF-β1-loaded microparticles. Samples marked by (*) exhibited significantly higher DNA content than all other groups (p<0.05). Error bars represent means ± standard deviation with n = 4.
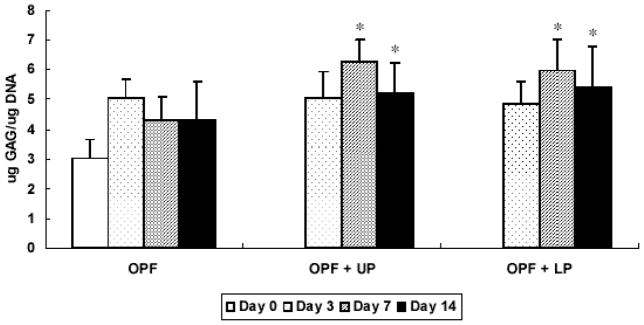
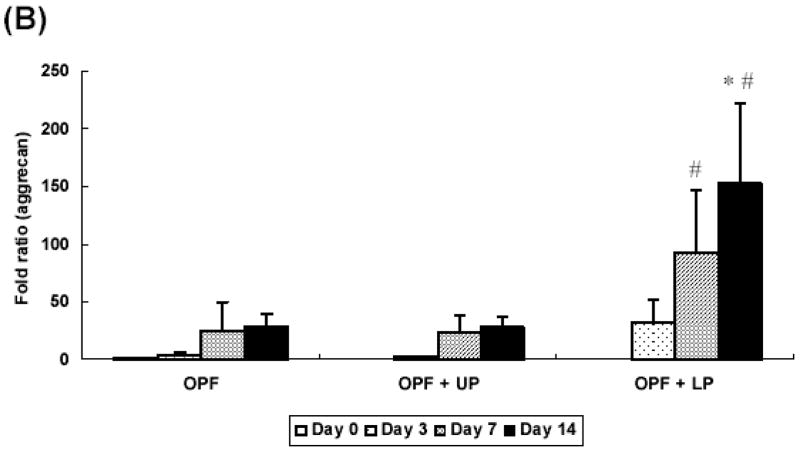
GAG content for each composite is shown in Figure 3. A significant increase in GAG/DNA content was found at day 7 and day 14 only in the groups containing gelatin microparticles as compared with day 0 controls.
Figure 3.
GAG/DNA content for OPF hydrogel composites encapsulating rabbit MSCs, rabbit MSCs and unloaded microparticles, or rabbit MSCs and TGF-β1-loaded microparticles. Significantly higher (p<0.05) GAG/DNA content than day 0 values (controls) are noted with (#). Error bars represent means ± standard deviation with n = 4.
Histology
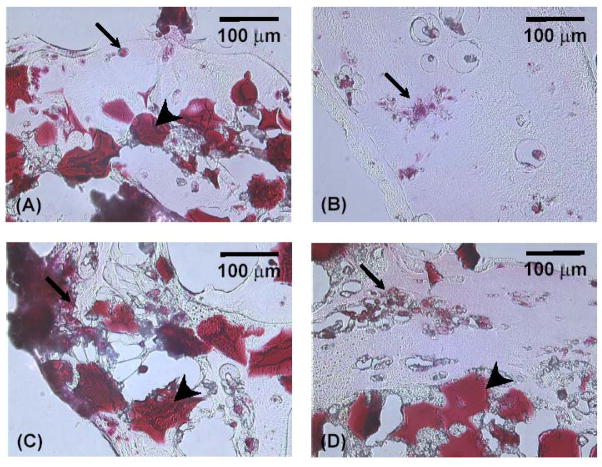
The GAG within cell-containing constructs was analyzed by Safranin-O. Figure 4 displays cross sections of these constructs at day 0 and 14. MSCs (indicated by small arrows) and gelatin microparticles (indicated by large arrows) were clearly stained with a deep red color. Due to the different swelling properties of the OPF network and gelatin microparticles, the preparation of samples for histology resulted in tearing of some samples. As seen in Figure 4, cells were homogenously distributed throughout hydrogel composites and, additionally, MSCs maintained a round shape and formed cell aggregates during culture period. Apparently larger cell aggregates were observed in sections of hydrogels with microparticles compared to cells in OPF hydrogel without microparticles. In addition, a light red background was seen around cell aggregates.
Figure 4.
Cross-sections of OPF hydrogel composites encapsulating rabbit MSCs and TGF-β1-loaded microparticles (A) at day 0. Figure (B), (C), and (D) represent OPF hydrogel composites encapsulating rabbit MSCs, rabbit MSCs and unloaded microparticles, and rabbit MSCs and TGF-β1-loaded microparticles at days 14, respectively. Sections were stained with Safranin-O. A scale bar represents 100 μm for all images.
Real time PCR
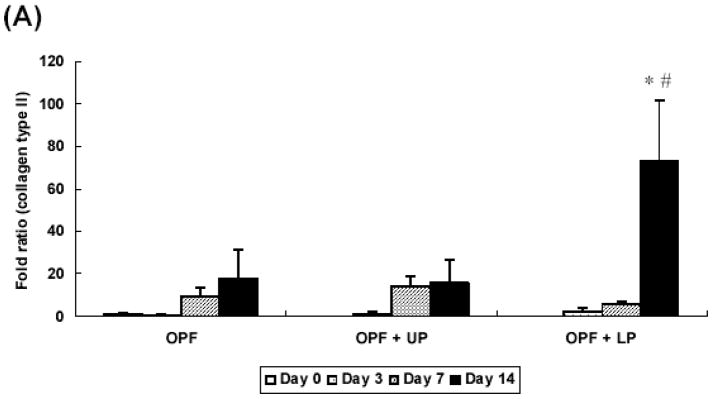
Groups containing TGF-β1-loaded microparticles showed a significant increase in collagen type II at day 14 as compared to the control group (no growth factor at day 0), while cells encapsulated in OPF hydrogels without microparticles and with microparticles without TGF-β1 did not demonstrate an increase in collagen type II gene expression (Figure 5A).
Figure 5.
Quantitative analysis of gene expression for OPF hydrogel composites encapsulating rabbit MSCs (OPF), rabbit MSCs and unloaded microparticles (OPF+UP), or rabbit MSCs and TGF-β1-loaded microparticles (OPF+LP): (A) Collagen type II; (B) Aggrecan; (C) Collagen type I. Data are presented as a fold ratio after normalized to GAPDH. The expression level of controls (Day 0) is represented as one. Within a given hydrogel formulation, significantly higher (p<0.05) gene expression than day 0 values (controls) are noted with (#). Samples indicated with (*) had significantly higher gene expression than the other two groups at the same time point (p<0.05). Error bars represent means ± standard deviation with n = 4.
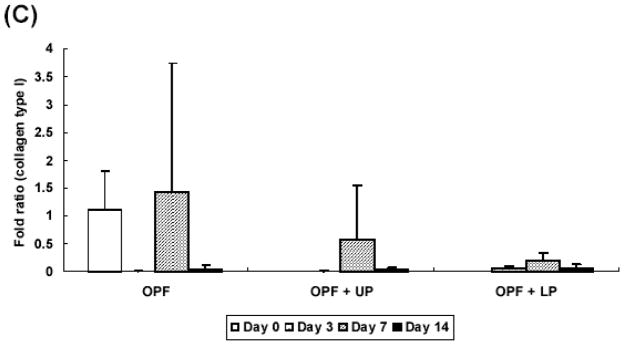
Aggrecan gene expression was more evident in groups with TGF-β1-loaded microparticles. Aggrecan expression showed 93 fold increase compared with controls at day 7 and increased up to 152 fold by day 14 (Figure 5B). The expression of collagen type I was not significantly different across the culture period (Figure 5C).
Effect of cell seeding density in hydrogel composites on chondrogenic differentiation of encapsulated MSCs
Biochemical assays
Although the use of a higher seeding density (20 million cells/ml) resulted in a higher DNA content than those measured for the lower seeding density (10 million cells/ml), no significant differences in DNA and GAG/DNA were observed in both groups over the culture period (data not shown).
Real time PCR
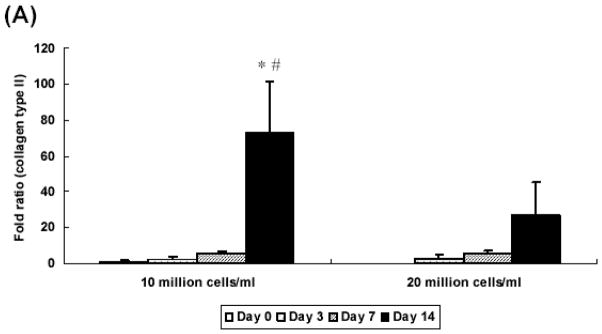
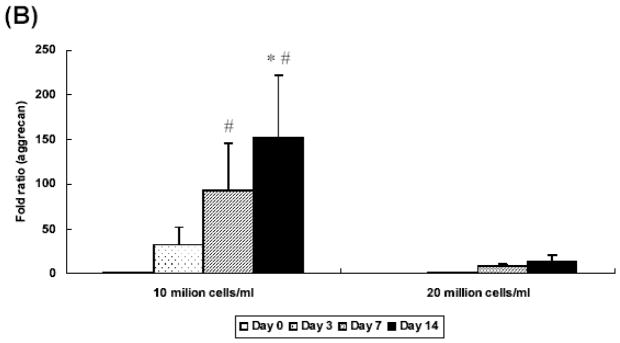
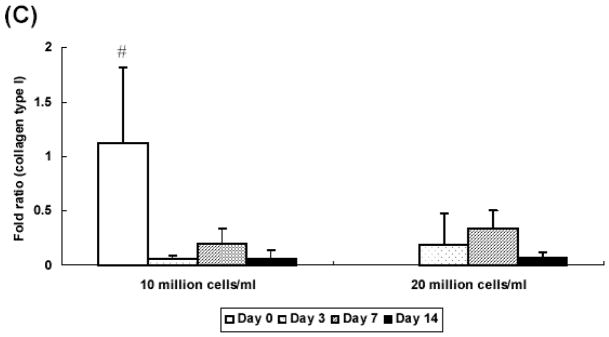
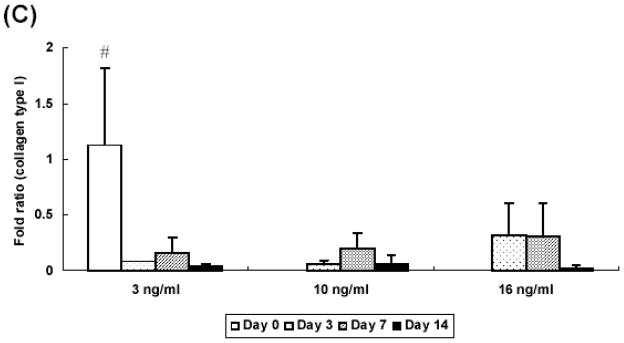
At 2 weeks, hydrogel constructs with low seeding density showed a 70 fold increase of collagen type II gene expression over controls prepared without growth factor at day 0 while those with high seeding density showed a 30 fold increase (Figure 6A). At day 7 and 14, only the low seeding density group had aggrecan gene expression that was significantly higher than control groups prepared without growth factor at day 0 (Figure 6B). Additionally, aggrecan expression was significantly lower in the high seeding density group (a 152 fold increase) as compared with the low seeding density group (a 14 fold increase) at day 14. Collagen type I gene expression was downregulated in all groups at day 3 and remained unchanged over culture period, as compared with controls (Figure 6C).
Figure 6.
Quantitative analysis of gene expression for OPF hydrogel composites encapsulating rabbit MSCs and TGF-β1-loaded microparticles with different seeding densities (10 and 20 million cells/ml): (A) Collagen type II; (B) Aggrecan; (C) Collagen type I. Data are presented as fold ratio after normalized to GAPDH. The expression level of controls (Day 0) is represented as one. Within a given hydrogel formulation, significantly higher (p<0.05) gene expression than day 0 values (controls) are noted with (#). Samples indicated with (*) had significantly higher gene expression than the other two groups at the same time point (p<0.05). Error bars represent means ± standard deviation with n = 4.
Effect of TGF-β1 loading concentration on chondrogenic differentiation of encapsulated MSCs
Biochemical assays
No significant differences in DNA and GAG/DNA were observed in all of the groups over the culture period (data not shown).
Real time PCR
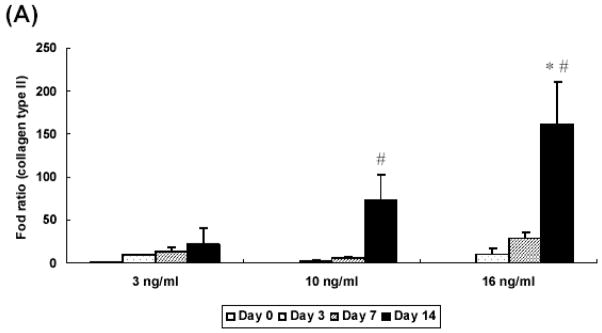
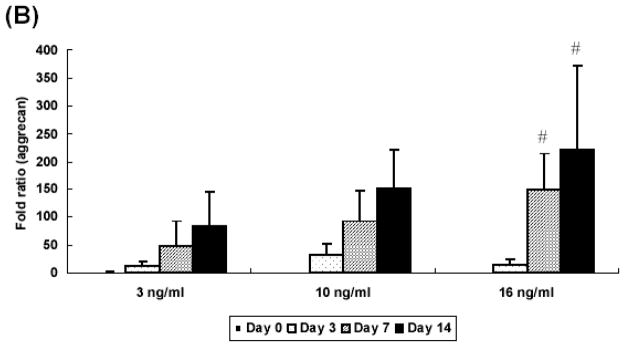
Experiments with different concentrations of TGF-β1 revealed that the expression of collagen type II was TGF-β1 concentration-dependent. Cells in high concentration (16 ng/ml) showed the greatest increase over the culture period, resulting in significantly higher expression as compared with low levels (3 ng/ml, 10 ng/ml) (Figure 7A). Aggrecan gene expression was also upregulated in concentration-dependent manner. The high concentration group (16 ng/ml) demonstrated a 200 fold increase at day 14, while the medium (10 ng/ml) and low (3 ng/ml) doses showed a 150- and 100- fold increase, respectively (Figure 7B). As shown in Figure 7C, type I collagen expression significantly decreased at day 3 than day 0 for all sample types and remained constant at that level during the culture period.
Figure 7.
Quantitative analysis of gene expression for OPF hydrogel composites encapsulating rabbit MSCs and TGF-β1-loaded microparticles with different concentrations of TGF-β1 (3 ng/ml, 10 ng/ml, and 16 ng/ml): (A) Collagen type II; (B) Aggrecan; (C) Collagen type I. Data are presented as fold ratio after normalized to GAPDH. The expression level of controls (Day 0) is represented as one. Within a given hydrogel formulation, significantly higher (p<0.05) gene expression than day 0 values (controls) are noted with (#). Samples indicated with (*) had significantly higher gene expression than the other two groups at the same time point (p<0.05). Error bars represent means ± standard deviation with n = 4.
Discussion
The objective of this study was to examine the effects of the presence of gelatin microspheres alone or microspheres loaded with TGF-β1 on the chondrogenic differentiation of rabbit MSCs embedded in OPF hydrogels. Additionally, cell proliferation, gene expression and matrix production from encapsulated MSCs were monitored at two cell seeding densities (10 million cells/ml and 20 million cells/ml) and three doses of TGF-β1 (3 ng/ml, 10 ng/ml and 16 ng/ml) over 14 days in vitro.
Due to the scope of this study, three separate experiments were devised (see Table 1). The first, designed to assess the effects of the presence of gelatin microparticles and the sustained release of TGF-β1 (10 ng/ml) on chondrogenic differentiation, included the following sample types: rabbit MSCs encapsulated in OPF, rabbit MSCs and microparticles without TGF-β1 encapsulated in OPF, and rabbit MSCs and TGF-β1-loaded microparticles in OPF.
Results of the DNA assay showed no significant differences in all groups during 14 days of in vitro culture except the first three days, suggesting that cell death may have occurred during the encapsulation process even though previous studies with OPF hydrogels showed that this system is cytocompatible [7, 14]. A similar decrease of DNA was reported with rat calvarial osteoblasts embedded in poly(ethylene glycol) diacrylate and rat MSCs encapsulated in OPF hydrogels [14, 20]. However, in a study with OPF hydrogels encapsulating bovine chondrocytes, a significant increase in DNA content was found in groups containing gelatin microparticles [7], indicating that rabbit MSCs experienced different cellular activity as compared with bovine chondrocytes under the same conditions.
GAG, matrix molecules associated with the chondrocytic phenotype, were measured to examine the chondrogenic differentiation of rabbit MSCs. As shown in Figure 3, hydrogels containing gelatin microparticles showed a significant increase in GAG/DNA content starting at day 7, indicating that gelatin microparticles may promote more GAG production compared to hydrogels without gelatin microparticles. A previous study with OPF hydrogel encapsulating chondrocytes and gelatin microparticles showed a significant increase in GAG production as compared with OPF hydrogels alone [7]. Additionally, it has been reported that rabbit MSCs embedded in PLGA scaffolds modified with gelatin and chondroitin sulfate also showed the increase in GAG production as compared to PLGA alone [21]. However, it appears that MSCs encapsulated in OPF hydrogel composites produced less GAG than when cultured on scaffolds with pore sizes of 500 μm [21]. It is possible that GAG production was limited by the polymer mesh surrounding MSCs.
Histology results showed that rabbit MSCs exhibited a round morphology similar to chondrocytes in hydrogel constructs and also formed cell aggregates in hydrogel composites, indicating that cells were intact, moved together, and interacted with each other throughout the constructs. It is well known that cell condensation occurs in joint development in vivo, leading to the formation of cartilage tissue[22]. Therefore, cell-cell contact may be important in promoting chondrogenic differentiation of MSCs in this system.
Additionally, the relative gene expression of collagen type II, aggrecan, and collagen type I was analyzed as an indicator for chondrogenic differentiation of rabbit MSCs with real time PCR. Production of collagen type II and aggrecan are markers of chondrogenic differentiation of MSCs, while collagen type I produced by fibroblasts or undifferentiated MSCs [23]. The results showed a significant increase in collagen type II expression at day 14 and aggrecan expression starting at day 7 in groups containing TGF-β1-loaded microparticles over control groups, indicating that TGF-β1-loaded microparticles promote chondrogenic differentiation of rabbit MSCs. Although GAG/DNA production was increased with the presence of gelatin microparticles alone, these results suggest that the presence of TGF-β1 is required to induce expression of chondrocyte-associated genes.
Collagen type I gene expression was consistently low over the culture period, regardless of sample type. A possible explanation for this is that MSCs encapsulated in hydrogels maintained a round morphology during the culture period. In these experiments, the change in shape from a spread morphology during preculture to a round morphology after encapsulation could have induced the decrease in collagen I expression seen in the first three days (and maintained over the remaining culture period). A study with chondrocytes embedded in alginate also showed a similar trend of decreasing collagen type I expression [24].
The effect of seeding density on cell differentiation was also investigated since the cell density is a critical factor for chondrogenic differentiation of MSCs embedded in a scaffold [25]. A previous study with bovine chondrocytes embedded in agarose hydrogels revealed that higher seeding densities (20 and 60 million cells/ml) showed more collagen and GAG content and better mechanical strength over a lower seeding density (10 million cells/ml). However, this study also showed that the highest seeding density (60 million cells/ml) may cause a limitation of nutrient availability [26].
Based on this study, as well as our previous work with bovine chondrocytes seeded at 9 million cells/ml [7], for these experiments we selected two different seeding densities (10 million cells/ml and 20 million cells/ml). Real time PCR revealed that OPF hydrogel constructs with a low seeding density demonstrated higher collagen type II and aggrecan gene expression at day 14 than those with higher seeding density. Although it has been reported that high cell seeding density promoted more GAG and collagen production by chondrocytes [26], it appears that the chondrocytic gene expression in OPF hydrogel composites was affected more by the TGF-β1 dose than by the seeding density. In this system, because the microparticles in both groups were loaded with the same amount of TGF-β1 (10 ng/ml) and the same amount of MPs were applied per gel, the concentration of TGF-β1 that each cell experienced in high density groups could have been relatively lower as compared with low density groups. To further explore this explanation of these results, we examined the effect of TGF-β1 concentration on the chondrogenic gene expression of MSCs embedded in hydrogel constructs in a third set of experiments.
In the third study, three different concentrations of TGF-β1 (3 ng/ml, 10 ng/ml, and 16 ng/ml) in OPF hydrogel composites were used. A pellet culture study with rabbit MSCs showed that lowering the TGF-β1 concentration in the medium decreased chondrogenesis. When rabbit MSC pellets were exposed to 0.5 ng/ml and 1 ng/ml TGF-β1, they demonstrated partial chondrogenesis in the outer layer of pellets. However, chondrogenesis was observed throughout pellets cultured with 10 ng/ml TGF-β1 [27]. Based on this, 10 ng/ml TGF-β1 was chosen as the “medium dose” for our study. A previous study in our laboratory showed that gelatin microparticles loaded with TGF-β1 at 3 ng/ml were able to promote cell proliferation and GAG production when encapsulated in OPF hydrogels with bovine chondrocytes. Thus, 3 ng/ml was selected as the lowest dose. 16 ng/ml of TGF-β1 was chosen because a prior experiment revealed that, at this loading concentration, that 10 ng/ml of TGF-β1 was expected to remain in the hydrogel composites at day 14 [7]. The level of gene expression corresponded to the concentration of TGF-β1 initially loaded into the gelatin microparticles. Higher gene expression of collagen type II and aggrecan was found in groups with the highest dose of TGF-β1 (16 ng/ml). However, changes in GAG production were not observed, indicating that the upregulation of aggrecan gene expression did not result in an increase in extracellular aggregating species over 14 days. Cells in hydrogel constructs with the lowest dose of TGF-β1 (3 ng/ml) did not show a significant increase over control groups during the culture period, indicating that there may be a threshold dose of TGF-β1 that influence the differentiation of cells in OPF hydrogels. Interestingly, the total amount of TGF-β1 required to have significant effects on chondrogenic differentiation of rabbit MSCs was lower than other in vitro studies, suggesting that the localized and sustained delivery of TGF-β1 from gelatin microparticles was still able to promote cartilage relevant gene expression of rabbit MSCs. This result indicates that not only does the OPF hydrogel encapsulating gelatin microparticles provide a carrier for cells and growth factors, but also functions as an effective way to deliver the growth factor to cells.
Conclusion
Rabbit MSCs encapsulated within OPF hydrogel composites remained viable and maintained a round morphology over 14 days in vitro. On a per-cell basis, the presence of gelatin microparticles promoted GAG production over OPF hydrogels alone. Real time RT-PCR demonstrated the upregulation of cartilage-relevant genes such as collagen type II and aggrecan when MSCs were encapsulated with microparticles loaded with TGF-β1. Additionally, gene expression was dependent on the dose of TGF-β1 originally loaded, suggesting a threshold level of TGF-β1 release is required to induce chondrogenic differentiation in this system, Taken together, these results indicate that OPF hydrogel composites are a promising material for concurrent cell and growth factor delivery for cartilage tissue regeneration.
Acknowledgments
This work has been supported by the National Institutes of Health (R01 AR48756).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21(5):431–440. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 2.Jackson DWST. Tissue engineering principles in orthopaedic surgery. Clin Orthop. 1999;367 (Suppl):31–45. doi: 10.1097/00003086-199910001-00005. [DOI] [PubMed] [Google Scholar]
- 3.Kisiday JMJ, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99(15):9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elisseeff JMW, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. Journal of Biomedical Materials Research. 2000;51(2):164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Lee KYMD. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 6.Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75(1):156–167. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 7.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26(34):7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 8.Holland TATJ, Tabata Y, Mikos AG. Transforming growth factor-1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. Journal of Controlled Release. 2003;94(1):101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Holland TATY, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. Journal of Controlled Release. 2003;91(3):299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 10.Jo S, Shin H, Mikos AG. Modification of oligo(poly(ethylene glycol) fumarate) macromer with a GRGD peptide for the preparation of functionalized polymer networks. Biomacromolecules. 2001;2(1):255–261. doi: 10.1021/bm000107e. [DOI] [PubMed] [Google Scholar]
- 11.Jo S, Shin H, Shung AK, Fisher JP, Mikos AG. Synthesis and characterization of oligo(poly(ethylene glycol) fumarate) macromer. Macromolecules. 2001;34(9):2839–2844. [Google Scholar]
- 12.Shin H, Quinten Ruhe P, Mikos AG, Jansen JA. In vivo bone and soft tissue response to injectable, biodegradable oligo(poly(ethylene glycol) fumarate) hydrogels. Biomaterials. 2003;24(19):3201–3211. doi: 10.1016/s0142-9612(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 13.Shin HZK, Farach-Carson MC, Yaszemski MJ, Mikos AG. Modulation of differentiation and mineralization of marrow stromal cells cultured on biomimetic hydrogels modified with Arg-Gly-Asp containing peptides. J Biomed Mater Res. 2004;69A(3):535–543. doi: 10.1002/jbm.a.30027. [DOI] [PubMed] [Google Scholar]
- 14.Temenoff JS, Park H, Jabbari E, Sheffield TL, LeBaron RG, Ambrose CG, et al. In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels. J Biomed Mater Res A. 2004;70(2):235–244. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 15.Temenoff JS, Park H, Jabbari E, Conway DE, Sheffield TL, Ambrose CG, et al. Thermally cross-linked oligo(poly(ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro. Biomacromolecules. 2004;5(1):5–10. doi: 10.1021/bm030067p. [DOI] [PubMed] [Google Scholar]
- 16.Ashcroft GS. Bidirectional regulation of macrophage function by TGF-beta. Microbes Infect. 1999;1(15):1275–1282. doi: 10.1016/s1286-4579(99)00257-9. [DOI] [PubMed] [Google Scholar]
- 17.Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, et al. Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng. 2002;8(2):333–347. doi: 10.1089/107632702753725085. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12(1):77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 19.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta (BBA) - General Subjects. 1986;883(2):173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 20.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23(22):4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 21.Fan H, Hu Y, Zhang C, Li X, Lv R, Qin L, et al. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials. 2006;27(26):4573. doi: 10.1016/j.biomaterials.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7–8):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 23.Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5(1):14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 24.Bonaventure J, Kadhom N, Cohen-Solal L, Ng KH, Bourguignon J, Lasselin C, et al. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res. 1994;212(1):97–104. doi: 10.1006/excr.1994.1123. [DOI] [PubMed] [Google Scholar]
- 25.Kavalkovich KW, Boynton RE, Murphy JM, Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002;38(8):457–466. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Mauck RL, Seyhan SL, Ateshian GA, Hung CT. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30(8):1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]