Endocrine-disrupting chemicals (EDCs) have captivated the attention of scientists, the public and the media [1; 2]. Since its inception, the endocrine disruption field has been controversial [3], and skeptics of the hypothesis have been just as vocal as the proponents. So why should neuroendocrinologists care about EDCs and why have a special issue on the subject? One fundamental reason is because the two fields are inextricably linked. As neuroendocrinologists, our history is already ingrained with the concept that there are critical periods of development, the disruption of which has permanent effects in adulthood. It has been known for decades that exogenous hormones, or interference with endogenous hormones, during these critical periods of organization and activation can have permanent effects on the physiological and behavioral pathways regulated by hypothalamic neuroendocrine circuits. Thus, neuroendocrinologists in some sense predicted that EDCs would disrupt homeostatic neuroendocrine processes, and that the critical developmental periods would be most sensitive, even before the term “endocrine disruptor” was coined. Another more immediate reason is that EDCs are inescapable. For example, EDCs are now recognized to be pervasive in the laboratory. They are present at high levels in soy-based animal feed and in soy supplements consumed by humans, they leach from plastics, lurk in tap water, and can interfere with hormone sensitive assays, such as MCF-7 breast cancer cells, potentially confounding experimental results. They are also common in house dust, fabrics, cookware, furniture, food containers, an assortment of other household products, and even in the air. We are exposed to a complex cocktail of these compounds every day, from conception to death.
Just because EDCs pervade our bodies does not automatically mean that they cause harm, and determining which do and which do not, and by what measure, is where the bulk of the controversy now lies. At issue are both the degree to which low dose exposures to chemicals with low hormonal potency can appreciably affect vertebrate physiology, and the degree to which the potential long term risks of chemicals with sex-, life stage-, and tissue-specific impacts can be swiftly and sufficiently gauged. In humans, both issues are difficult to address experimentally because the timing, duration and level of human exposure are often uncertain, particularly during fetal life. Moreover, the latency between EDC exposure and the emergence of consequential health effects can be markedly long, often decades, and the degree to which some groups might be more sensitive than others, resulting in inter-individual variability, is poorly understood. Finally, predicting human responses from sentinel wildlife cases, or experimental animal and in vitro tests of endocrine action is not straightforward and frequently contested [3]. We believe that rapidly emerging data from numerous labs conducting basic animal research, studies of inadvertent human exposures, and epidemiological analyses overwhelmingly point to the inevitable conclusion that EDC exposures are pervasive, and cause both short- and long-term harm to humans and wildlife. But what is the extent of the problem and what should be done to correct it? Neuroendocrinologists are uniquely poised to tackle this question.
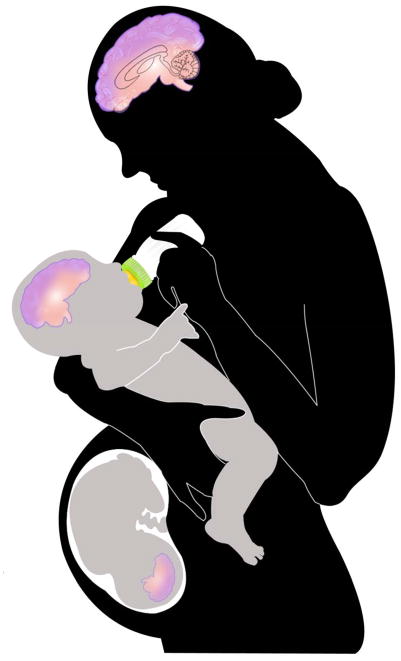
In this special edition of Frontiers in Neuroendocrinology, eight articles are devoted to the effects of EDCs on reproductive health, neuroendocrine function, thyroid hormones, energy balance, cognition, and maternal behavior in rodents, non-human primates and humans. These articles underscore the message that neuroendocrine disruption, especially during critical periods of the life cycle, can result in a broad array of effects that may not manifest for years or decades. A few highlight recent evidence for transgenerational effects of EDCs [4; 5] and discuss previously unsuspected mechanisms, including molecular epigenetic changes, for the transmission of EDC effects to future generations even if the exposure to the EDC can be identified and removed [Figure 1]. This alarming possibility makes it all the more imperative for neuroendocrinologists to familiarize themselves with the EDC literature and weigh in on the issue.
Figure 1.
Schematic representation of exposure of neuroendocrine systems in humans to EDCs. Depicted here is how exposure of multiple generations can occur via maternal exposure. EDCs can directly modify the mother’s brain, hormones and the germ cells in her ovary. The mother’s hormones, brain and behavior can be altered by EDCs, and her germ cells can be subject to epigenetic programming through a variety of molecular mechanisms. At the same time, her developing fetus is exposed to EDCs through placental transfer, and infants are exposed via breast milk. Further exposure to suckling infants may occur through formula feeding (soy products, plastics in baby bottles). In addition, the germ cells of these developing infants and fetuses, which represent the third generation, may be modified through behavioral, hormonal, and epigenetic mechanisms of the previous two generations.
Defining and Testing the Endocrine Disrupting Hypothesis – A Role for Neuroendocrinologists
The term “endocrine disruption” was first coined in 1991 by a diverse group of 21 scientists who had gathered at the Wingspread conference center in Racine, Wisconsin, USA, to discuss what was known about the issue at the time (the history of which is detailed in the book Our Stolen Future). They released an opinion, now referred to as the Wingspread Consensus Statement, which became the foundation of the field [6; 7; 8; 9] and defined many of its key principles including the concept of critical windows of susceptibility, the potential for bioaccumulation and a long latency between exposure and effect. Although the concept of critical windows during which hormones can influence the organization of neuroendocrine systems is fundamental to neuroendocrinology and predates the endocrine disruption field by decades, it represented a paradigm shift in toxicology. Well-known neuroendocrinologists including Arnold Berthold (1803–1861), William Young (1899–1965), Frank Beach (1911–1988), Robert Goy (1924–1999) and their contemporaries have clearly established that during embryonic and early postnatal life, the gonads and adrenals produce hormones that influence ontogeny of the body and brain. Differences between testicular and ovarian hormones play a crucial role in brain sexual differentiation [10]. As the hypothalamus develops and differentiates, structural, neurochemical and functional differences are programmed early in life, referred to as organization. Later in life at puberty and into adulthood, the developing adrenals and gonads begin to produce increasing levels of steroids, which continue to organize and then activate those pathways formed at birth [11]. It thus seems obvious to us as basic researchers in the field that neuroendocrine tissues should be vulnerable to endocrine disruption, but obtaining broad support for this inference from scientists outside the field has been a struggle. Although still an emerging concept, there is now published evidence for the exquisite sensitivity of hypothalamic neuroendocrine systems to developmental EDC exposures (reviewed in [12; 13]) making this a hot area of investigation. One experimental barrier is that neuroendocrine endpoints are difficult to assess directly because of the relative inaccessibility of the hypothalamus and pituitary, and because direct experimentation in humans is unethical and thus virtually impossible. Recent studies in animal models, however, have shown neuroendocrine effects of EDCs in numerous species. Because the mechanisms of action of EDCs are largely conserved among humans, other mammals, and even across the vertebrate classes, we believe it is reasonable to draw inferences from these studies about potential human impacts. Others have argued that such corollaries are not justified, making this one of the most pressing controversies to overcome.
The best evidence for both the importance of animal models for predicting human outcomes and causality between early life exposure and adult disease has been definitively provided by the unfortunate case of the estrogenic pharmaceutical, diethylstilbestrol (DES). Prescribed to pregnant women to avert miscarriage (a practice subsequently discovered to be ineffective), it exposed their fetuses to high levels of a powerful synthetic estrogen. While the children appeared anatomically normal at birth, the daughters grew up to have a high incidence of reproductive tract structural abnormalities, and an increased prevalence of very rare clear cell vaginal and cervical carcinomas. It is now recognized that DES sons experience higher rates of reproductive disorders and cancers as well. This tragic event was critically informative because it illuminated several crucial concepts embedded in the endocrine disruption hypothesis. First, it provided a direct cause-and-effect relationship between prenatal exposure to an estrogenic compound, and the later development of an endocrine cancer [14]. Second, it also emphasized that the human fetus is not fully protected from exogenous hormones, as once believed, and that human health is just as vulnerable to endocrine disruption as wildlife and laboratory species. Finally, it demonstrated that animal models can in fact be good predictors of human health outcomes, as comparisons of results of perinatal DES exposures between mice and humans reveal very similar results [15]. Of particular relevance to neuroendocrinologists, actions of DES on the hypothalamus and pituitary have been known for over sixty years [16; 17], a predictable finding based on the abundance of estrogen receptors in these regions [18]. The DES model, as currently studied in the DES daughters, sons, and now grandchildren, together with laboratory animal models of DES exposure, has provided translational links to the developmental origins of adult disease. Current research on EDCs has been informed by DES, including recent work by some of the original investigators who first described DES’s effects. Still, an understanding of the impacts of EDCs on neuroendocrinology is still in its infancy, and it is imperative that this data gap be filled by future research.
An Evolving Field
The Environmental Protection Agency (USA) now defines an EDC (in part) as “an exogenous chemical substance or mixture that alters the structure or function(s) of the endocrine system and causes adverse effects.” Recognition by scientists in the field that effects may extend beyond the endocrine system has forced this definition to evolve into the broader hypothesis that there are “developmental origins of adult disease,” (also referred to as the “fetal basis of adult disease (FeBAD) hypothesis”) [19; 20; 21]. This concept was first articulated by David Barker in describing the links between adult coronary artery or type 2 diabetes and previous poor fetal or infant growth [22]. The DES tragedy is also consistent with this hypothesis and demonstrates that fetal exposure to estrogens can lead to cancer, infertility and other adverse outcomes in later life. There is now concern that the fetal environment, including exposure to toxicants, can contribute to obesity and metabolic syndrome. Skeptics insist that endocrine disruption cannot account for such effects, but neuroendocrinologists are well aware that disruption of hypothalamic-pituitary regulation can undermine body systems controlling reproductive, thyroid, metabolic, obesity and pancreatic hormones, water/electrolyte balance, lactation and growth.
Research in neuroendocrinology has additional roots in several concepts that are now the basis for current work on environmental endocrine disruption. The neuroendocrine systems have elements of both nervous and endocrine systems. As such, they enable the organism to interact with and respond to the environment, with the nervous system level mediating the most immediate and rapid effects, and the endocrine level enabling the organism to maintain and prolong the response, but ultimately to restore homeostasis. Internal secretions such as hormones play key roles as effectors of environmental triggers, and themselves trigger responses in the organism, thus resulting in the feedforward/feedback mechanisms with which endocrinologists are familiar. These properties of neuroendocrine systems are critical to the effects of environmental endocrine disruptors, which can interfere with natural hormonally-mediated processes in the body and disrupt homeostasis.
There is now strong biological evidence for effects on EDCs in vitro and in vivo, and experiments seeking to understand the molecular and cellular mechanisms targeted by EDCs show that along with the more predictable targets such as estrogen receptors, EDCs can affect other hormone receptors (androgen, thyroid, retinoid and orphan receptors involved in metabolism), coregulatory factors, metabolic enzymes, and neurotransmitters/receptors. Exposures to EDCs typically involve lifelong contact with hundreds of chemicals. Although there are rare examples of toxic exposures of humans to a single EDC compound, such as the release of dioxin in high concentrations from an industrial plant in Seveso, Italy, or contamination of cooking oil with polychlorinated biphenyls (PCBs) in Taiwan and Japan, most EDC exposures are sub-toxic. The fact that exposure to a single EDC predicts exposure to multiple EDCs makes the issue of complex mixtures very relevant [23]. At the same time, there is now epidemiological evidence for adverse links between EDCs and human disease, including for the controversial compound bisphenol A (BPA) used in food containers and plastics in the food industry [24]. Biomonitoring studies show the pervasiveness of EDCs in human tissues, serum, urine and amniotic fluid [25; 26; 27; 28].
Human Health Trends
In her 1985 dystopian novel, The Handmaid’s Tale, Margaret Atwood described a human population rendered largely infertile by overwhelming chemical and nuclear pollution. Sometimes science fiction is too close to non-fiction for comfort. Global trends indicate that over the past half century human health has been declining and chronic disease is on the rise. For example, two pivotal studies independently concluded that mean seminal volume and sperm concentration have steadily decreased over the last 50 years [29; 30]. In Denmark, it is now estimated that more than 10% of men have sperm counts in the infertile range and upwards of 30% are in the subfertile range [31]. Alarming reproductive health trends are also emerging among women and girls [32]. For example, in the United States, the median age at menarche, first breast development, and sexual precocity has steadily advanced, particularly among minority populations [33; 34], such that is no longer unusual for girls to obtain their first bras in second or third grade. Similar effects have been noted in Europe and among children adopted from developing countries by Western parents [35; 36; 37]. Although somewhat weak, there is emerging evidence for a decline in female fecundity, even among young women who have not elected to delay childbearing [38; 39; 40]. Concomitant with this decline in reproductive health is a similarly paced increase in the incidence of childhood psychological and behavioral disorders such as attention deficit disorder and autism spectrum disorders (ASD) (see CDC database at http://www.cdc.gov/ncbddd/adhd/data.html) [41]. A recent survey of over 78,000 families conducted by the National Survey of Children’s Health revealed that as many as 1.1% of all children born in the US are now diagnosed with ASD, with rates in boys nearly four times that of girls [42]. In their ground-breaking 2010 report, the President’s Cancer Panel highlighted that rates of cancer in children continue to increase, and argued for a greater research focus on the relationship between exposure to toxicants and cancer. In all cases, the underlying cause of such alarming human health trends is likely multi-faceted, and although lifestyle factors such as delayed childbearing, diet, stress, and body composition likely play a role, the rapidity of the increase in reproductive and behavioral disorders, and cancer, suggests an environmental component.
It is now widely hypothesized that exposure to EDCs, both synthetic and naturally occurring, are at least partially responsible. We believe this a plausible hypothesis but robustly testing it is not straightforward. For example, did the decline in male fecundity result from exposure in the womb, when the gonads were forming; during puberty, when the reproductive system was maturing; in adulthood, when conception is desired; or all of the above? Obviously, obtaining absolute proof of endocrine disruption in humans by a chemical (or a mixture of chemicals) with weak hormonal activity is likely impossible because it would be unethical to conduct a double-blind study where one group is exposed to a suspected toxicant and the other is not. So how can data obtained from animal models be used to inform human risk assessment? Can health effects that result from environmental factors be improved or corrected once diagnosed? Could effects be transmitted to subsequent generations by epigenetic or other mechanisms? Such questions are on the minds of policy-makers and the general public, and will be touched upon throughout this special issue.
Regulatory Action and Public Policy
Endocrine disruptors are critically important for another reason-they illuminate how public trust in regulatory agencies charged with protecting human health has eroded and how assessment paradigms must change to deal with emerging, and unanticipated threats. The groundbreaking book Silent Spring by Rachel Carson, published in 1962, highlighted the devastation to wildlife caused by the pesticide, DDT. Although tremendously successful at killing disease-carrying pests such as lice and mosquitoes, the unintended impacts of DDT on vertebrates nearly led to the extinction of the brown pelican and the bald eagle. This event highlights the important point that endocrine disrupting compounds were not designed to cause harm, but rather to improve the human condition by reducing disease and famine, for example. It also emphasized the need to consider unintended consequences on the endocrine system when designing new products. Unfortunately, this lesson has gone largely unheeded and to this day, pest-control products including insecticides, rodenticides, fungicides and nematocides known to have endocrine-disrupting properties are still used liberally, making Carson’s book all the more relevant nearly fifty years later. We have only just begun to quantify the costs associated with the widespread use of these compounds. In the US, products are not currently tested for endocrine disrupting activity before coming to market, thus it is up to scientists to discover which chemicals are endocrine disrupting and where they are found only after the fact. It is now obvious that they are everywhere. This is unacceptable and must change. The President’s 2010 Cancer Panel emphatically argued for reform of federal chemical policy and for stronger regulation of chemicals, with a greater emphasis on precaution and prevention. To those of us that have been in the endocrine disruption field for many years, this argument sounds very familiar.
This is an historically important time, when scientists and policymakers are talking and listening to one another with the intent of identifying a solution. The discussion is too often cantankerous and not without controversy, but it is generating bold and meaningful change. This cooperation has undeniably been spurred by growing public awareness of the prevalence of these compounds in popular products, and shifts in consumer habits (as exemplified by the recent explosive popularity of stainless steel water bottles). The 2008 announcement by Walmart that it would no longer sell plastic baby bottles or food containers containing the additive Bisphenol A (BPA) arguably had the greatest impact on manufacturing methods than any FDA, EPA or other governmental decision, and bottles bearing a “BPA-Free” sticker are now ubiquitous in major retail stores. In January of 2010 the FDA reversed its long held ruling that BPA exposure was inconsequential and adopted the position that it has “some concern about the potential effects of BPA on the brain, behavior, and prostate gland of fetuses, infants and children.” The move was revolutionary and signifies a major shift in how regulatory agencies are thinking about endocrine disruptors. Journalists are paying attention too and articles on the subject have appeared in publications as diverse as The Christian Science Monitor, The New York Times, The New Yorker, Time Magazine, The Daily Mail, Men’s Health and Vogue, further fueling public interest and concern. Momentum to quantify the scale and scope of the problem has never been greater.
The Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) was formed by Congress back in 1996 to make specific recommendations to the EPA about how to test and screen compounds for endocrine disrupting properties, but progress has been frustratingly slow. A list of compounds to be screened was not compiled until April of 2009 and only 67 chemicals were included, a tiny fraction of the thousands of compounds now believed to have endocrine disrupting properties. There is also concern that the screening program itself is insufficient, poorly designed and unlikely to reliably identify potential endocrine disruptors, further bogging down the implementation of the program. In response to this, there are currently bills pending in both the US House (“The Endocrine Disruption Prevention Act” H.R.4190) and the US Senate (S2828) to give the NIH the power to develop an endocrine disruption screening program and specifically generate data to guide better regulatory control over their use. NIEHS is currently working with the FDA to develop a mechanism by which academic scientists could cooperatively work in conjunction with FDA scientists to conduct risk assessment work [43]. The hope is that, by working together, the rapidity of the screening process, the incorporation of innovative experimental approaches, and the number of endpoints screened can be dramatically enhanced. At the moment this emerging partnership focuses on BPA but could ultimately be expanded to include other compounds of concern.
Scientific societies and medical organizations have also taken note of the biological evidence for EDCs, and they have begun to weigh in. The publication of the Faroes Statement, a consensus statement authored by a group of eminent researchers and clinicians, focused on developmental exposure to EDCs and emphasized the particular vulnerability of the developing infant [44]. This statement challenged toxicologists to move from a testing paradigm centered around the idea that “the dose makes the poison” to one that appreciates that “the timing makes the poison.” Of even greater impact was the Endocrine Society’s Scientific Statement on EDCs [1], which was rapidly endorsed by the American Medical Association and garnered considerable media coverage. The European Union and Canada have made moves to ban some EDCs, and some states in the U.S. are following suit. The Johnson Foundation again hosted a workshop at Wingspread to revisit the state of the science and develop ideas to mitigate the threat posed by “contaminants of emerging concern.” A formal statement from that group, one of whom also attended the original 1991 Wingspread meeting, is forthcoming.
Concluding Thoughts About Where to Go From Here
Although the two of us are basic researchers, we are concerned about the regulation of putative EDCs. The identification of EDCs is not necessarily straightforward due to their existence as complex mixtures and their actions through multiple biological pathways. In its Scientific Statement [1], The Endocrine Society invoked the “Precautionary Principle” in the regulatory process, advocating that new compounds being introduced into products that come into contact with human tissues or in our food/water containers be considered harmful unless proven otherwise. This puts the burden of proof on the manufacturer, something that has caused resistance from the chemical industry. Some of the responsibility can be assumed by consumers who can make informed choices about storing and heating their food products in safe containers, washing produce that may come into contact with pesticides, and choosing organic alternatives and unprocessed food when available/affordable. Unfortunately, it is extraordinarily difficult for individuals to make informed choices about how to reduce their potential exposure because it is often impossible to determine which plastics, cosmetics, toys, or other household items contain endocrine disrupting compounds, so consumers have no adequate way to avoid them if desired. This powerlessness is one major reason why the topic of endocrine disruption continues to receive global attention by scientists and the general public, and why the time has never been better for neuroendocrinologists to get involved and weigh in both through performing high-quality basic research, for making translational links to humans, and by getting out of their comfort zone of the laboratory/clinic and getting engaged in a dialogue with policymakers. The articles presented in this special issue describe some of the seminal findings within the endocrine disruption field and highlight the critical data gaps that remain to be addressed. This landmark issue of Frontiers in Neuroendocrinology is intended to be a first step in the directions that we have advocated.
Acknowledgments
Work discussed in this article was supported by NIH 1RC1 ES018139 (ACG) and NIH R01 ES016001 (HBP). We are grateful to Belinda Lehmkuhle for assistance with Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocrine Reviews. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biology of Reproduction. 2009;81:807–13. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beronius A, Ruden C, Hakansson H, Hanberg A. Risk to all or none? A comparative analysis of controversies in the health risk assessment of Bisphenol A. Reproductive Toxicology. 2010;29:132–46. doi: 10.1016/j.reprotox.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anway M, Skinner M. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147:S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- 6.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environmental Health Perspectives. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markey CM, Rubin BS, Soto AM, Sonnenschein C. Endocrine disruptors: from Wingspread to environmental developmental biology. J Steroid Biochem Mol Biol. 2002;83:235–44. doi: 10.1016/s0960-0760(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 8.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread”--environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicological Sciences. 2008;105:235–59. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gore AC. Developmental programming and endocrine disruptor effects on reproductive neuroendocrinology. Front Neuroendocrinol. 2008;29:358–374. doi: 10.1016/j.yfrne.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 12.Gore AC. Neuroendocrine targets of endocrine disruptors. Hormones. 2010;9:16–27. doi: 10.14310/horm.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:10. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst A, Ulfelder H, Poskanzer D. Adenocarcinoma of vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 15.McLachlan JA, Dixon RL. Toxicologic comparison of experimental and clinical exposure to diethylstilbestrol during gestation. Advance in Sex Steroid Hormone Research. 1977;3:309–336. [PubMed] [Google Scholar]
- 16.Smith OW, Vanderlinde RE. Oxidation product of stilbestrol; effects upon the rat pituitary of a non-estrogenic oxidation product of diethylstilbestrol. Endocrinology. 1951;49:742–754. doi: 10.1210/endo-49-6-742. [DOI] [PubMed] [Google Scholar]
- 17.Maurer RA, Woolley DE. Demonstration of nuclear 3H-estradiol binding in hypothalamus and amygdala of female, androgenized-female, and male rats. Neuroendocrinol. 1974;16:137–147. doi: 10.1159/000122560. [DOI] [PubMed] [Google Scholar]
- 18.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 19.Heindel J, Lawler C. In: Role of exposure to environmental chemicals in developmental origins of health and disease. Gluckman P, Hanson M, editors. Developmental Origins of Health and Disease, Cambridge University Press; Cambridge: 2006. [Google Scholar]
- 20.Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–13. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 21.Heindel JJ. The fetal basis of adult disease: Role of environmental exposures--introduction. Birth Defects Res A Clin Mol Teratol. 2005;73:131–2. doi: 10.1002/bdra.20119. [DOI] [PubMed] [Google Scholar]
- 22.Barker DJP. The developmental origins of adult disease. Eur J Epidemiol. 2003;18:733–736. doi: 10.1023/a:1025388901248. [DOI] [PubMed] [Google Scholar]
- 23.Willingham E, Crews D. The slider turtle: An animal model for the study of low doses and mixtures. American Zoologist. 2000;40:421–429. [Google Scholar]
- 24.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 25.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. Exposure to Bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113:e429–34. doi: 10.1542/peds.113.5.e429. [DOI] [PubMed] [Google Scholar]
- 28.Chun OK, Chung SJ, Song WO. Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. J Am Diet Assoc. 2009;109:245–54. doi: 10.1016/j.jada.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 29.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Bmj. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect. 2000;108:961–6. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joensen UN, Jorgensen N, Rajpert-De Meyts E, Skakkebaek NE. Testicular dysgenesis syndrome and Leydig cell function. Basic Clin Pharmacol Toxicol. 2008;102:155–61. doi: 10.1111/j.1742-7843.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- 32.Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ., Jr Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–40. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–12. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 34.Partsch CJ, Sippell WG. Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens. Hum Reprod Update. 2001;7:292–302. doi: 10.1093/humupd/7.3.292. [DOI] [PubMed] [Google Scholar]
- 35.Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123:e932–9. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 36.Proos LA, Hofvander Y, Tuvemo T. Menarcheal age and growth pattern of Indian girls adopted in Sweden. I. Menarcheal age. Acta Paediatr Scand. 1991;80:852–8. doi: 10.1111/j.1651-2227.1991.tb11960.x. [DOI] [PubMed] [Google Scholar]
- 37.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 38.Brannian J, Hansen K. Assisted reproductive technologies in South Dakota: the first ten years. S D Med. 2006;59:291–3. [PubMed] [Google Scholar]
- 39.Nyboe Andersen A, Erb K. Register data on Assisted Reproductive Technology (ART) in Europe including a detailed description of ART in Denmark. Int J Androl. 2006;29:12–6. doi: 10.1111/j.1365-2605.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- 40.Frey KA, Patel KS. Initial evaluation and management of infertility by the primary care physician. Mayo Clin Proc. 2004;79:1439–43. doi: 10.4065/79.11.1439. quiz 1443. [DOI] [PubMed] [Google Scholar]
- 41.Fombonne E. The prevalence of autism. JAMA. 2003;289:87–9. doi: 10.1001/jama.289.1.87. [DOI] [PubMed] [Google Scholar]
- 42.Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, Singh GK, Strickland BB, Trevathan E, van Dyck PC. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124:1395–403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- 43.Borrell B. Toxicology: The big test for bisphenol A. Nature. 2010;464:1122–4. doi: 10.1038/4641122a. [DOI] [PubMed] [Google Scholar]
- 44.Grandjean P, Bellinger D, Bergman A, Cordler S, Davey-Smith G, Eskenazi B, Gee D, Gray K, Hanson M, van den Hazel P, Heindel JJ, Heinzow B, Hertz-Picciotto I, Hu H, Huang TTK, Jensen TK, Landrigan PJ, McMillen IC, Murata K, Ritz B, Schoeters G, Skakkebaek N, Skerfving S, Weihe P. The Faroes Statement: Human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol. 2007;102:73–75. doi: 10.1111/j.1742-7843.2007.00114.x. [DOI] [PubMed] [Google Scholar]