Abstract
BACE1 and presenilin (PS)/γ-secretase are primary proteolytic enzymes responsible for the generation of pathogenic amyloid β-peptides (Aβ) in Alzheimer’s disease. We and others have found that β-subunits of the voltage-gated sodium channel (Navβs) also undergo sequential proteolytic cleavages mediated by BACE1 and PS/γ-secretase. In a follow-up study, we reported that elevated BACE1 activity regulates total and surface expression of voltage-gated sodium channels (Nav1 channels) and thereby modulates sodium currents in neuronal cells and mouse brains. In this review, we focus on the molecular mechanism of how BACE1 and PS/γ-secretase regulate Nav1 channels in neuronal cells. We will also discuss potential physiological and pathological roles of BACE1- and PS/γ-secretase-mediated processing of Navβs in relation to Nav1 channel function.
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly [2]. AD patients lose their ability to acquire new memories and the capacities for reasoning, abstraction, and language skills. In addition, AD patients in late stages frequently show severe personality changes and various neuropsychiatric symptoms, including depression, aggressiveness, agitation, and generalized anxiety [1, 70]. Epileptic and myoclonic seizures are common in early-onset AD patients with familial presenilin mutations, but are also frequently found in late-onset forms of the disease [9, 17, 18, 31].
Two major pathological hallmarks of AD are extracellular amyloid deposits (senile plaques) and hyperphosphorylated tau protein in neurofibrillary tangles [54]. Amyloid deposits are composed predominantly of amyloid β peptides (Aβ), central in AD pathogenesis [16, 47]. Aβ is generated from sequential cleavage of the amyloid β precursor protein (APP), mediated by β–site APP cleaving enzyme 1 (β-secretase, memapsin 2, BACE1) and presenilin/γ-secretase (PS/γ-secretase).
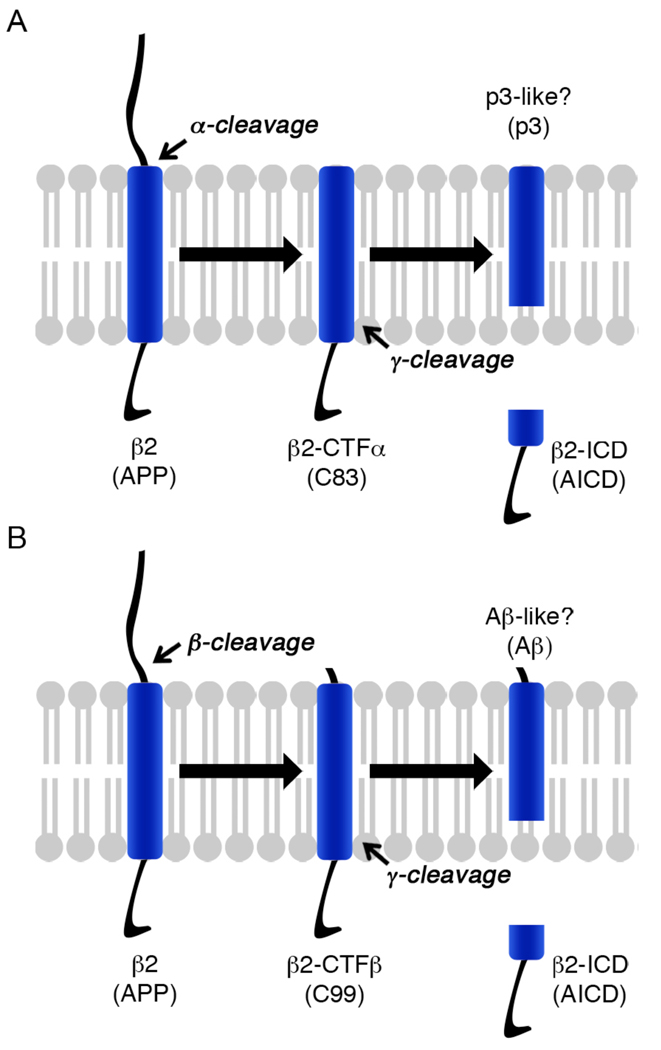
In neuronal as well as non-neuronal cells, APP undergoes two distinctive cleavage pathways mediated by α-/γ- or β-/γ- secretases (Fig. 1). Cleavage by α-secretase gives rise to a soluble extracellular domain (sAPP-α) and a C-terminal fragment (C83) that is further processed by PS/γ-secretase to generate p3 fragment and an intracellular domain (AICD, Fig 1A). In β-/γ- secretase pathway, APP is first cleaved by BACE1 to generate sAPPβ and C99 that is then converted to AICD and Aβ by PS/γ-secretase activity (Fig 1B).
Fig. 1.
APP and Navβ2 are processed via similar cleavage pathways. (A) In the nonamyloidogenic pathway, APP/β2 undergo extracellular domain shedding by α- secretase and is subsequently cleaved by PS/γ-secretase to produce β2/APP intracellular domain (AICD/β2-ICD). (B) In the amyloidogenic pathway, APP/β2 first undergo an extracellular domain shedding by BACE1. A membrane-tethered C-terminal fragment, C99/ β2-CTFβ, is then cleaved by PS/γ-secretase to produce AICD/β2-ICD and the amyloid β peptide (Aβ).
BACE1 is a membrane-bound aspartic protease that is highly expressed in the brain [22, 51, 56, 67]. PS/γ-secretase is a membrane protease complex consisting of Nicastrin, Aph-1, Pen-2, and a catalytic component PS, which is ubiquitously expressed in various tissues [49]. These proteases have been extensively studied to understand their pathological roles in Alzheimer’s disease. A number of studies have suggested that altered BACE1 and/or PS/γ-secretase activity play important roles in the pathogenesis of sporadic and familial AD by modulating Aβ generation [3, 12, 14, 49, 55, 68]. In addition to their pathological roles, mouse knockout studies have also demonstrated that these proteases play important physiological roles in brain function. BACE1-null mice show cognitive and behavioral deficits together with altered electrophysiological properties in neurons [11, 30, 46, 59]. BACE1-null mice even display spontaneous behavioral seizures [21]. Deficits in sodium channel may contribute to these phenotypes since hippocampal neurons from BACE1-null mice display a positive shift in voltage-dependent sodium current inactivation as well as an increase in sodium current densities as compared to control wild-types [11, 21]. Adult-specific deletion of PS also induces deficits in synaptic plasticity and presynaptic function and even neurodegeneration in mice [45, 50, 69, 71]. These deficits likely derive from altered cleavages of neuronal substrate proteins of BACE1 and/or PS/γ-secretase.
In addition to APP, dozens of additional BACE1 substrates and PS/γ-secretase substrates have been reported to date. This supports the proposed multifunctional roles of BACE1 and PS/γ-secretase [35, 49, 57]. While most PS/γ-secretase substrates undergo a sequential cleavage pathway regulated by α-/γ- secretase, only a few PS/γ-secretase substrate proteins also undergo an alternative β-/γ- secretase cleavage pathway similar to APP. In brains, Neuregulins 1, 3 (NRG-1, 3) and β-subunits of the voltage-gated sodium channel (Navβs) are reported as cleaved by both BACE1 and PS/γ-secretase under physiological conditions [19, 20, 28, 29, 64, 65].
Similar to APP, Navβ2 is processed by two distinctive cleavage cascades mediated by α-/γ- secretase or β-/γ- secretase (Fig 1). Cleavage by α-secretase (ADAM10) gives rise to a C-terminal fragment (β2-CTFα) that is further processed by PS/γ-secretase activity to generate an intracellular domain (β2-ICD, Fig 1A). In the β-/γ- pathway, Navβ2 is first cleaved by BACE1 to generate β2-CTFβ and then converted to β2-ICD by PS/γ-secretase activity (Fig 1B). It is not clear yet whether PS/γ-secretase -meditated processing of β2-CTFs also generates Aβ-like peptides.
Navβs assemble with channel-forming α-subunits and regulate cell surface expression and inactivation channel kinetics of the voltage-gated sodium channels (Nav1 channels) [6, 7, 24, 25]. In addition, Navβs interact with neuronal adhesion molecules and therefore play a role in neuronal adhesion and migration [4, 5, 25, 34]. While all the β-subunits (Navβ1–4) are cleaved by BACE1 and PS/γ-secretase in vitro, only Navβ2 and Navβ4 have been shown as physiological BACE1 substrates in mouse brains [65]. Our laboratory focused on Navβ2 because of its neuron-specific expression and primary role in regulating sodium channel α-subunits in hippocampus and cortex [8, 26].
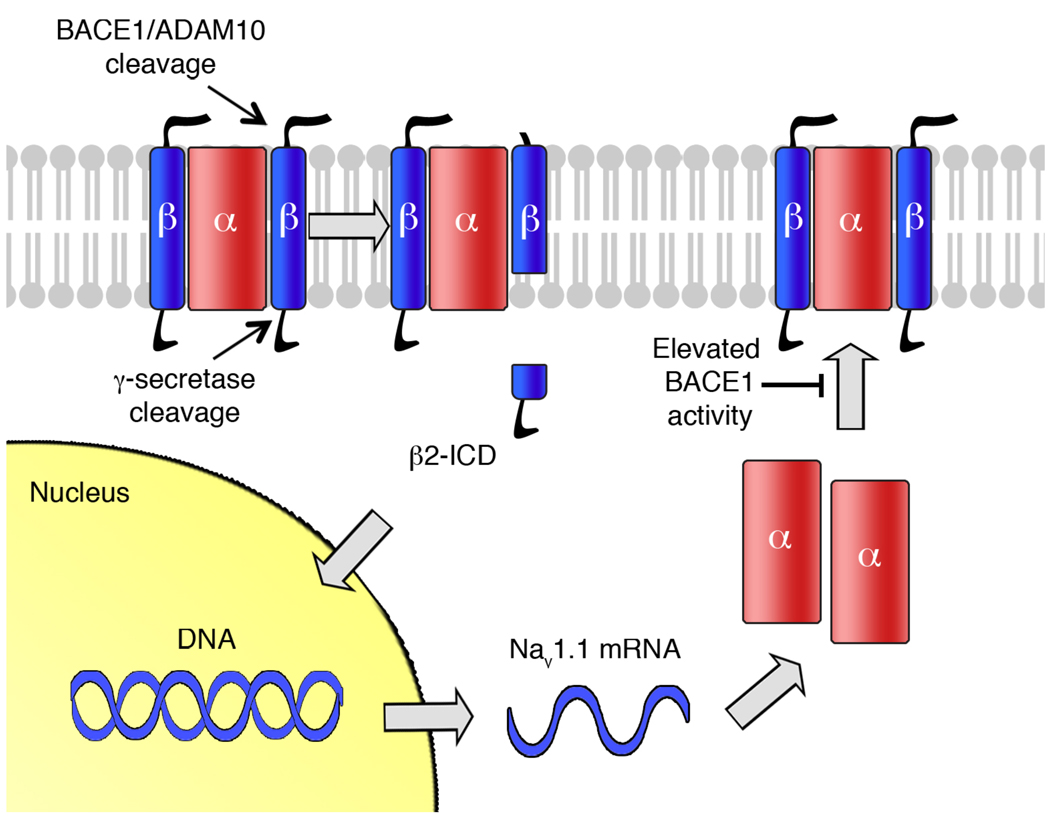
One of the well-known PS/γ-secretase functions is to modulate signaling cascades via “regulated intramembrane proteolysis” or RIP of specific proteins [35, 48]. For example, PS/γ-secretase-mediated cleavage of the Notch receptor releases a membrane-bound intracellular domain (NICD) that localizes to the nucleus and regulates target gene transcription [48]. Since PS/γ-secretase-mediated cleavage of Navβ2 also releases β2-ICD, we investigated whether PS/γ-secretase can initiate RIP by cleaving Navβ2. To directly address a potential nuclear function of β2-ICD, we generated a recombinant β2-ICD fragment and expressed it in rat and human neuroblastoma cells. We found that this recombinant β2-ICD fragment localized to the nucleus and specifically increased both protein and mRNA levels of a sodium channel α-subunit, Nav1.1 [28]. Elevated BACE1 activity increased β2-ICD levels and thereby Nav1.1 levels while the inhibition of BACE1 or PS/γ-secretase activity significantly decreased Nav1.1 levels in neuroblastoma cells and cultured primary neurons [28, 29]. We also found that levels of Navβ2 cleavage products and Nav1.1 α-subunits were increased in brains of BACE1-trangenic mice as compared to wild type controls [28, summarized in table 1]. Although further studies are required to see whether β2-ICD interacts with other transcriptional machinery to regulate gene transcription under physiological conditions, our data suggest a novel mechanism of Nav1 channel regulation through RIP of Navβ2.
Table 1.
Summary of Nav1 channel changes by elevated BACE1 activity.
| Neuroblastoma cells with BACE1 overexpression |
BACE1-transgenic mouse brains |
|
|---|---|---|
| Full-length Navβ2 | ↓1 | ↓1 |
| Navβ2-CTF | ↑ | ↑ |
| β2-ICD | ↑ | N/D 2 |
| scn1a (Nav1.1) mRNA | ↑ | ↑ |
| Nav1.1 total protein | ↑ | ↑ |
| Nav1.1 surface protein | ↓ | ↓3 |
| Na+ current density | ↓ | ↓3 |
Moderate or slight decrease;
not detected;
Nav1 channel surface levels and sodium current density are analyzed in hippocampal neurons acutely dissociated from BACE1-transgenic mice
When we checked whether elevated total Nav1.1 levels increased voltage-dependent sodium currents, paradoxically we found that BACE1 elevation dramatically decreased sodium current density and surface levels of Nav1 α-subunits in neuroblastoma cells and hippocampal neurons [28, please see Fig. 2]. These results strongly suggest that Nav1.1 α-subunits resulting from Navβ2 cleavage followed by increased Nav1.1 α-subunit mRNA, are not translocated into the plasma membrane (Fig 2). One possibility is that the intracellular accumulation of Nav1.1 precursors may directly or indirectly interfere with Nav1 channel trafficking. In the same publication, we have shown that elevated BACE1 activity induces Nav1.1 accumulation in an unusual HSP70-positive intracellular compartment [28]. It is interesting to note that elevated BACE1 activity induces impaired trafficking of APP along axons, similar to the impaired trafficking of Nav1 α-subunits to the cell surface [32]. It is also possible that highly elevated BACE1 depletes Navβs, and in particular Navβ2, that is required for surface expression of Nav1 α-subunits. However, we were still able to detect significant amount of intact Navβ2 in brains of BACE1-transgenic mice [8, 28].
Fig. 2.
Schematic representation of Nav1 channel regulation by BACE1/ADAM10 and γ- secretase. Navβ2 undergoes ectodomain shedding by either α- (ADAM10) or β-secretase (BACE1). The resulting membrane-tethered C-terminal fragments are further cleaved by PS/γ-secretase to release β2-ICD, which then localizes to the nucleus. β2-ICD increases mRNA and protein levels of Nav1.1 α-subunit levels. However, elevated BACE1 activity also interferes with the trafficking of Nav1 α-subunits to cell surface.
BACE1 may also alter sodium currents by modulating additional α-subunits in addition to Nav1.1. Hu et al have recently shown that axonal and surface levels of Nav1.2 are significantly increased in hippocampal neurons from BACE1-null mice [21]. Similarly, elevated BACE1 activity may decrease surface levels of Nav1.2, contributing to the dramatic decrease of sodium currents that we have observed in BACE1-transgenic mice [28]. In addition, Huth et al. reported that overexpressed BACE1 can induce a hyperpolarizing shift of Nav1.2 current activation in cultured cell lines [23]. However, this finding does not explain the observed BACE1-mediated decrease of sodium current density in adult hippocampal neurons [28]. Further studies are required to clarify the molecular mechanism underlying BACE1-mediated regulation of Nav1 channel trafficking. These data suggest the interesting possibility that elevated BACE1 activity specifically modulates the surface expression of membrane proteins essential for neuronal function, such as sodium channels and APP.
Navβs interacts with various neuronal proteins including contactin, tenascin, neurofascin, NrCAM, receptor protein tyrosine phosphatase β (RPTPβ), and ankyrinG [4, 5, 10, 25, 27, 34, 36, 37, 66]. These interactions are reported to modulate axonal localization of Nav channels, surface expression, and even neuronal cell adhesion and migration [5, 10, 27, 34, 36]. BACE1 and/or PS/γ-secretase mediated cleavage of Navβs may affect these interactions and potentially modulate these functions. Interestingly, we found that blockage of Navβ2 cleavage by PS/γ-secretase inhibitors interferes with cell-cell adhesion and migration in cultured cells [29]. In addition, BACE1-mediated processing of Navβ4 increases neurite extention in Neuro2a cells [38]. These data suggest an additional molecular mechanism potentially contributing to BACE1/γ-secretase mediated regulation of neuronal function.
BACE1 and presenilins are directly involved in AD pathology in specific groups of patients. BACE1 activity and levels are significantly increased in brains of a subset of AD patients, possibly contributing to Aβ accumulation [12, 55, 68]. Elevated BACE1 levels in AD mouse models increase Aβ generation and deposition [72]. Mutations in PS1 and PS2 are tightly associated with early-onset familial Alzheimer’s disease (FAD) and all PS FAD mutations alter Aβ generation by modulating PS/γ-secretase activity [3, 14, 49]. Altered activities of these secretases may affect Nav1 channel metabolism as well as Aβ generation through the regulation of Navβ cleavages. We have already found that Navβ2 C-terminal fragment and Nav1.1 α-subunit levels are significantly increased in brains of AD patients with elevated BACE1 activity as compared to age-matched controls [28]. Altered Nav1 channel metabolism may contribute to neuronal dysfunction and/or neurodegeneration in the course of the disease. Indeed, epileptic and myoclonic seizures are common in early-onset AD with familial presenilin mutations, but are also found in late-onset forms of the disease [17, 52, 58]. Increased excitatory neuronal activities, so called ”silent seizures”, are also detected in AD animal models [41–43]. Further studies will be required to test whether elevated BACE1 contributes to AD pathogenesis by altering Nav1 channels.
Altered expression of sodium channels were reported in multiple sclerosis and chronic pain after nerve injuries [15, 60–62]. Interestingly, animal model studies have shown that Navβ2 contributes to the pathogenic mechanism in these conditions by regulating sodium channel metabolism [40, 44]. BACE1 levels and activity are increased in some other injury conditions including brain trauma [33] and ischemia [13, 53, 63]. O’Conor et al proposed that BACE1 functions as a stress-response protein elevated by phosphorylation of the Translation Initiation Factor eIF2α [39]. The fact that BACE1 regulates Nav1 channel metabolism through Navβ2 suggests the interesting possibility that BACE1 might modulate sodium channel metabolism not only in AD but also other disease conditions in which BACE1 levels are increased.
In summary, current data strongly suggest that BACE1 and PS/γ-secretase regulate Nav1 channel metabolism at multiple levels in vitro and in vivo conditions. Further studies will be required to characterize the exact molecular mechanism underlying this function of Alzheimer’s secretases.
Acknowledgements
This work is supported by grants from the NIH/NIA to DMK and DYK and from the Cure Alzheimer’s Fund to DMK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Assal F, Cummings J. Neuropsychiatric symptoms in the dementias. Curr Opin Neurol. 2002;15:445–450. doi: 10.1097/00019052-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 2.A.s. Association2010. Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Borchelt D, Thinakaran G, Eckman C, Lee M, Davenport F, Ratovitsky T, Prada C, Kim G, Seekins S, Yager D, Slunt H, Wang R, Seeger M, Levey A, Gandy S, Copeland N, Jenkins N, Price D, Younkin S, Sisodia S. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 4.Brackenbury W, Davis T, Chen C, Slat E, Detrow M, Dickendesher T, Ranscht B, Isom L. Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. J Neurosci. 2008;28:3246–3256. doi: 10.1523/JNEUROSCI.5446-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brackenbury WJ, Djamgoz MBA, Isom LL. An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist. 2008;14:571–583. doi: 10.1177/1073858408320293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 7.Catterall WA. Molecular mechanisms of gating and drug block of sodium channels. Novartis Found Symp. 2002;241:206–218. discussion 218–232. [PubMed] [Google Scholar]
- 8.Chen C, Bharucha V, Chen Y, Westenbroek R, Brown A, Malhotra J, Jones D, Avery C, Gillespie P, Kazen-Gillespie K, Kazarinova-Noyes K, Shrager P, Saunders T, Macdonald R, Ransom B, Scheuer T, Catterall W, Isom L. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proc Natl Acad Sci U S A. 2002;99:17072–17077. doi: 10.1073/pnas.212638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark DG, Mendez MF, Farag E, Vinters HV. Clinicopathologic case report: progressive aphasia in a 77-year-old man. J Neuropsychiatry Clin Neurosci. 2003;15:231–238. doi: 10.1176/jnp.15.2.231. [DOI] [PubMed] [Google Scholar]
- 10.Davis T, Chen C, Isom L. Sodium channel beta 1 subunits promote neurite 0utgrowth in cerebellar granule neurons. J Biol Chem. 2004 doi: 10.1074/jbc.M410830200. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho I, Marjaux E, Craessaerts K, Roebroek A, Schwake M, D'Hooge R, Bach P, Kalinke U, Moechars D, Alzheimer C, Reiss K, Saftig P, De Strooper B. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem. 2005;280:30797–30806. doi: 10.1074/jbc.M505249200. [DOI] [PubMed] [Google Scholar]
- 12.Fukumoto H, Cheung B, Hyman B, Irizarry M. beta-Secretase Protein and Activity Are Increased in the Neocortex in Alzheimer Disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 13.Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, Tabaton M. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J Neurochem. 2009;108:1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- 14.Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993;75:1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- 15.Hains BC, Waxman SG. Sodium channel expression and the molecular pathophysiology of pain after SCI. Prog Brain Res. 2007;161:195–203. doi: 10.1016/S0079-6123(06)61013-3. [DOI] [PubMed] [Google Scholar]
- 16.Hardy J, Selkoe D. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 17.Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer's disease. Neurology. 1986;36:1226–1230. doi: 10.1212/wnl.36.9.1226. [DOI] [PubMed] [Google Scholar]
- 18.Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology. 1996;46:727–730. doi: 10.1212/wnl.46.3.727. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, He W, Diaconu C, Tang X, Kidd G, Macklin W, Trapp B, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Hicks C, He W, Wong P, Macklin W, Trapp B, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Zhou X, He W, Yang J, Xiong W, Wong P, Wilson CG, Yan R. BACE1 deficiency causes altered neuronal activity and neurodegeneration. J Neurosci. 2010;30:8819–8829. doi: 10.1523/JNEUROSCI.1334-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain I, Powell D, Howlett D, Tew D, Meek T, Chapman C, Gloger I, Murphy K, Southan C, Ryan D, Smith T, Simmons D, Walsh F, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 23.Huth T, Schmidt-Neuenfeldt K, Rittger A, Saftig P, Reiss K, Alzheimer C. Non-proteolytic effect of beta-site APP-cleaving enzyme 1 (BACE1) on sodium channel function. Neurobiol Dis. 2009;33:282–289. doi: 10.1016/j.nbd.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Isom L. Sodium channel beta subunits: anything but auxiliary. Neuroscientist. 2001;7:42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- 25.Isom L. The role of sodium channels in cell adhesion. Front Biosci. 2002;7:12–23. doi: 10.2741/isom. [DOI] [PubMed] [Google Scholar]
- 26.Isom L, Catterall W. Na+ channel subunits and Ig domains. Nature. 1996;383:307–308. doi: 10.1038/383307b0. [DOI] [PubMed] [Google Scholar]
- 27.Kazarinova-Noyes K, Malhotra J, McEwen D, Mattei L, Berglund E, Ranscht B, Levinson S, Schachner M, Shrager P, Isom L, Xiao Z. Contactin associates with Na+ channels and increases their functional expression. J Neurosci. 2001;21:7517–7525. doi: 10.1523/JNEUROSCI.21-19-07517.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Carey B, Wang H, Ingano L, Binshtok A, Wertz M, Pettingell W, He P, Lee V, Woolf C, Kovacs D. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nature cell biology. 2007;9:755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D, Ingano L, Carey B, Pettingell W, Kovacs D. Presenilin/{gamma}-Secretase-mediated Cleavage of the Voltage-gated Sodium Channel {beta}2-Subunit Regulates Cell Adhesion and Migration. J Biol Chem. 2005;280:23251–23261. doi: 10.1074/jbc.M412938200. [DOI] [PubMed] [Google Scholar]
- 30.Laird F, Cai H, Savonenko A, Farah M, He K, Melnikova T, Wen H, Chiang H, Xu G, Koliatsos V, Borchelt D, Price D, Lee H, Wong P. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer's disease associated with mutations of the presenilin-1 gene. J Neurol. 2006;253:139–158. doi: 10.1007/s00415-005-0019-5. [DOI] [PubMed] [Google Scholar]
- 32.Lee E, Zhang B, Liu K, Greenbaum E, Doms R, Trojanowski J, Lee V. BACE overexpression alters the subcellular processing of APP and inhibits Abeta deposition in vivo. J Cell Biol. 2005;168:291–302. doi: 10.1083/jcb.200407070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, Burns MP. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra J, Kazen-Gillespie K, Hortsch M, Isom L. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J Biol Chem. 2000;275:11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy JV, Twomey C, Wujek P. Presenilin-dependent regulated intramembrane proteolysis and gamma-secretase activity. Cell Mol Life Sci. 2009;66:1534–1555. doi: 10.1007/s00018-009-8435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen D, Meadows L, Chen C, Thyagarajan V, Isom L. Sodium channel beta1 subunit-mediated modulation of Nav1.2 currents and cell surface density is dependent on interactions with contactin and ankyrin. J Biol Chem. 2004;279:16044–16049. doi: 10.1074/jbc.M400856200. [DOI] [PubMed] [Google Scholar]
- 37.Meadows L, Malhotra J, Stetzer A, Isom L, Ragsdale D. The intracellular segment of the sodium channel beta 1 subunit is required for its efficient association with the channel alpha subunit. J Neurochem. 2001;76:1871–1878. doi: 10.1046/j.1471-4159.2001.00192.x. [DOI] [PubMed] [Google Scholar]
- 38.Miyazaki H, Oyama F, Wong H-K, Kaneko K, Sakurai T, Tamaoka A, Nukina N. BACE1 modulates filopodia-like protrusions induced by sodium channel beta4 subunit. Biochem Biophys Res Commun. 2007;361:43–48. doi: 10.1016/j.bbrc.2007.06.170. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, Lichtenthaler SF, Hébert SS, De Strooper B, Haass C, Bennett DA, Vassar R. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Malley HA, Shreiner AB, Chen G-H, Huffnagle GB, Isom LL. Loss of Na+ channel beta2 subunits is neuroprotective in a mouse model of multiple sclerosis. Mol Cell Neurosci. 2009;40:143–155. doi: 10.1016/j.mcn.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palop J, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- 42.Palop J, Chin J, Roberson E, Wang J, Thwin M, Bien-Ly N, Yoo J, Ho K, Yu G, Kreitzer A, Finkbeiner S, Noebels J, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palop J, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pertin M, Ji R, Berta T, Powell A, Karchewski L, Tate S, Isom L, Woolf C, Gilliard N, Spahn D, Decosterd I. Upregulation of the voltage-gated sodium channel beta2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons. J Neurosci. 2005;25:10970–10980. doi: 10.1523/JNEUROSCI.3066-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saura C, Choi S, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao B, Chattarji S, Kelleher R, Kandel E, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 46.Savonenko A, Melnikova T, Laird F, Stewart K, Price D, Wong P. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci U S A. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selkoe D. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 48.Selkoe D, Kopan R. Notch and Presenilin: Regulated Intramembrane Proteolysis Links Development and Degeneration. Annu Rev Neurosci. 2003 doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 49.Selkoe D, Wolfe M. Presenilin: running with scissors in the membrane. Cell. 2007;131:215–221. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS Defects in Presenilin-1-Deficient Mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 51.Sinha S, Anderson J, Barbour R, Basi G, Caccavello R, Davis D, Doan M, Dovey H, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari S, Wang S, Walker D, John V, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 52.Takao M, Ghetti B, Murrell JR, Unverzagt FW, Giaccone G, Tagliavini F, Bugiani O, Piccardo P, Hulette CM, Crain BJ, Farlow MR, Heyman A. Ectopic white matter neurons, a developmental abnormality that may be caused by the PSEN1 S169L mutation in a case of familial AD with myoclonus and seizures. J Neuropathol Exp Neurol. 2001;60:1137–1152. doi: 10.1093/jnen/60.12.1137. [DOI] [PubMed] [Google Scholar]
- 53.Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62:1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- 55.Tyler S, Dawbarn D, Wilcock G, Allen S. alpha- and beta-secretase: profound changes in Alzheimer's disease. Biochem Biophys Res Commun. 2002;299:373–376. doi: 10.1016/s0006-291x(02)02635-9. [DOI] [PubMed] [Google Scholar]
- 56.Vassar R, Bennett B, Babu-Khan S, Kahn S, Mendiaz E, Denis P, Teplow D, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski M, Biere A, Curran E, Burgess T, Louis J, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 57.Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29:12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Velez-Pardo C, Arellano JI, Cardona-Gomez P, Jimenez Del Rio M, Lopera F, De Felipe J. CA1 hippocampal neuronal loss in familial Alzheimer's disease presenilin-1 E280A mutation is related to epilepsy. Epilepsia. 2004;45:751–756. doi: 10.1111/j.0013-9580.2004.55403.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Song L, Laird F, Wong P, Lee H. BACE1 knock-outs display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to CA3 synapses in the hippocampus. J Neurosci. 2008;28:8677–8681. doi: 10.1523/JNEUROSCI.2440-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waxman SG. Acquired channelopathies in nerve injury and MS. Neurology. 2001;56:1621–1627. doi: 10.1212/wnl.56.12.1621. [DOI] [PubMed] [Google Scholar]
- 61.Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci. 2006;7:932–941. doi: 10.1038/nrn2023. [DOI] [PubMed] [Google Scholar]
- 62.Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and their genes: dynamic expression in the normal nervous system, dysregulation in disease states(1) Brain Res. 2000;886:5–14. doi: 10.1016/s0006-8993(00)02774-8. [DOI] [PubMed] [Google Scholar]
- 63.Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004;1009:1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 64.Willem M, Garratt A, Novak B, Citron M, Kaufmann S, Rittger A, Destrooper B, Saftig P, Birchmeier C, Haass C. Control of Peripheral Nerve Myelination by the {beta}-Secretase BACE1. Science. 2006 doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 65.Wong H, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. {beta} Subunits of Voltage-gated Sodium Channels Are Novel Substrates of {beta}-Site Amyloid Precursor Protein-cleaving Enzyme (BACE1) and {gamma}-Secretase. J Biol Chem. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 66.Xiao Z, Ragsdale D, Malhotra J, Mattei L, Braun P, Schachner M, Isom L. Tenascin-R is a functional modulator of sodium channel beta subunits. J Biol Chem. 1999;274:26511–26517. doi: 10.1074/jbc.274.37.26511. [DOI] [PubMed] [Google Scholar]
- 67.Yan R, Bienkowski M, Shuck M, Miao H, Tory M, Pauley A, Brashier J, Stratman N, Mathews W, Buhl A, Carter D, Tomasselli A, Parodi L, Heinrikson R, Gurney M. Membrane-anchored aspartyl protease with Alzheimer's disease beta- secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 68.Yang L, Lindholm K, Yan R, Citron M, Xia W, Yang X, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 69.Yu H, Saura C, Choi S, Sun L, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson M, Younkin S, Kandel E, Kirkwood A, Shen J. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- 70.Zayas E, Grossberg G. Treating the agitated Alzheimer patient. J Clin Psychiatry. 1996;57 Suppl 7:46–51. discussion 52–44. [PubMed] [Google Scholar]
- 71.Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Sudhof T, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O'Connor T, Logan S, Maus E, Citron M, Berry R, Binder L, Vassar R. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer's disease pathogenesis. J Neurosci. 2007;27:3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]