Abstract
Considerable evidence indicates that native neuronal voltage-gated K+ (Kv) currents reflect the functioning of macromolecular Kv channel complexes, composed of pore-forming (α) subunits, cytosolic and transmembrane accessory subunits, together with regulatory and scaffolding proteins. The individual components of these macromolecular complexes appear to influence the stability, the trafficking, the localization and/or the biophysical properties of the channels. Recent studies suggest that Kv channel accessory subunits subserve multiple roles in the generation of native neuronal Kv channels. Additional recent findings suggest that Kv channel accessory subunits can respond to changes in intracellular Ca2+ or metabolism and thereby integrate signaling pathways to regulate Kv channel expression and properties. Although studies in heterologous cells have provided important insights into the effects of accessory subunits on Kv channel expression/properties, it has become increasingly clear that experiments in neurons are required to define the physiological roles of Kv channel accessory and associated proteins. A number of technological and experimental hurdles remain that must be overcome in the design, execution and interpretation of experiments aimed at detailing the functional roles of accessory subunits and associated proteins in the generation of native neuronal Kv channels. With the increasing association of altered Kv channel functioning with neurological disorders, the potential impact of these efforts is clear.
Keywords: Kv channels, proteomics, neuronal excitability, macromolecular complex, signaling
Introduction
Voltage-gated K+ (Kv) currents are key regulators of neuronal membrane excitability, functioning to control resting membrane potentials [12], spontaneous firing rates [45], the back propagation (into dendrites) of action potentials [18], neurotransmitter release [20], synaptic integration [17, 53], and even apoptosis [40]. Neurons typically express multiple types of Kv channels with distinct time- and voltage-dependent properties and subcellular distributions that differentially contribute to the regulation of firing properties and signal integration. Molecular cloning has revealed even greater potential for generating functional Kv channel diversity than was anticipated based on the physiology [10, 45]. A large number of Kv channel pore forming (α) subunits and a variety of Kv channel accessory subunits have been identified [10, 45], and studies in heterologous cells have revealed multiple, sometimes overlapping, effects of accessory subunits on the expression, distributions and properties of Kv α-subunit-encoded channelses of Kv α-subunit-encoded channels [25, 32]. Electrophysiological and biochemical evidence indicates that native neuronal K+ currents reflect the functioning of macromolecular K+ channel complexes comprising four α subunits, cytosolic and transmembrane accessory subunits, as well as regulatory and scaffolding proteins [25]. Recent proteomic studies, for example, demonstrated that native (mouse) brain Kv4 channel complexes contain many different types of accessory and regulatory subunits [33], each of which may influence channel targeting, trafficking and/or biophysical properties. It has also become clear that the properties of Kv α subunit-encoded channels and the modulatory effects of channel associated proteins vary in different cellular backgrounds. Thus, studies need to be carried out in neurons to understand the functions of Kv channel accessory subunits in the generation and regulation of native Kv channels. This review focuses on recent advances made in exploring the roles of Kv channel associated proteins in neurons with an emphasis on studies suggesting that Kv channel accessory subunits can serve as points of integration for signaling pathways, as well as link membrane excitability to other cellular processes.
Kv channel accessory subunits integrate multiple signaling pathways
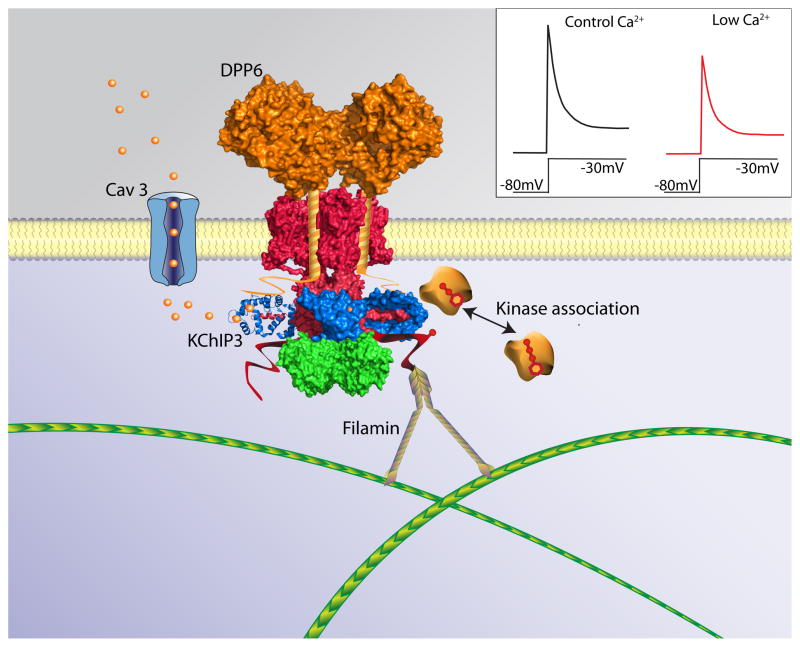
Several recent studies, focused on examining the roles of K+ Channel Interacting Protein 3 (KChIP3), have highlighted the potential for Kv channel accessory subunits to serve as points of signal integration in the regulation of neuronal function [1, 3, 51]. KChIP3, like the other members of the Neuronal Ca2+ Sensor super family [5], binds Ca2+ through multiple EF-hand motifs [31, 44]. KChIP3 also promotes the surface expression of heterologously expressed Kv4 channels [56] and regulates the functional expression of neuronal Kv4-encoded currents [29, 39]. Exciting recent findings also demonstrate that KChIP3 regulates the voltage-dependence of inactivation of Kv4-encoded channels in cerebellar stellate cells in response to Ca2+ entry through voltage-gated Ca2+ (Cav) channels [3]. In the presence of KChIP3 (but not the other KChIPs), Ca2+ entry through Cav3 channels induced a depolarizing shift in the voltage-dependence of Kv4 channel inactivation, thereby linking Kv4 channel avaialbility to local changes in Ca2+ concentration [2] (Figure 1 inset). Together, these observations suggest that KChIP3 may regulate functional Kv4-encoded current densities in neurons, both by controlling the numbers of Kv4 channels expressed at the cell surface and by dynamically regulating Kv 4 channel availability in response to changes in Ca2+ entry.
Figure 1. Multiple components of Kv4 channel macromolecular protein complexes.
Schematic representation of Kv4 channel α-subunits (red) bound to Kv β (green), DPP6 (orange) and KChIP3 (blue) accessory subunits. The channel complex is shown anchored to the actin cytoskeleton by filamin via the carboxyl-terminus of one of the Kv4 α-subunits. Kinases also associate with Kv4 channel complexes and phosphorylate α and/or accessory subunits, modifications that can affect channel stability, trafficking and/or properties. It has been reported that association of Kv4 α-subunits with KChIP accessory subunits is required for the modification of channel function by some kinases [51, 52]. Cav3 channels can also associate with Kv4 channel complexes by binding to Kv4.2 [2]. In Kv4.2-Cav3 complexes identified in stellate neurons, KChIP3 acts as a Ca2+ sensor, and Ca2+ entry through Cav3 channels, shifts voltage dependence of Kv4.2 channel inactivation to more depolarized potentials [2, 3]. Consistent with a role for Ca2+ entry through Cav3 channels in the regulation of Kv4.2 channels, reductions in extracellular Ca2+ concentration result in attenuation of Kv4.2 currents (inset) because more Kv4.2 channels are inactive at the holding potential when Ca2+ entry is reduced. (Inset adapted with permission from Macmillian Publishers Ltd: Nature Neuroscience copyright 2010, [2])
Additional experiments suggest that KChIP3 also regulates Kv4-channel modulation by kinases [52]. The association of G protein-coupled receptor kinases (GRK) with KChIP3, for example, is dependent on Ca2+ [51], suggesting that KChIP3 could function to integrate local changes in Ca2+ levels with kinase signaling pathways. In addition, the phosphorylation of Kv4 α-subunits has been suggested to be related to surface expression [19, 54, 61]. The possibility that KChIP3 acts as a point of signal integration to regulate the surface expression of Kv4 channels through multiple pathways is exciting, particularly given the importance of dynamic alterations in Kv4-encoded currents in modulating dendritic integration and synaptic potentiation [8, 30]. Interestingly, it has also been reported that KChIPs regulate the surface expression and the functioning of voltage-gated Ca2+ channels [57], NMDA receptors [65] and other Kv channels [27], suggesting that these proteins are multifunctional, differentially regulating excitability in different cell types and/or subcellular compartments. Clearly, experiments in which the expression levels of the KChIPs are altered in situ are needed to explore these hypotheses directly.
Importantly, KChIP3 has also been demonstrated to function in other cellular processes and has been given additional names reflecting these roles: including regulating gene transcription (downstream regulatory element antagonist modulator or DREAM) [7], modulating the production of amyloid precursor protein by binding to presenilin (calsenlinin) [6, 28], and regulating gamma secretase activity and apoptosis [21, 22]. The possibility that KChIP3 may help coordinate long-term changes, through regulating gene transcription, and short-term changes, in response to alterations in cellular activity (intracellular Ca2+ levels and phosphorylation state), in neuronal excitability is intriguing and warrants further investigation.
A number of recent studies also suggest that the inclusion of Kvβ subunits in Kv channel complexes has broad ramifications for channel expression and functioning. Similar to KChIPs, Kvβ subunits also appear to subserve multiple roles including: directly altering the time- and voltage-dependent properties of Kv currents [37, 49], regulating the trafficking of Kv1 and Kv4 channels [14, 15, 64], and modulating changes in Kv currents in response to signaling pathways. Numerous kinases have been shown to phosphorylate Kv1 α-subunits, as well as Kvβ subunits, and thereby modify the effects of Kvβ subunits on Kv1-encoded currents [35]. Interestingly, Kvβ subunits also reportedly associate with Kv4 α-subunits in brain [33], and coexpression of Kvβ subunits with Kv4 α-subunits in heterologous cells alters the cell surface expression of Kv4-encoded channels [62, 64]. Little is presently known, however, about the physiological role(s) of Kvβ subunits in regulating the expression or the properties of native neuronal Kv4 channels.
Given that the Kvβ subunits have a high degree of structural homology to aldo-keto reductases, it is perhaps not surprising that Kvβ2 was crystallized bound to the cofactor nicotinamide adenine dinucleotide phosphate (NADP+) [9, 16]. The Kvβ subunits appear positioned, therefore, to serve as sensors of the oxidative state of cells, suggesting a direct link to metabolism [63]. Mutations in the NADP+ binding site have been reported to disrupt Kvβ mediated alterations in the trafficking of Kv1 α-subunits [42]. In addition, changes in the oxidization state of bound NADP+ were shown to result in changes in the expression/properties of heterologously expressed Kv1-encoded currents [26, 63]. Interestingly, Kvβ subunits reportedly confer sensitivity to oxygen concentration to heterologously expressed Kv4, but not Kv1,-encoded currents [41]. Accumulating evidence also suggests that Kvβ subunits may couple changes in cellular metabolism to the regulation of membrane excitability [58], although a direct link in neurons has yet to be demonstarted.
Experimental Challenges to Determining the Functions of Kv Channel Accessory Subunits
As highlighted by the results of several recent studies [15, 24, 36], a number of challenges exist to efforts focused on defining the physiological roles of Kv channel accessory subunits in the generation of native neuronal Kv channels. Recent proteomic studies, focused on Kv4 channel complexes isolated from brain, indicated that Kv4 α-subunits channels interact with multiple different proteins. Many of the proteins traditionally thought of as Kv channel accessory subunits, such as DPP6 and the KChIPs were identified, but several additional, unexpected proteins, such as the GABA receptor subunit, Gabra-6, and the poorly understood metabotropic glutamate receptor, Gpr158, were also found to co-immunoprecipitate with the Kv4.2 protein from brain [33]. Consistent with findings from studies on Kv4.2, other Kv channels have been suggested to interact with scaffolding proteins like ADAM22 and PSD-95. Thus, it seems likely that native Kv channel macromolecular complexes contain numerous accessory and regulatory proteins. In efforts to determine the functional roles of the proteins associated with Kv channels, experiments utilizing heterologous cells will be important, but experiments in native cells will be required to understand fully how each Kv channel accessory subunit functions in the generation of native neuronal Kv channel complexes.
There are, however, a number of potential challenges to the design, execution and interpretation of experiments focused on exploring the functioning of Kv channel accessory subunits in neurons. Among the potential challenges is the fact that many families of Kv channel accessory subunits, including the KChIPs, Kvβs and MinK/MiRPs, have multiple members with properties (and functional effects) that are likely conserved throughout the family. The individual subunits may be able to compensate for the loss of other family members. The disruption of the expression of an individual accessory subunit, therefore, may not reveal defects in the expression or the targeting of α-subunits and/or in the properties of expressed Kv currents dramatic enough to be resolved. Recent studies focused on exploring the contributions of the various KChIPs to the expression of Kv4-encoded channels in (mouse) cortical pyramidal neurons, in fact, revealed that these cells express KChIP2, 3 and 4 and that when the expression of an individual KChIP was disrupted, the protein expression levels of the remaining two KChIPs are up regulated and Kv4 currents were only modestly affected (Norris et al 2010). Simultaneous RNAi mediated knockdown of all three KChIPs, however, caused a marked reduction in Kv4-encoded current densities (Norris et al. 2010).
Functional compensation may also occur in other families of Kv channel accessory subunits. There are, for example, three members of the Kvβ family in mammals [46]. Expression of mutant Kvβ subunits has revealed a critical role for Kvβ subunits in the trafficking of Kv1 channels [14]. In animals lacking Kvβ2, the most abundantly expressed β subunit [50], however, Kv1.1 and Kv1.2 α-subunits traffic normally. Mice lacking Kvβ2, however, display cold swim-induced tremors, suffer occasional seizures and have a reduced average lifespan [36], suggesting that the loss of Kvβ2 produces defects that are undetected in the molecular studies focused on examining the effects of Kvβ2 on the trafficking Kv1 channels.
An additional challenge to determining the functional roles of Kv channel associated proteins is the potential for these proteins to interact with multiple channel types and to participate in a number of cellular processes. This point is well illustrated with proteins like the AKAPs and filamins, which interact with specific types of ion channels [13, 43], but are also scaffolding proteins generally involved in the formation of macromolecular protein complexes [43, 47]. Several Kv channel accessory subunits are also involved in multiple cellular processes. Both KChIP3 and KChIP4, for example, were independently identified as binding partners for the presenilins and were termed calsenilin and calsenilin related protein, respectively, suggesting roles for both proteins in the regulation of gamma secretase activity [6, 38]. Disruption of the expression of molecules like the filamins and the KChIPs, therefore, could potentially have numerous consequences for cellular functioning not directly related to Kv channels.
Kv channel accessory subunits in neurological disorders and diseases
Mutations in K+ channel α-subunits (for example, Kv7.2 and Kv7.3 in epilepsy [48], and Kv1.1 in Episodic Ataxia Type 1 [4]) have been associated with neurological disorders/diseases. It is perhaps not surprising then that recent results from genetic studies in patient populations and studies using genetically altered mice suggest roles for altered expression and/or functioning of Kv channel accessory subunits in a number of different neurological disorders/diseases. Structural variants of both of the Kv channel accessory subunit genes, DPP6 and DPP10, for example, were identified in a genome-wide association study as candidate genes in autism spectrum disorder [34]. Interestingly, the DPP6 gene has also been associated with increased risk of progressive spinal muscular atrophy [60] and sporadic amyotrophic lateral sclerosis [11, 59]. Mutations in another K+ channel associated protein, leucine-rich glioma-inactivated 1 (Lgi1), have been linked to multiple epilepsy syndromes [23]. As the functional roles of channel accessory/associated proteins are increasingly appreciated, it seems certain that alterations in the expression and/or properties of these proteins will be linked to more disease states. Clearly, carefully crafted experiments, combining molecular genetic, pharmacological, electrophysiological and biochemical approaches, will be needed to define the roles of Kv channel accessory and associated proteins in the functioning of native neuronal Kv channel macromolecular complexes and to further delineate the contributions of Kv channel accessory subunits to neuronal functioning under normal and pathologic conditions.
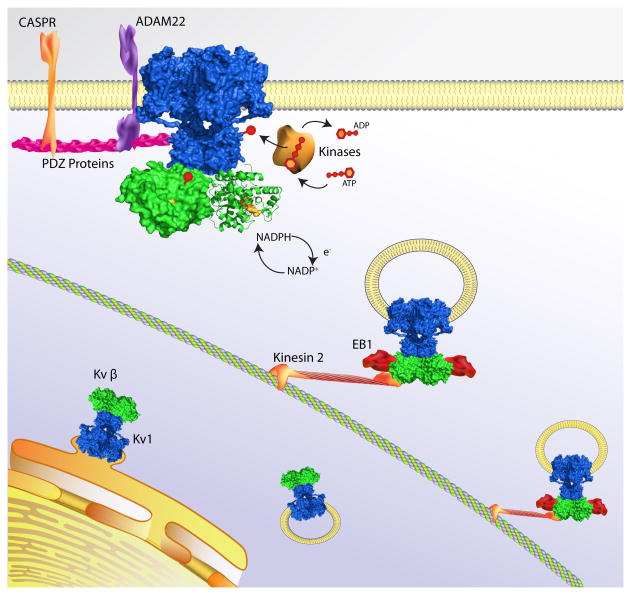
Figure 2. Kv1 channel complexes targeted to axons.
Schematic representation of the assembly and targeting of Kv1 channel macromolecular complexes. Previous studies have shown that Kv1 channel α-subunits (blue) associate with Kv β subunits (green) in the endoplasmic reticulum early during biosynthesis [55]. Through the binding of Kvβ to EB-1 and Kinesin-2 channel complexes then are trafficked down the axon on microtubules. At nodes of Ranvier, Kv1-encoded channels associate with PDZ-domain containing proteins (e.g., PSD-95 and PSD-93) and with the transmembrane proteins, ADAM22 and CASPR. The functioning of Kv1-encoded channels can also be modulated by phosphorylation of both the Kv1 α and the Kvβ subunit [35]. In addition, Kvβ subunits can also regulate channel properties and functioning in response to changes in the oxidation state of the bound co-factor NADP+, providing a potential link between cell metabolism and membrane excitability [42, 63].
Acknowledgments
The authors thank Mr. Rick Wilson for technical assistance in the creation of the figures. We would also like to thank Dr. Yarimar Carrasquillo for many valuable discussions and for comments on the manuscript. In addition, the authors acknowledge the financial support provided by the National Institutes of Health (NS030676 and NS065295 to JMN); AJN was supported by an institutional training grant (T32-EY013360) from the National Eye Institute and NCF was supported by an institutional training grant (T32-HL007275) from the National Heart Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Alexander JC, McDermott CM, Tunur T, Rands V, Stelly C, Karhson D, Bowlby MR, An WF, Sweatt JD, Schrader LA. The role of calsenilin/DREAM/KChIP3 in contextual fear conditioning. Learn Mem. 2009;16:167–177. doi: 10.1101/lm.1261709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, Zamponi GW, Turner RW. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci. 2010;13:333–337. doi: 10.1038/nn.2493. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D, Rehak R, Hameed S, Mehaffey WH, Zamponi GW, Turner RW. Regulation of the Kv4.2 complex by Cav3.1 calcium channels, Channels (Austin) Vol. 4. 2010. [DOI] [PubMed] [Google Scholar]
- 4.Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- 5.Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 2007;8:182–193. doi: 10.1038/nrn2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxbaum JD, Choi EK, Luo Y, Lilliehook C, Crowley AC, Merriam DE, Wasco W. Calsenilin: a calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat Med. 1998;4:1177–1181. doi: 10.1038/2673. [DOI] [PubMed] [Google Scholar]
- 7.Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouinard SW, Wilson GF, Schlimgen AK, Ganetzky B. A potassium channel beta subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila hyperkinetic locus. Proc Natl Acad Sci U S A. 1995;92:6763–6767. doi: 10.1073/pnas.92.15.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 11.Del Bo R, Ghezzi S, Corti S, Santoro D, Prelle A, Mancuso M, Siciliano G, Briani C, Murri L, Bresolin N, Comi GP. DPP6 gene variability confers increased risk of developing sporadic amyotrophic lateral sclerosis in Italian patients. J Neurol Neurosurg Psychiatry. 2008;79:1085. doi: 10.1136/jnnp.2008.149146. [DOI] [PubMed] [Google Scholar]
- 12.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 13.Gravante B, Barbuti A, Milanesi R, Zappi I, Viscomi C, DiFrancesco D. Interaction of the pacemaker channel HCN1 with filamin A. J Biol Chem. 2004;279:43847–43853. doi: 10.1074/jbc.M401598200. [DOI] [PubMed] [Google Scholar]
- 14.Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science. 2003;301:646–649. doi: 10.1126/science.1086998. [DOI] [PubMed] [Google Scholar]
- 15.Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron. 2006;52:803–816. doi: 10.1016/j.neuron.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Gulbis JM, Mann S, MacKinnon R. Structure of a voltage-dependent K+ channel beta subunit. Cell. 1999;97:943–952. doi: 10.1016/s0092-8674(00)80805-3. [DOI] [PubMed] [Google Scholar]
- 17.Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol. 2005;64:75–90. doi: 10.1002/neu.20144. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 19.Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RWt. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T, Nakamura Y, Saitoh N, Li WB, Iwasaki S, Takahashi T. Distinct roles of Kv1 and Kv3 potassium channels at the calyx of Held presynaptic terminal. J Neurosci. 2003;23:10445–10453. doi: 10.1523/JNEUROSCI.23-32-10445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo DG, Kim MJ, Choi YH, Kim IK, Song YH, Woo HN, Chung CW, Jung YK. Pro-apoptotic function of calsenilin/DREAM/KChIP3. Faseb J. 2001;15:589–591. doi: 10.1096/fj.00-0541fje. [DOI] [PubMed] [Google Scholar]
- 22.Jo DG, Lee JY, Hong YM, Song S, Mook-Jung I, Koh JY, Jung YK. Induction of pro-apoptotic calsenilin/DREAM/KChIP3 in Alzheimer’s disease and cultured neurons after amyloid-beta exposure. J Neurochem. 2004;88:604–611. doi: 10.1111/j.1471-4159.2004.02159.x. [DOI] [PubMed] [Google Scholar]
- 23.Kalachikov S, Evgrafov O, Ross B, Winawer M, Barker-Cummings C, Martinelli Boneschi F, Choi C, Morozov P, Das K, Teplitskaya E, Yu A, Cayanis E, Penchaszadeh G, Kottmann AH, Pedley TA, Hauser WA, Ottman R, Gilliam TC. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet. 2002;30:335–341. doi: 10.1038/ng832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, Rudy B, Hoffman DA. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2008;100:1835–1847. doi: 10.1152/jn.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- 26.Leicher T, Bahring R, Isbrandt D, Pongs O. Coexpression of the KCNA3B gene product with Kv1.5 leads to a novel A-type potassium channel. J Biol Chem. 1998;273:35095–35101. doi: 10.1074/jbc.273.52.35095. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Guo W, Mellor RL, Nerbonne JM. KChIP2 modulates the cell surface expression of Kv 1.5-encoded K(+) channels. J Mol Cell Cardiol. 2005;39:121–132. doi: 10.1016/j.yjmcc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Lilliehook C, Bozdagi O, Yao J, Gomez-Ramirez M, Zaidi NF, Wasco W, Gandy S, Santucci AC, Haroutunian V, Huntley GW, Buxbaum JD. Altered Abeta formation and long-term potentiation in a calsenilin knock-out. J Neurosci. 2003;23:9097–9106. doi: 10.1523/JNEUROSCI.23-27-09097.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and KChip3.1 transcription. Embo J. 2001;20:5715–5724. doi: 10.1093/emboj/20.20.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 31.Lusin JD, Vanarotti M, Li C, Valiveti A, Ames JB. NMR structure of DREAM: Implications for Ca(2+)-dependent DNA binding and protein dimerization. Biochemistry. 2008;47:2252–2264. doi: 10.1021/bi7017267. [DOI] [PubMed] [Google Scholar]
- 32.Maffie J, Rudy B. Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol. 2008;586:5609–5623. doi: 10.1113/jphysiol.2008.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marionneau C, LeDuc RD, Rohrs HW, Link AJ, Townsend RR, Nerbonne JM. Proteomic analyses of native brain Kv4.2 channel complexes. Channels (Austin) 2009;3:284–294. doi: 10.4161/chan.3.4.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y, Thiruvahindrapduram B, Fiebig A, Schreiber S, Friedman J, Ketelaars CE, Vos YJ, Ficicioglu C, Kirkpatrick S, Nicolson R, Sloman L, Summers A, Gibbons CA, Teebi A, Chitayat D, Weksberg R, Thompson A, Vardy C, Crosbie V, Luscombe S, Baatjes R, Zwaigenbaum L, Roberts W, Fernandez B, Szatmari P, Scherer SW. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martens JR, Kwak YG, Tamkun MM. Modulation of Kv channel alpha/beta subunit interactions. Trends Cardiovasc Med. 1999;9:253–258. doi: 10.1016/s1050-1738(00)00037-2. [DOI] [PubMed] [Google Scholar]
- 36.McCormack K, Connor JX, Zhou L, Ho LL, Ganetzky B, Chiu SY, Messing A. Genetic analysis of the mammalian K+ channel beta subunit Kvbeta 2 (Kcnab2) J Biol Chem. 2002;277:13219–13228. doi: 10.1074/jbc.M111465200. [DOI] [PubMed] [Google Scholar]
- 37.Morales MJ, Wee JO, Wang S, Strauss HC, Rasmusson RL. The N-terminal domain of a K+ channel beta subunit increases the rate of C-type inactivation from the cytoplasmic side of the channel. Proc Natl Acad Sci U S A. 1996;93:15119–15123. doi: 10.1073/pnas.93.26.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morohashi Y, Hatano N, Ohya S, Takikawa R, Watabiki T, Takasugi N, Imaizumi Y, Tomita T, Iwatsubo T. Molecular cloning and characterization of CALP/KChIP4, a novel EF-hand protein interacting with presenilin 2 and voltage-gated potassium channel subunit Kv4. J Biol Chem. 2002;277:14965–14975. doi: 10.1074/jbc.M200897200. [DOI] [PubMed] [Google Scholar]
- 39.Norris AJ, Foeger NC, Nerbonne JM. Interdependent Roles for Accessory KChIP2, KChIP3 and KChIP4 Subunits in the Generation of Kv4-encoded IA Channels in Cortical Pyramidal Neurons. JNeurosci. 2010 doi: 10.1523/JNEUROSCI.2487-10.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Garcia MT, Lopez-Lopez JR, Gonzalez C. Kvbeta1.2 subunit coexpression in HEK293 cells confers O2 sensitivity to kv4.2 but not to Shaker channels. J Gen Physiol. 1999;113:897–907. doi: 10.1085/jgp.113.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peri R, Wible BA, Brown AM. Mutations in the Kv beta 2 binding site for NADPH and their effects on Kv1.4. J Biol Chem. 2001;276:738–741. doi: 10.1074/jbc.M008445200. [DOI] [PubMed] [Google Scholar]
- 43.Petrecca K, Miller DM, Shrier A. Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin. J Neurosci. 2000;20:8736–8744. doi: 10.1523/JNEUROSCI.20-23-08736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pioletti M, Findeisen F, Hura GL, Minor DL., Jr Three-dimensional structure of the KChIP1-Kv4.3 T1 complex reveals a cross-shaped octamer. Nat Struct Mol Biol. 2006;13:987–995. doi: 10.1038/nsmb1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pongs O. Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS Lett. 1999;452:31–35. doi: 10.1016/s0014-5793(99)00535-9. [DOI] [PubMed] [Google Scholar]
- 46.Pongs O, Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev. 2010;90:755–796. doi: 10.1152/physrev.00020.2009. [DOI] [PubMed] [Google Scholar]
- 47.Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31:411–419. doi: 10.1016/j.tibs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Reid CA, Berkovic SF, Petrou S. Mechanisms of human inherited epilepsies. Prog Neurobiol. 2009;87:41–57. doi: 10.1016/j.pneurobio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 50.Rhodes KJ, Monaghan MM, Barrezueta NX, Nawoschik S, Bekele-Arcuri Z, Matos MF, Nakahira K, Schechter LE, Trimmer JS. Voltage-gated K+ channel beta subunits: expression and distribution of Kv beta 1 and Kv beta 2 in adult rat brain. J Neurosci. 1996;16:4846–4860. doi: 10.1523/JNEUROSCI.16-16-04846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruiz-Gomez A, Mellstrom B, Tornero D, Morato E, Savignac M, Holguin H, Aurrekoetxea K, Gonzalez P, Gonzalez-Garcia C, Cena V, Mayor F, Jr, Naranjo JR. G protein-coupled receptor kinase 2-mediated phosphorylation of downstream regulatory element antagonist modulator regulates membrane trafficking of Kv4.2 potassium channel. J Biol Chem. 2007;282:1205–1215. doi: 10.1074/jbc.M607166200. [DOI] [PubMed] [Google Scholar]
- 52.Schrader LA, Anderson AE, Mayne A, Pfaffinger PJ, Sweatt JD. PKA modulation of Kv4.2-encoded A-type potassium channels requires formation of a supramolecular complex. J Neurosci. 2002;22:10123–10133. doi: 10.1523/JNEUROSCI.22-23-10123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrader LA, Anderson AE, Varga AW, Levy M, Sweatt JD. The other half of Hebb: K+ channels and the regulation of neuronal excitability in the hippocampus. Mol Neurobiol. 2002;25:51–66. doi: 10.1385/MN:25:1:051. [DOI] [PubMed] [Google Scholar]
- 54.Schrader LA, Ren Y, Cheng F, Bui D, Sweatt JD, Anderson AE. Kv4.2 is a locus for PKC and ERK/MAPK cross-talk. Biochem J. 2009;417:705–715. doi: 10.1042/BJ20081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- 56.Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278:36445–36454. doi: 10.1074/jbc.M306142200. [DOI] [PubMed] [Google Scholar]
- 57.Thomsen MB, Wang C, Ozgen N, Wang HG, Rosen MR, Pitt GS. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ Res. 2009;104:1382–1389. doi: 10.1161/CIRCRESAHA.109.196972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueda A, Wu CF. Effects of hyperkinetic, a beta subunit of Shaker voltage-dependent K+ channels, on the oxidation state of presynaptic nerve terminals. J Neurogenet. 2008;22:1–13. doi: 10.1080/01677060701807954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Es MA, van Vught PW, Blauw HM, Franke L, Saris CG, Van den Bosch L, de Jong SW, de Jong V, Baas F, van’t Slot R, Lemmens R, Schelhaas HJ, Birve A, Sleegers K, Van Broeckhoven C, Schymick JC, Traynor BJ, Wokke JH, Wijmenga C, Robberecht W, Andersen PM, Veldink JH, Ophoff RA, van den Berg LH. Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nat Genet. 2008;40:29–31. doi: 10.1038/ng.2007.52. [DOI] [PubMed] [Google Scholar]
- 60.van Es MA, van Vught PW, van Kempen G, Blauw HM, Veldink JH, van den Berg LH. Dpp6 is associated with susceptibility to progressive spinal muscular atrophy. Neurology. 2009;72:1184–1185. doi: 10.1212/01.wnl.0000345368.01098.7e. [DOI] [PubMed] [Google Scholar]
- 61.Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci. 2004;24:3643–3654. doi: 10.1523/JNEUROSCI.0154-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Takimoto K, Levitan ES. Differential association of the auxiliary subunit Kvbeta2 with Kv1.4 and Kv4.3 K+ channels. FEBS Lett. 2003;547:162–164. doi: 10.1016/s0014-5793(03)00705-1. [DOI] [PubMed] [Google Scholar]
- 63.Weng J, Cao Y, Moss N, Zhou M. Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase. J Biol Chem. 2006;281:15194–15200. doi: 10.1074/jbc.M513809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang EK, Alvira MR, Levitan ES, Takimoto K. Kvbeta subunits increase expression of Kv4.3 channels by interacting with their C termini. J Biol Chem. 2001;276:4839–4844. doi: 10.1074/jbc.M004768200. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Su P, Liang P, Liu T, Liu X, Liu XY, Zhang B, Han T, Zhu YB, Yin DM, Li J, Zhou Z, Wang KW, Wang Y. The DREAM protein negatively regulates the NMDA receptor through interaction with the NR1 subunit. J Neurosci. 30:7575–7586. doi: 10.1523/JNEUROSCI.1312-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]