Abstract
Humans and lower animals time as if using a stopwatch that can be “stopped” or “reset” on command. This view is challenged by data from the peak-interval procedure with gaps: Unexpected retention intervals (gaps) delay the response function in a seemingly continuous fashion, from stop to reset. We evaluated whether these results are an artifact of averaging over trials, or whether subjects use discrete alternatives or a continuum of alternatives in individual-trials: A Probability-of-Reset hypothesis proposes that in individual gap trials subjects stochastically use discrete alternatives (stop/reset), such that when averaged over trials, the response distribution in gap trials falls in between “stop” and “reset”. Alternatively, a Resource Allocation hypothesis proposes that during individual gap trials working memory for the pregap duration decays, such that the response function in individual gap trials is shifted rightward in a continuous fashion. Both hypotheses provided very good fits with the observed individual-trial distributions, although the Resource Allocation hypothesis generated reliably better fits. Results provide support for the usefulness of individual-trial analyses in dissociating theoretical alternatives in interval timing tasks.
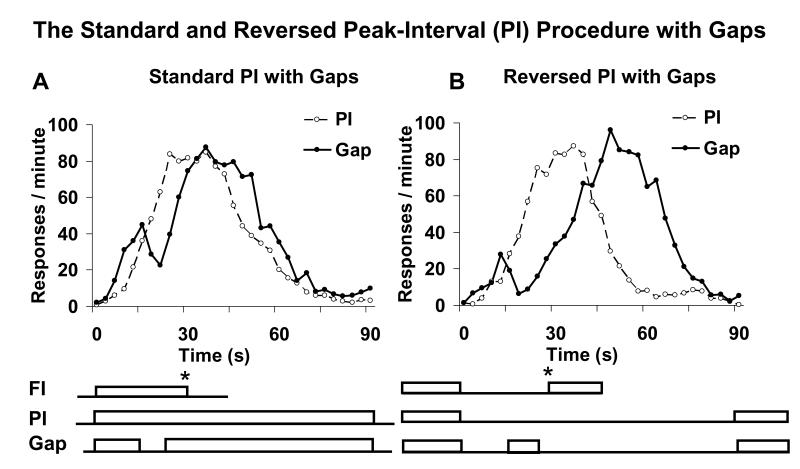
Humans and lower animals time as if using an internal clock that can be “stopped” or “reset” on command (reviewed by Buhusi & Meck, 2005). The internal clock concept assumes that pulses emitted by a pacemaker are stored in an accumulator whose content represents the current subjective time (Fraisse, 1957; Francois, 1927; Hoagland, 1933; Treisman, 1963; Woodrow, 1930). A case in point that the internal clock can be used in discrete (stop/reset) modes is given by the response pattern in the peak-interval (PI) procedure with gaps, or PI-GAP procedure (Church, 1978; S. Roberts, 1981) (Figure 1). In the standard version of this procedure, during fixed-interval (FI) trials, the first response after a specified criterion, say 30 s, from the onset of a to-be-timed signal is reinforced (Figure 1A). In PI trials, the to-be-timed signal is presented but subject’s responses are not reinforced; typically, in PI trials the average response rate increases after the onset of the to-be-timed signal, reaches a peak about the time when subjects’ responses were (sometimes) reinforced, and declines afterwards (Catania, 1970; Church, 1978) (Figure 1A). During gap trials, a brief interruption (gap) of the to-be-timed signal prompts a delay in response relative to PI trials by about the duration of the gap, suggesting that on average subjects retain in working memory the pre-gap interval and resume timing after the gap where they left off before the gap, a response mode (alternative) called stop (e.g., Church, 1978; S. Roberts & Church, 1978) (Figure 1A). However, a quite different response pattern is observed in the reversed version of the PI procedure (Buhusi & Meck, 2000), in which subjects time the absence of a signal (e.g., in the dark) and their timing is interrupted by a signaled (e.g., illuminated) gap (Figure 1B): Subjects delay their response function after the gap for a duration that is approximately the sum of the gap and pre-gap intervals, suggesting that on average they restart the entire timing process after the gap, using a reset response (Buhusi & Meck, 2000).
Figure 1. The peak-interval (PI) procedure with gaps.
Subjects time the presence of a signal (Standard, panel A) or the absence of a signal (Reversed, panel B). Subjects are randomly presented with fixed-interval (FI) trials, peak-interval (PI) trials, and gap trials in which the to-be-timed signal is interrupted by a retention-interval (gap). The upper graphs show that in PI trials the average response rate peaks at the to-be-timed interval, and that the presentation of a gap delays the response function. * = reinforcement.
Because the results presented in Figure 1 were obtained by averaging response rate over many trials, there is the possibility that the stop / reset modes represent averaging artifacts that do not readily reflect the behavior of the subjects in the experimental box. Indeed, while the average response function in PI trials is Gaussian-like (Figure 1), during individual PI trials both pigeons (Cheng & Westwood, 1993) and rats (Church, Meck, & Gibbon, 1994; Gibbon & Church, 1990) use a low-high-low pattern of response (Figure 2B). Similarly, during individual gap trials the pattern of response is relatively complex: Some gap trials follow a low-high-low-high-low pattern, while some lack pre-gap responses altogether, and have a pattern similar to that from PI trials, but delayed. Such complex response patterns challenge current interval timing theories, developed to address data averaged over many trials (e.g., Gibbon, Church, & Meck, 1984). To address the complex behavior in the PI-GAP procedure, two classes of theoretical interpretations have been proposed, attributing response variability to either the internal clock or to the interaction between the internal clock and other cognitive processes: Initial investigations indicated that on average rodents tend to stop (e.g., Church, 1978; S. Roberts & Church, 1978), while birds tend to reset (Bateson & Kacelnik, 1998; Brodbeck, Hampton, & Cheng, 1998; Cabeza de Vaca, Brown, & Hemmes, 1994; W. A. Roberts, Cheng, & Cohen, 1989), suggesting that the stop/reset response alternatives reflect discrete modes of the internal clock (Church, 1978), accounted for by a switch connecting the pacemaker and the accumulator, allowing or not pulse accumulation (Church, 1978; Gibbon et al., 1984).
Figure 2. Example of singe-trial statistics distributions and for an individual subject.
Upper panel: Normalized distributions of start points, S1 (continuous gray curve), stop points, S2 (continuous black curve), and m12 (dashed gray curve) for an individual subject from Buhusi & Meck (2000). Lower panel: Raster plot of responses in the same individual. Responses are marked by dots. High states are marked by segments delineated by the extracted start and stop points. While peak trials are characterized by a single high state (marked by a S1-S2 line segment), some gap trials also include a pre-gap “high” state (marked by an additional S3-S4 line segment). The location of the gap is marked by vertical dashed lines.
In contrast, recent results indicate that rats reset their timing in PI trials upon presentation of reinforcement (Matell & Meck, 1999; Thorpe, Petrovic, & Wilkie, 2002), that both rats and pigeons stop or reset depending on gap content (Buhusi & Meck, 2000), gap discriminability (Buhusi, Perera, & Meck, 2005), gap/signal contrast (Buhusi & Cerutti, 2005; Buhusi, Sasaki, & Meck, 2002), and subjects’ visual acuity (Buhusi et al., 2005), and that similar delays are obtained when the procedure includes distracter events rather than gaps (Buhusi & Meck, 2006a, 2006b). These departures from the stop/reset modes support the alternative interpretation that the average response after a gap does not reflect discrete modes of the internal clock, but rather that working memory for time is sensitive to Resource Allocation (RA) (Buhusi, 2003), a process external to the clock, actively controlled by the perceived salience of events. This interpretation accounts not only for the discrete stop and reset responses, but also for a continuum of responses in between stop and reset.
Here we evaluate whether the pattern of response in individual gap trials is consistent with either discrete modes of the internal clock (Gibbon et al., 1984) or a continuum of alternatives (Buhusi, 2003). To reconcile these views, Cabeza de Vaca et al. (1994) proposed a “stochastic” model, denoted here as the Probability-of-Reset hypothesis (PR), which assumes that during individual gap trials subjects use discrete alternatives (stop / reset) with a certain probability, such that the average response functions in gap trials is a mix of the “stop and “reset” responses, thus falling in between stop and reset, and giving the appearance of a continuous range of alternatives. This distinction is lost when analyzing average response functions, and requires special individual-trial analyses.
Evidence for the PR hypothesis was previously sought by Cabeza de Vaca et al. (1994), by estimating the time of response initiation S1 (“start”) and the time of response termination S2 (“stop”) for each individual trial using Gibbon & Church’s (1990) algorithm (Figure 3A). Acknowledging that applying this algorithm to gap trials is problematic because they do not have a consistent start-stop pattern (Figure 2B), Cabeza de Vaca et al. focused on the stop time S2, which is reliably identified by the algorithm irrespective of trial type (PI or gap). Because the PR hypothesis assumes that subjects stochastically use stop and reset alternatives in gap trials, it predicts that the distributions of individual-trial statistics (e.g., S2) should be bi-modal in gap trials and wider in gap trials relative to PI trials (Cabeza de Vaca et al., 1994). However, analyses of pigeon’s responses failed to indicate bi-modality of S2 distribution in gap trials. Nevertheless, the S2 distribution was found to be wider for late gaps than for early gaps, thus providing partial support the PR hypothesis (Cabeza de Vaca et al., 1994).
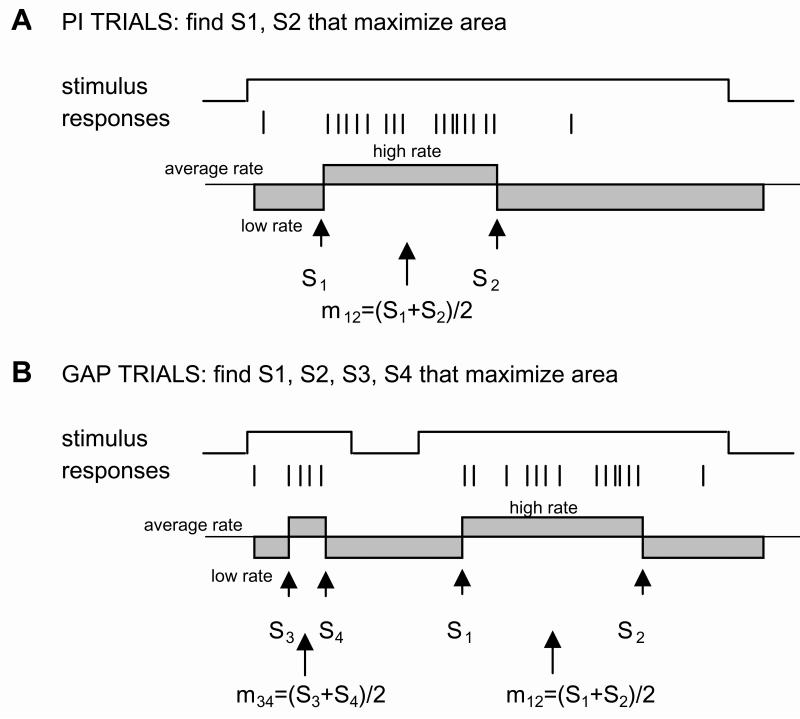
Figure 3. Single-trial analysis of the response pattern in the PI procedure with gaps.
A. The single-trial algorithm originally proposed by Church et al. (1994) assumes that in individual PI trials the response rate has a low-high-low pattern. The algorithm searches for the start (S1) and stop point (S2) that maximizes Eq.1 (the gray area). B. During individual gap trials, the pattern of response follows a low-high-low-high-low pattern. Our extended algorithm searches for two sets of start and stop points, before the gap, S3 and S4, and after the gap S1 and S2, which maximizes Eq.2 (the gray area). Using the start and stop points one can also compute a middle point, m=(start+stop)/2, and response duration, d=stop-start.
The mixed results obtained Cabeza de Vaca et al. (1994) may be equally due to the algorithm used (Church et al., 1994; Gibbon & Church, 1990), since gap trials do not follow a consistent start-stop pattern, but also due to the species investigated, since pigeons have a known tendency to reset (W. A. Roberts et al., 1989) and are possibly less prone to stochastic variations in their responses. Therefore, here we extended the Church & Gibbon (1990) algorithm to gap trials with multiple start-stops (Figure 3B), and we applied this new algorithm to the analysis of rat’s response pattern in individual gap trials in the standard and reversed PI-GAP procedure (Buhusi & Meck, 2000) to evaluate the predictions of the PR hypothesis. Finally, we evaluated how well the PR and RA hypotheses fit the observed distributions of start and stop times in individual trials by computing an index of superposition (intra-class correlation, ICC) between observed and hypothetical distributions. By evaluating response variability as predicted by PR – an account relying on discrete modes of the internal clock – and by RA – an account based on the continuum of interactions between the internal clock and other cognitive processes, our study speaks more generally about the complex cognitive processes at work in behavioral tasks involving interval timing.
Materials and Methods
Experimental data
We analyzed data reported in Buhusi & Meck (2000), evaluating the effect of gap duration in the standard and reversed PI-GAP procedure (Figure 1). Rats were trained to time the presence (standard group) or the absence (reversed group) of a 30-s visual signal, and were tested by 1-, 5-, and 15-s gaps (dark for the standard group, and illuminated for the reversed group), presented 15-s after signal onset. The response (lever press) time (10-ms resolution) in PI trials and in the 3 types of gap trials was passed to the single-trial analysis algorithm described below.
An individual-trials analysis algorithm for gap trials
The statistics of the pattern of response in individual PI and gap trials were estimated using an extension of the algorithm described by Church et al. (1994). For PI trials the algorithm assumes that responses follow a “low-high-low” pattern (Figure 3A), and exhaustively searches for the transition from a low to a high response state, S1 (“start”), and the subsequent transition from a high to a low response state, S2 (“stop”), to maximize the function
| (Eq.1) |
where t1, t2, and t3 are the ranges of the low and high areas, and r1, r2, and r3 are the average response rates during these ranges, r being the overall average response rate on that individual trial (Figure 3A). Trials in which subjects lack temporal control, i.e., start responding after the criterion (S1>T) or stop responding before the criterion (S2<T) were excluded from analyses (Church et al. 1994).
For gap trials, our algorithm aimed at identifying two “high states”, one for the pre-gap interval and one for the post-gap interval, by an exhaustive search for the pre-gap start (S3) and stop (S4), and for the post-gap start (S1) and stop (S2) which maximize the function
| (Eq.2) |
where t1, t2, t3, t4, and t5 are the respective ranges of the low and high states, and r1, r2, r3, r4, and r5 are the average response rates during these ranges, r being the overall average response rate on that individual gap trial (Figure 3B). Gap trials in which the subjects start responding very late after the gap (S1> T + gap + pregap), or stop responding before the criterion (S2<T) were eliminated from analyses. Because in some gap trials there is vigorous pre-gap activity, while in others there is sparse pre-gap activity, analyses for gap trials were run in two steps. Individual gap trials were first analyzed using Eq.2 to identify S3, S4, S1, and S2; if the pre-gap stop S4 exceeded the to-be-timed criterion T, the trial was re-analyzed using Eq.1 to generate only the points S1 and S2. Finally, trials with less than 4 responses were eliminated. In all, including both PI and gap trials, the algorithm rejected 6% of trials for having too few responses, 3% of trials for having an early stop, and 14% of trials for having a late start.
For all trials we calculated the median response time and the duration of the response bout as follows: m34 = (S3 + S4)/2, m12 = (S1 + S2)/2, d34 = S4 - S3, d12 = S2 - S1. The S1, S2, m12, and d12 statistics were submitted to further analyses to evaluate the predictions of the PR hypothesis, and the degree by which the RA or PR hypotheses fit their distributions. An example of the distributions of these statistics for an individual subject is shown in Figure 2A.
Basic individual-trials statistics
Means, standard errors of the mean, and coefficient of variations for S1, S2, m12, and d12 were computed first over individual trials, and then averaged over subjects (Cheng & Westwood, 1993; Church et al., 1994; Gibbon & Church, 1990). Individual subject average S1, S2, m12, and d12 were submitted to mixed ANOVAs with between factor group (standard v. reversed) and within factor trial type (PI, 1-, 5-, and 15-s gap). A “shift in gap trials relative to PI trials” value was computed for S1, S2, and m12 by subtracting from the value in gap trials the value in PI trials and the duration of the gap, e.g., , and submitted to mixed ANOVAs with factors group and trial type.
Evaluating the probability-of-reset hypothesis
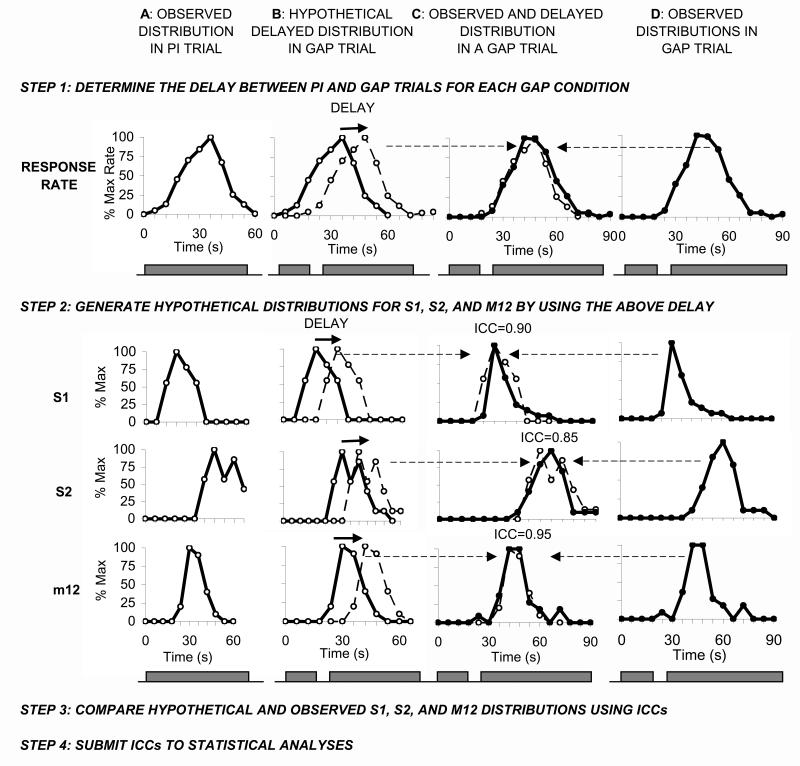
The PR hypothesis assumes that in individual gap trials subjects stochastically use the stop and reset alternatives (Cabeza de Vaca et al. 1994). The following steps were applied for each individual for each gap type and for each statistic S1, S2, and m12 (Figure 4). First, the normalized average response distribution in PI trials (in 3-s bins) was used to generate a hypothetical average “stop” distribution (by shifting the average PI response function by gap duration) and an average “reset” distribution (by shifting the average PI response function by the sum of the gap and pre-gap durations). Afterwards, we computed the optimal “proportion of average stop and reset distributions” which best fits (least-squares minimization) the observed average response function in that gap type (an example of a 40% stop and 60% reset mix is shown in Figure 4). Second, for each gap type we generated hypothetical PR individual-trial distributions for S1, S2, and m12, by mixing the individual’s S1, S2, and m12 distributions observed in individual PI trials in the proportions obtained in step 1. Third, for each gap type and for each statistic S1, S2, and m12, we computed an intra-class correlation coefficient (ICCPR) as a measure of the degree of superposition between the hypothetical individual-trial PR distribution generated at step 2 and the observed individual-trial distribution in the given gap type (not including responses which were classified as within the first “high response region”, between S3 and S4, since the PR hypothesis does not address these responses). ICCPR indices were submitted to statistical analyses over groups and subjects.
Figure 4. General evaluation method for a probability of reset (PR) hypothesis.
A. Observed distributions in peak-interval (PI) trials. B. Hypothetical distributions in a “stop” trial, generated by shifting the “observed distributions” in panel A with the duration of gap relative to PI trials. C. Observed (thick curves) and hypothetical PR distributions obtained by mixing the “stop” and “reset” distributions (panels B and D) by the proportions that provide a best fit for the response rate. D: Hypothetical distributions in a “reset” trial, generated by shifting the “observed distributions” in panel A with sum of the gap and pre-gap intervals relative to PI trials. All steps are performed for each individual and for each gap type.
The ICC index is interpretable as the percentage of overall variance attributable to time-bin-to-time-bin variation as opposed to within-time-bin variation, and can be used directly in hypothesis testing irrespective of the shape of the response function (Landis & Koch, 1977). The closer to 1 the ICC the better the fit between the hypothetical and observed distributions. The ICC index is considered poor for values < .2, fair > .2, good > .4, very good > .6, and excellent > .8, with a value of 1 indicating perfect superposition of distributions for all time bins (Andresen, 2000; Landis & Koch, 1977).
Evaluating the Resource Allocation hypothesis
The Resource Allocation hypothesis assumes that the gap delays the subject’s response in a continuous fashion between stop and reset. The following steps were applied for each individual for each gap type and for each statistic S1, S2, and m12 (Figure 5). First, the normalized average response distributions in PI and gap trials were used to compute the optimal “delay” by which shifting the average PI response function best fits the average response function for the given gap (least-squares minimization). Second, for each gap type we generated hypothetical RA distributions for S1, S2, and m12 by delaying their distributions observed in individual PI trials by the delay(s) obtained in step 1. Third, for each individual and for each statistic S1, S2, and m12, an intra-class correlation coefficient (ICCRA) was used to measure the degree of superposition between the hypothetical and observed distributions for the given gap type. ICCRA indices were submitted to statistical analyses over groups and subjects.
Figure 5. General evaluation method for a Resource Allocation (RA) hypothesis.
A. Observed distributions in peak-interval (PI) trials. B. Hypothetical distributions in a gap trial, generated by shifting the “observed distributions” in panel A by the delay obtained in step 1 of the algorithm. C. Observed (thick curves) and hypothetical RA distributions (from panels B and D). D. Observed distributions in a gap trial. All steps are performed for each individual and for each gap type.
Hypothesis comparison
For each individual for each gap type and for each of the S1, S2, and m12 statistics we computed the difference between ICCRA and ICCPR, ΔICC= ICCRA - ICCPR, which was further used in hypothesis testing to evaluate the model that better fits the observed individual-trial statistics. For each trial type we evaluated the percent of cases (10 subjects × 3 statistics) for which ΔICC is positive, and contrasted it with the null hypothesis (50% of cases) using a Χ2 test. Additionally, to test for possible interactions between group variables, individual ICCPR and ICCRA values were submitted to a mixed ANOVA with between factor group (standard v. reversed) and within factors hypothesis (PR v. RA) and gap duration (1s, 5s, 15s).
All statistical tests were evaluated at a significance level of 0.05.
Results
Evaluation of the basic assumptions of the individual-trial analysis algorithm
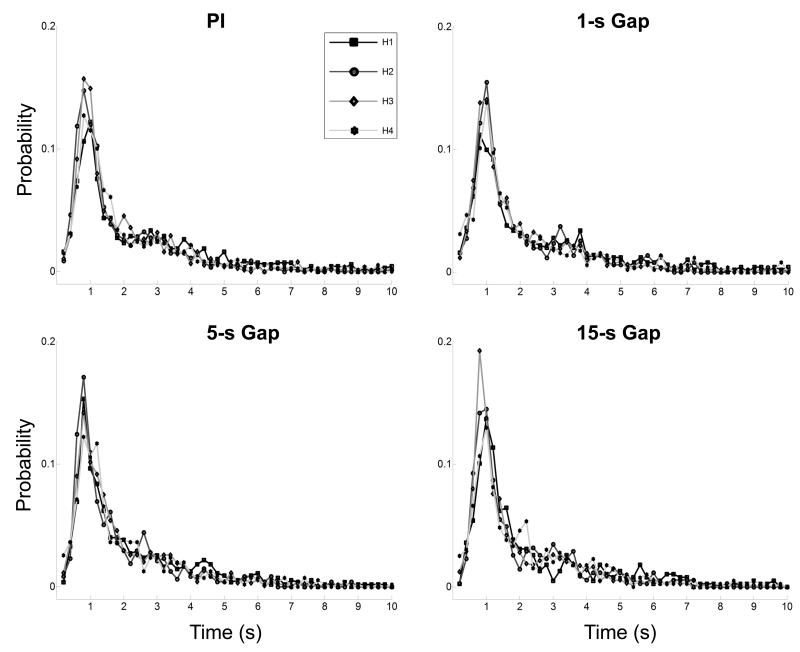
The assumption that response rates are constant during “high” regions was evaluated as in Church et al. (1994) by splitting the region of interest into four quartiles, and evaluating their inter-response time (IRT) distributions. Figure 6 shows the average IRT distributions for each quartile of the “high” states for the standard group across trial types. The IRT distributions overlap well across quartiles for each trial type, suggesting that the response rates are constant throughout the identified “high” region in both PI and gap trials. Similar analyses performed for the reversed group (not shown here) indicate that the quartile IRT distributions superimpose well over all conditions, thus allowing the use of the individual-trial analyses based on Eq.1 and Eq.2.
Figure 6. Inter-response time distributions.
Plot of the inter-response time distributions across subjects in the standard group. Each plot is split into four quarters (H1-H4), which overlap showing consistent responses throughout the high response region.
Individual-trial statistics
We evaluated the effect of the gap duration and stimulus modality on the individual-trial statistics: We expected S1 and S2 to increase with gap duration; we also expected S1 and S2 to be larger in the Reversed group relative to the Standard group (Buhusi & Meck, 2000). A confirmation of these expectations would provide confidence in our individual-trial analysis algorithm. Most importantly, according to the PR hypothesis, in gap trials subjects stochastically mix stop and reset alternatives, so that d12 should be larger in gap trials relative to PI trials. The means and variance of the individual-trial statistics S1, S2, m12, and d12 are summarized in Table 1. As expected, S1, S2, and m12 increase with gap duration, and are larger in the Reversed than in the Standard group; in contrast, d12 seems to be constant across trials.
Table 1.
Mean, standard error, and coefficient of variation for S1, S2, m12, and d12 for the Standard and Reversed groups were first computed for each individual-trial, then averaged across subjects (units = seconds). PI: peak-interval.
| Standard | PI | 1-s Gap | 5-s Gap | 15-s Gap | |
|---|---|---|---|---|---|
| S1 | Mean ± SEM | 15.4 ± 1.2 | 19.8 ± 1.7 | 27.7 ± 1.7 | 41.3 ± 2.0 |
| CV | 0.39 | 0.45 | 0.34 | 0.24 | |
|
| |||||
| S2 | Mean ± SEM | 47.4 ± 1.7 | 48.6± 1.8 | 56.6± 2.1 | 68.2 ± 2.1 |
| CV | 0.18 | 0.19 | 0.19 | 0.16 | |
|
| |||||
| m12 | Mean ± SEM | 31.4 ± 1.3 | 34.2 ± 1.5 | 42.1± 1.6 | 54.8± 1.8 |
| CV | 0.20 | 0.23 | 0.20 | 0.17 | |
|
| |||||
| d12 | Mean ± SEM | 32.1± 1.6 | 28.7 ± 1.7 | 28.9 ± 2.1 | 26.9± 2.0 |
| CV | 0.25 | 0.31 | 0.37 | 0.37 | |
|
| |||||
| Reversed | |||||
|
| |||||
| S1 | Mean ± SEM | 18.3± 1.4 | 29.12 1.7 | 35.2 ± 2.1 | 45.2 ± 2.1 |
| CV | 0.33 | 0.28 | 0.28 | 0.20 | |
|
| |||||
| S2 | Mean ± SEM | 45.6 ± 2.0 | 54.9± 2.3 | 60.9 ± 2.5 | 73.1 ± 1.7 |
| CV | 0.18 | 0.17 | 0.17 | 0.11 | |
|
| |||||
| m12 | Mean ± SEM | 31.9 ± 1.5 | 42.0 ± 1.7 | 48.1 ± 1.9 | 59.2± 0.4 |
| CV | 0.19 | 0.18 | 0.17 | 0.11 | |
|
| |||||
| d12 | Mean ± SEM | 27.3 ± 1.9 | 25.7 ± 2.3 | 25.7 ± 2.7 | 27.9 ± 2.6 |
| CV | 0.29 | 0.36 | 0.46 | 0.39 | |
These suggestions were confirmed by statistical analyses. One way ANOVAs on S1, S2, m12, and d12 in PI trials revealed no differences between the groups, Fs(1,8)<4.64, p>0.16. For both groups the median m12 in PI trials was not statistically different from the 30s criterion, ts(4)<1.86, p>0.14, suggesting that both groups learned the timing task. Moreover, mixed ANOVAs of S1, S2, and m12 with between factor group and within factor trial type revealed reliable effects of trials, Fs(3,24)>137.62, p<.001, suggesting that the longer the gap the larger the delay in response, and group × trial interactions, Fs(3,24)>4.06, p<.02, suggesting that a reliable delay was observed for the 1-s gap in the Reversed group relative to the Standard group. Finally, a reliable group effect was revealed for the S1 and m12 statistics, Fs(1,8)>6.96, p<.03, suggesting that subjects in the Reversed group started later and responded longer than those in the Standard group, but not for the S2 statistic, suggesting both groups stopped at about the same time. Most importantly, analyses of the width statistic, d12, failed to suggest any reliable effects of group, F(1,8)>2.63, p=.14, trial, F(3,24)=1.36, p=.28, or group × trial interaction, F(3,24)=1.45, p=.25.
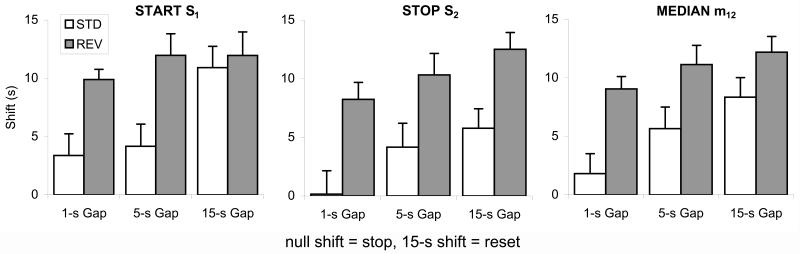
Finally, a shift value was computed for S1, S2, and m12 for each gap type by subtracting the value in PI trials and the duration of the gap from the value in gap trials (Buhusi & Meck, 2000). A null shift suggests the use of a discrete stop response, a value equal to the pre-gap duration (15s) suggests the use of a discrete reset response, while values in between stop and reset are consistent with the use of a continuum of response alternatives. As shown in Figure 7, subjects the standard groups showed smaller shifts relative to subjects the reversed group, suggesting that these statistics are sensitive to stimulus modality. However, shift values were mostly in between stop and reset, suggesting the use of continuous rather than discrete response alternatives.
Figure 7. Average shift in individual-trial start (S1), stop (S2) and median (m12) in gap trials relative to PI trials.
A null shift indicates the use of a discrete stop alternative, a value equal to the pre-gap duration (15s) indicates the use of a discrete reset alternative, while values in between 0 and 15 indicate possible use a continuum of response alternatives.
These suggestions were supported by statistical analyses of the shift in S1, S2, and m12. Mixed ANOVAs with between factor group and within factor gap duration revealed reliable effects of group, Fs(1,8)>17.89, p<.01, suggesting that the Reversed group tended to delay more than the Standard group, reliable effects of gap, Fs(2,16)>6.58, p<.01, suggesting that the delay increased with gap duration, but no reliable group × gap interactions, Fs(2,16)<1.84, p>.19, except for the start statistic S1, F(2,16)=4.00, p<.05, suggesting that the Standard group shifted response initiation less than the Reversed group at short gaps, 1-s and 5-s, but not at the 15-s gap, when both groups delayed response initiation equally after the gap.
Taken together, these results validate our algorithm, and suggest that the individual-trial trial statistics S1, S2, and m12 are sensitive to both stimulus modality and gap duration. In particular, the start (response initiation) S1 was found to be more sensitive to gap manipulations than the stop (response termination) S2, which may explain the lack of reliability in evaluating the PR hypothesis by analyzing only the S2 statistic in the Cabeza de Vaca et al. (1994) study. Individual-trial statistics were consistent with the interpretation that responses were merely shifted rightward relative to PI trials: the longer the gap the more subjects delayed response initiation after the gap, but they responded equally long irrespective of trial type (PI or gap). The later finding is at odds with the PR hypothesis, because the PR hypothesis assumes that distributions in gap trials are a mixture of stop and reset distributions, and predicts larger widths for the gaps than for PI trials (Cabeza de Vaca et al., 1994). However, this finding is consistent with the RA hypothesis, which predicts that responses are simply “shifted” in gap trials relative to PI trials, thus maintaining their duration.
Correlations among individual-trial statistics
The reliability of the our new algorithm was further tested by evaluating whether the pattern of correlations between individual-trial statistics matches previous studies (Cheng & Westwood, 1993; Church et al., 1994; Gibbon & Church, 1990). The correlations between the S1, S2, m12, and d12 statistics in PI and gap trials are shown in Table 2. The pattern of correlation was qualitatively similar in both groups: We found consistent positive S1 : S2 and S1 : m12 correlations suggesting that subjects tended to stop later on trials where they start later, thus having a later median, and consistent negative S1 : d12 correlations suggesting longer response durations when subjects started earlier. We also found consistent positive S2 : m12 and S2 : d12 correlations suggesting that when subjects stopped later their response duration and median increased. This pattern matches that found for the PI trials both in our study (Table 2) and in previous studies (Cheng & Westwood, 1993; Church et al., 1994; Gibbon & Church, 1990) with one exception. The d12 : m12 correlation, which was positive in PI trials in our study and in previous research, was unreliable in gap trials, a result due to the occasional very long high states in gap trials (Figure 2B). The latter finding confirms the high variability and complexity of response patterns in gap trials, and provides incentive for further attempts to understand the pattern of response in gap trials using individual-trial or other types of analyses.
Table 2.
Mean correlations between S1, S2, m12, and d12 were first computed for each individual, then averaged across subjects.
| STANDARD | S1 : S2 | S1 : d12 | d12 : m12 | S1 : m12 | S2 : d12 | S2 : m12 |
|---|---|---|---|---|---|---|
| PI | 0.47 | −0.25 | 0.36 | 0.80 | 0.72 | 0.90 |
| 1-s Gap | 0.51 | −0.47 | 0.04 | 0.86 | 0.51 | 0.88 |
| 5-s Gap | 0.43 | −0.44 | 0.15 | 0.81 | 0.61 | 0.87 |
| 15-s Gap | 0.53 | −0.38 | 0.10 | 0.87 | 0.55 | 0.86 |
| Mean ± SEM | 0.48±0.02 | −0.38±0.05 | 0.16±0.07 | 0.84±0.02 | 0.60±0.05 | 0.88±0.01 |
| REVERSED | ||||||
| PI | 0.40 | −0.36 | 0.26 | 0.79 | 0.68 | 0.87 |
| 1-s Gap | 0.39 | −0.40 | 0.18 | 0.76 | 0.60 | 0.86 |
| 5-s Gap | 0.37 | −0.50 | 0.06 | 0.81 | 0.58 | 0.83 |
| 15-s Gap | 0.17 | −0.62 | −0.03 | 0.78 | 0.64 | 0.72 |
| Mean ± SEM | 0.33±0.06 | −0.47±0.06 | 0.11±0.06 | 0.79±0.01 | 0.63±0.02 | 0.82±0.03 |
| LITERATURE | ||||||
| Gibbon&Church1990 | 0.34 | −0.34 | 0.38 | 0.74 | 0.77 | 0.89 |
| Cheng & Westwood 1993 | 0.38 | −0.36 | 0.31 | 0.76 | 0.71 | 0.88 |
| Church et al. 1994 | 0.31 | −0.34 | 0.41 | 0.70 | 0.78 | 0.89 |
Testing the Resource Allocation and Probability of Reset Hypothesis
To test the RA and PR hypotheses we first generated RA and PR hypothetical distributions for S1, S2, and m12 for each individual and for each gap type, as described in Figures 4 and 5. The hypothetical PR distribution for each subject and gap type was based on finding the best “proportion of stop and reset distributions”, as follows: For the 1-, 5- and 15-s gaps respectively, the best stop : reset mix were 85:15% ± 2%, 56:44% ± 7%, and 32:68% ± 5% for the Standard group, 37:63% ± 5%, 23:77% ± 7%, and 20:80% ± 6% for the Reversed group. For each individual and for each gap type we then computed the degree of fit (superposition) between the PR hypothetical distribution based on the given proportion and the observed S1, S2, and m12 distribution in the given condition, ICCPR. Similarly, the hypothetical RA distribution for each subject and gap type was based on finding the best “delay” of the distribution in PI trials that fits the average response distribution in the given gap trial, as follows: For the 1-, 5- and 15-s gaps respectively, the delays were 3.7 ± .4s, 12.9 ± 1s, and 25.2 ± 1.4 s for the Standard group, and 10.2 ± 1s, 16.1 ± 2.1s, and 27.3 ± 1s for the Reversed group, the. For each individual and for each gap type we then computed the degree of fit (superposition) between the RA hypothetical distribution based on the given delay and the observed distribution in the given condition, ICCRA.
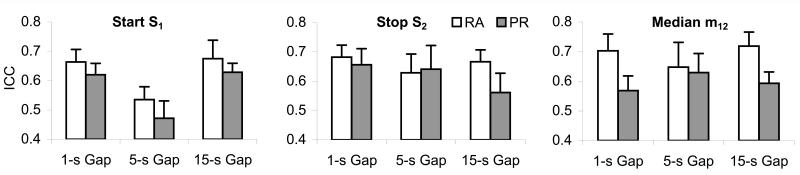
Basic ICC statistics are shown in Figure 8. ICCs > .4 are considered good, ICCs > .6 are considered very good, and ICCs > .8 are considered excellent, with a value of 1 indicating perfect superposition (Andresen, 2000; Landis & Koch, 1977). In absolute terms, both the PR and RA hypotheses provided very good fits for most conditions: The RA provided very good fits for 8 out of 9 conditions, while the PR provides very good fits for only 5 out of 9 conditions, with only good fits for the rest of the conditions. In relative terms, RA seems to provide better fits than PR: On average, 73% of the conditions (across subjects and gaps) were better fit by the resource allocation model; we failed to find any cases where the PR model performed better than the RA model (across subjects and gaps).
Figure 8. Average ICC for the start (S1), stop (S2), and median (m12) over gap durations and hypotheses.
The ICCs for the RA hypothesis exceed the ICCs for the PR hypothesis under all conditions but S2 for the 5-s gap.
These suggestions were supported by separate mixed ANOVAs for the ICCs obtained for the S1, S2, and m12, with between factor group and within factors hypothesis and gap duration. Because no significant main effect of interactions were found for the group variable, data were collapsed across groups and re-submitted to repeated ANOVAs with factors hypothesis and gap duration, which indicated a significant main effect of hypothesis for m12, F(1,9)=6.25, p<.05, and a close to significance main effects of hypothesis S1, F(1,9) = 3.84, p=.08, suggesting better fits for these individual-trial statistics by the RA hypothesis. Moreover, analyses indicated a significant main effect of gap duration on S1, F(2,18)=11.39, p<.01, suggesting that both hypotheses performed unevenly at different gap values; both hypotheses provided very good fits (ICCs > .6) at short, 1-s, and long, 15-s, gaps rather than at intermediate, 5-s, gaps. Finally, analyses indicated a significant interaction between the hypothesis and gap variables for the in S2 statistic, F(2,18)=3.67, p <.05, suggesting that the PR hypothesis provides a significantly worse fit for S2 at the 15-s long gaps.
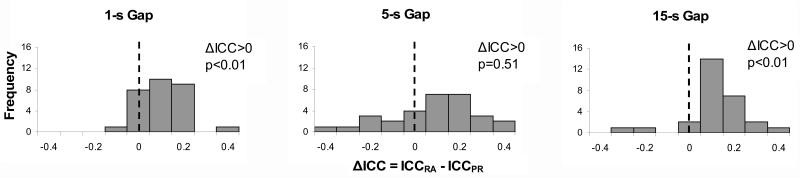
To further evaluate which model better fits the observed individual–trial statistics, we computed for each individual for each statistic the difference in ICC between the RA and PR hypotheses ΔICC= ICCRA - ICCPR. For each gap type we computed the frequency of ΔICC >0 (better fit by the RA relative to PR), and the expected frequency under the null hypothesis (RA = PR, ΔICC = 0), and submitted them to Χ2 tests. For example, for the 1-s gap there were in all 30 cases (10 subjects × 3 statistics), of which ΔICC >0 in 21 cases (better fit by RA), more than the expected 15 cases under the null hypothesis. Analyses revealed reliable effects for the 1-s gap, Χ2(1)=4.03, p<.05, and the 15-s gap, Χ2(1)=14.7, p<.01, but not for the 5-s gap, Χ2(1)=1.63, p=.20. Histograms of the individual ΔICC values for each gap duration are shown in Figure 9, which indicate a significant rightward shift in mean for the 1- and 15-s gaps, ts(29)>3.1, ps<.01, but not for 5-s gap, t(29)=0.67, p=.51. These results suggest that the RA hypothesis provides better fits for the 1-s and 15-s gaps, but that at the 5-s gap there is no distinction between hypotheses.
Figure 9. Distribution of the difference in ICC between RA and PR hypotheses.
Frequency distributions of difference in ICC for observed cases (10 subjects × 3 statistics: S1, S2, m12) for the 1-s, 5-s, and 15-s gaps. Positive differences are supportive of the RA hypothesis, while negative differences are supportive of the PR hypothesis.
Taken together, these results suggest that both the PR and RA and hypotheses provide very good fits to the observed individual-trial statistics in most of the cases, and that the distributions generated using the RA hypothesis are reliably better relative to the PR hypothesis. Interestingly, the PR hypothesis provides a reliably lower fit for the stop time S2 than the RA hypothesis, which may explain the relative inability of Cabeza de Vaca et al (1994) to evaluate the PR hypothesis using solely the S2 statistic. In turn, it seems that further research in understanding the pattern of response in PI-GAP procedure needs to focus on the start (response initiation), which seems most sensitive to the gap manipulations.
Discussion
The effects of gap duration and modality were previously investigated in a PI-GAP procedure by averaging responses over trials. On average, when timing the presence of a visual stimulus rats stop timing during a dark gap, but when timing the absence of a visual stimulus (dark) rats tend to reset (re-start) the entire timing process after an illuminated gap (Buhusi & Meck, 2000). While these average results are typically interpreted as evidence for discrete stop and reset modes of an internal clock (PR hypothesis), the fact that gap duration and modality modulate these results provide support for the alternative interpretation that the internal clock interacts with other cognitive processes resulting in a continuum of response alternatives (RA hypothesis). This distinction is lost when averaging responses over many gap trials, and requires analyzing the pattern of response in individual gap trials.
Here we developed a new algorithm for the analysis of individual gap trials, and we applied this algorithm to evaluate response initiation S1, response termination S2, response duration d12, and reward expectation m12 in individual gap trials, to further investigate the role of stimulus modality and gap duration on individual-trial statistics. Moreover, we generated hypothetical distributions for the individual-trial statistics under the PR and RA hypotheses, and we evaluated which hypothesis better fits the observed distributions of the individual-trial statistics, by computing an index of superposition between the observed and hypothetical distributions (an intra-class correlation). Specifically, the Probability-of-Reset (PR) hypothesis assumes that in individual gap trials responses are stochastically controlled by discrete stop or reset modes of the internal clock, such that the average response falls in between stop and reset; however, individual-trial statistics are predicted to be bi-modal and wider in gap trials relative to PI trials. On the other hand, a Resource Allocation (RA) hypothesis assumes that gaps simply “delay” responses due to processes external to the clock; both when averaged over trials and at the individual-trial level, the RA hypothesis predicts that in gap trials distributions are delayed but have same width and characteristics as in PI trials. Therefore, we evaluated whether distributions in gap trials can be generated by delaying the distribution in PI trials (irrespective of their shape, thus preserving their width) as suggested by the RA hypothesis, or whether distributions in gap trials can be generated by mixing stop and reset distributions (thus obtaining bi-modal, wider functions) as suggested by the PR hypothesis.
The pattern of response in the PI-GAP procedure in rats
While in PI trials responses followed a low-high-low pattern as reported previously (Cabeza de Vaca et al., 1994; Cheng & Westwood, 1993; Church et al., 1994; Gibbon & Church, 1990), in gap trials, the pattern was considerably more complex (Figure 2B). This pattern was evaluated by the distributions of S1, S2, d12, m12 and inter-response times. First, analyses of the inter-response times in gap trials (Figure 6), and the pattern of correlations between the S1, S2, m12, and d12 statistics in gap trials (Table 2), indicated that in individual gap trials responses follow a similar pattern as previously reported in individual PI trials (Cheng & Westwood, 1993; Church et al., 1994; Gibbon & Church, 1990). Second, analyses of S1, S2, and m12 statistics by gap duration and stimulus modality (Table 1) indicated that the distributions of these statistics are shifted more after illuminated (Reversed group) than dark gaps (Standard group), and after longer gaps. Third, and most importantly for differentiating discrete modes of the internal clock from a continuum of response alternatives, response duration in individual gap trials d12 was not reliably affected by either gap duration or modality, contrary to the PR hypothesis, and in accord with the RA hypothesis. Fourth, analyses of the shift in gap trials relative to PI trials for these statistics indicated response in between the stop and reset extremes (Figure 7), again in contrast to the PR hypothesis, and in accord with the RA hypothesis. Finally, the start (response initiation) S1 was the most sensitive statistic. In contrast, response termination S2 was less sensitive to manipulations; this may explain previous failure to test the predictions of the PR hypothesis solely based on analyzing S2 (Cabeza de Vaca et al., 1994). In summary, the pattern of response in gap trials was found similar to those in PI trials, but delayed in a continuous, rather than discrete, fashion, as suggested by the RA hypothesis.
Evaluating the PR and RA hypotheses
To evaluate these hypotheses, we generated hypothetical distributions for the S1, S2, and m12 statistics under each hypothesis (Figures 4 and 5), and we contrasted them against the observed distributions using intra-class correlations (ICCs) (Landis & Koch, 1977). Both the PR and RA hypotheses provided good fits for most conditions, although the RA hypothesis provided better fits than the PR hypothesis, with more very good fits (ICCs>.6) for more conditions (8/9) (Figure 8). The RA hypothesis provided better fits for the start and median statistics, but not for the stop statistic, further supporting the general unreliability of using S2 to evaluate individual-trial behavior (Cabeza de Vaca et al., 1994). Interestingly, both hypotheses provided very good fits (over 0.7) at the short and long gaps—possibly because the pattern of response is less variable under these conditions—but only good fits (around 0.5) at the middle gap duration (Figure 9), possibly because the pattern of response is more complex (Figure 2B). Overall, these analyses provided evidence in favor of the RA hypothesis. Together with the analysis of the inter-response times (Figure 6) which suggested similar response patterns in PI and gap trials, and the analysis of the response duration d12 which did not change with gap duration and modality (contrary to PR), these results strongly suggest that in gap trials response distributions are simply shifted relative to PI trials, as suggested by the RA hypothesis. Importantly, the RA provided better than PR despite having the same number of parameters (one): best “stop : reset” mix for PR, and best “delay” for RA.
Implications for Interval Timing Theories
Most interval timing theories address data obtained from averaging responses over many trials, and rarely address what the animal is actually doing in the box in each individual trial, which understandably is very variable and complex. Three notable departures are worth discussing. First, the Scalar Expectancy Theory (SET) (Gibbon, 1977) proposes that timing results from accumulation of pulses emitted by a pacemaker, and as such, addresses only average timing behavior. Gibbon and Church (1990) extended SET to individual-trial behavior by introducing an attentional switch mechanism that selects on a trial-by-trial basis the stop and reset modes of the internal clock (Church et al., 1994; Gibbon et al., 1984). We evaluated this proposal in the form of the stochastic PR hypothesis (Cabeza de Vaca et al., 1994). Second, the Behavioral Timing (BeT) theory (Killeen & Fetterman, 1988) proposes that timing emerges from the progression through behavioral chains. One can apply BeT to the description of average behavior in the gap procedure as follows: Subjects temporarily interrupt the behavioral chain during the gap and resume the chain where they left off (stop alternative), or they restart the chain all over again (reset alternative). This interpretation is compatible with the stochastic PR hypothesis, although evidence for such behavioral chains in timing procedures is scarce (Fetterman, Killeen, & Hall, 1998). Finally, from the handful of theories addressing behavior in real-time (Buhusi & Schmajuk, 1999; Kirkpatrick & Church, 2003; Machado, 1997), only one, the Packet Theory (PT) (Kirkpatrick, 2002; Kirkpatrick & Church, 2003), is particularly concerned with the temporal pattern of response in individual-trials(Figure 2). PT is also the only interval timing theory that recognizes and deals with the considerable variability in interval timing behavior (Church & Guilhardi, 2005; Guilhardi, Keen, MacInnis, & Church, 2005; Kirkpatrick, 2002; Kirkpatrick & Church, 2003; MacInnis, 2007). In a recent example, MacInnis (2007) applied PT to the apparent differences in timing empty and filled events (as in the Standard and Reversed PI-GAP procedure, Figure 1), and found that PT accounts for 60% individual-trial variability, which is very promising, considering the considerable variability in the response pattern in individual-trials (Figure 2B). Interestingly, in our study the best ICCs were in the range of 0.7, further suggesting that there is still significant variability unexplained in interval timing procedures. In summary, both SET, BeT, and PT account for average interval timing behavior by assuming that the only source of behavioral variability is the internal clock, and are amenable to addressing individual-trial behavior when coupled with the PR hypothesis. The present results suggest that a stochastic PR hypothesis (implemented within SET, BeT, or PT) provides a very good absolute fit to the data, but explains significantly less variability than the RA hypothesis, which attributes (some) variability to sources external to the clock. Such external sources of behavioral variability may be found in the form of collateral behaviors, which may be less under the influence of the internal clock, and more under the influence of other cognitive processes.
Indeed, current timing theories are concerned with the internals of the clock, rather than with the way animals integrate time with other cognitive processes. For example, SET, BeT and PT (to name a few) assume that the sole source of variability in timing behavior is the internal clock, and propose various clock mechanisms to address the results. In contrast, theories like the Resource Allocation model, suggest two sources of variability in timing behavior, having to do with separable processes: the clock and the resource allocation mechanism, which is sensitive to non-temporal factors. The current results suggest that while a considerable amount of variability is accounted for by an internal clock with discrete response modes, a significant amount of extra variability can be accounted for by assuming another process external to the clock. This interpretation has an important implication for timing theories, suggesting the need for integration with other cognitive theories. Recent work along this line include integrating timing and associative learning (Buhusi & Schmajuk, 1999), decision making (Harrington et al., 2004), contextual processing (Buhusi & Meck, 2009b), emotional processing (Droit-Volet & Meck, 2007), habit formation (Williamson, Cheng, Etchegaray, & Meck, 2008), and resource allocation (Buhusi, 2003). The present work extends the horizon of timing theories to the individual-trial level, and poses a challenge to current theories, considering that we did not get excellent fits by either RA or PR account.
Caveats
In this report we did not directly evaluate the second prediction of the PR hypothesis, namely bi-modality of the distributions in gap trials, because under close scrutiny it is not as clear-cut as originally thought (Cabeza de Vaca et al. 1994). First, individual-trial distributions in PI trials were not clearly uni-modal for all subjects. For example, m12 seems to be bi-modal in the PI trials in the subject presented in Figure 2A. Nevertheless, for this subject the individual gap trial distributions seem relatively sharp and uni-modal. Such observations suggest that no correlation exists between bi-modality and trial type (PI v. gap). Therefore, rather than focusing on this prediction we evaluated whether the individual-trial distributions superimpose well irrespective of their shape. Because our report is silent with respect to the uni- or bi-modality of the distributions, some level of caution is required in regard to the PR hypothesis.
Conclusions
Together with previous reports investigating the pattern of response in the PI or PI-GAP procedure in rats (Church et al., 1994) and pigeons (Cabeza de Vaca et al., 1994; Cheng & Westwood, 1993; Gibbon & Church, 1990), the present study shows that average timing behavior emerges from complex trial-to-trial behavior, and provides a new window into the effect of interruptions on interval timing. Although generally favorable to the interpretation that gaps prompt rats to use a continuum of response alternatives—as opposed to discrete stop and reset alternatives—present results indicate that the complex pattern of response following the presentation of gaps is still far from being fully understood. For example, neither the continuum nor the discrete alternatives provide excellent fits (ICCs > 0.8) for the distribution of start times at intermediate gap durations.
Nevertheless, the algorithm presented here, and the strategy to use ICCs to evaluate the degree of superposition between response distributions is useful in elucidating behavioral and neural correlates of interval timing. For example, the ICC statistic was recently used to investigate scalar property of timing in house mice in the PI procedure (Buhusi et al., 2009). Similarly, the use of individual-trial statistics has been shown to reveal the intricate mechanisms involved in interval timing, both at the neurophysiological (Drew, Fairhurst, Malapani, Horvitz, & Balsam, 2003; Drew et al., 2007; Gooch, Wiener, Portugal, & Matell, 2007; Matell, Bateson, & Meck, 2006; Wiener, Magaro, & Matell, 2008) and behavioral levels (Buhusi & Meck, 2009b; Cheng & Westwood, 1993; Church et al., 1994; Gooch et al., 2007; Matell & Portugal, 2007). Future studies following behavioral (e.g., Buhusi & Meck, 2000, 2009a, 2009b; Church, 1978; S. Roberts & Church, 1978) and neurobiological (e.g., Buhusi & Meck, 2002; Buhusi & Meck, 2007; Meck, Church, & Olton, 1984) manipulations in the PI-GAP procedure will reveal which theories provide appropriate descriptions of the flexible interval timing behavior not only when averaged over many gap trials, but also at the level of individual gap trials.
Acknowledgments
This work was supported by National Institutes of Health grants R01MH065561 and R01MH073057 to CVB. We would like to thank two anonymous reviewers for helpful comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/xan.
References
- Andresen EM. Criteria for assessing the tools of disability outcomes research. Archives of Physical Medicine and Rehabilitation. 2000;81(12):S15–S20. doi: 10.1053/apmr.2000.20619. [DOI] [PubMed] [Google Scholar]
- Bateson M, Kacelnik A. Risk-sensitive foraging: Decision making in variable environments. In: Dukas R, editor. Cognitive ecology: The evolutionary ecology of information processing and decision making. Chicago University Press; Chicago: 1998. pp. 297–341. [Google Scholar]
- Brodbeck DR, Hampton RR, Cheng K. Timing behaviour of blackcapped chickadees (Parus atricapillus) Behavioural Processes. 1998;44:183–195. doi: 10.1016/s0376-6357(98)00048-5. [DOI] [PubMed] [Google Scholar]
- Buhusi CV. Dopaminergic mechanisms of interval timing and attention. In: Meck WH, editor. Functional and neural mechanisms of interval timing. CRC Press; Boca Raton, FL: 2003. pp. 317–338. [Google Scholar]
- Buhusi CV, Aziz D, Winslow D, Carter RE, Swearingen JE, Buhusi MC. Interval timing accuracy and scalar timing in C57BL/6 mice. Behav Neurosci. 2009;123(5):1102–1113. doi: 10.1037/a0017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Cerutti DT. Concurrent time accumulation and memory decay in pigeons. Behavioural Processes. 2005 submitted. [Google Scholar]
- Buhusi CV, Meck WH. Timing for the absence of a stimulus: the gap paradigm reversed. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26(3):305–322. doi: 10.1037//0097-7403.26.3.305. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behavioral Neuroscience. 2002;116(2):291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Interval timing with gaps and distracters: Evaluation of the ambiguity, switch, and time-sharing hypotheses. Journal of Experimental Psychology: Animal Behavior Processes. 2006a;32(3):329–338. doi: 10.1037/0097-7403.32.3.329. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Time sharing in rats: A peak-interval procedure with gaps and distracters. Behavioural Processes. 2006b;71(2-3):107–115. doi: 10.1016/j.beproc.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Effect of clozapine on interval timing and working memory for time in the peak-interval procedure with gaps. Behavioural Processes. 2007;74(2):159–167. doi: 10.1016/j.beproc.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Relative time sharing: new findings and an extension of the resource allocation model of temporal processing. Philosophical Transactions of Royal Society B. 2009a doi: 10.1098/rstb.2009.0022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Relativity theory and time perception: single or multiple clocks? PLoS One. 2009b;4(7):e6268. doi: 10.1371/journal.pone.0006268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Perera D, Meck WH. Memory for timing visual and auditory signals in albino and pigmented rats. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31(1):18–30. doi: 10.1037/0097-7403.31.1.18. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Sasaki A, Meck WH. Temporal integration as a function of signal and gap intensity in rats (Rattus norvegicus) and pigeons (Columba livia) Journal of Comparative Psychology. 2002;116(4):381–390. doi: 10.1037/0735-7036.116.4.381. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Schmajuk NA. Timing in simple conditioning and occasion setting: a neural network approach. Behavioural Processes. 1999;45(1-3):33–57. doi: 10.1016/s0376-6357(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Brown BL, Hemmes NS. Internal clock and memory processes in animal timing. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:184–198. doi: 10.1037//0097-7403.20.2.184. [DOI] [PubMed] [Google Scholar]
- Catania AC. Reinforcement schedules and psychophysical judgements: A study of some temporal properties of behavior. In: Schoenfeld WN, editor. The theory of reinforcement schedules. Appleton-Century-Crofts; New York: 1970. pp. 1–42. [Google Scholar]
- Cheng K, Westwood R. Analysis of single trials in pigeons’ timing performance. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:56–67. [Google Scholar]
- Church RM. The internal clock. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Erlbaum; Hillsdale, NJ: 1978. pp. 277–310. [Google Scholar]
- Church RM, Guilhardi P. A Turing test of a timing theory. Behav Processes. 2005;69(1):45–58. doi: 10.1016/j.beproc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Church RM, Meck WH, Gibbon J. Application of scalar timing theory to individual trials. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20(2):135–155. doi: 10.1037//0097-7403.20.2.135. [DOI] [PubMed] [Google Scholar]
- Drew MR, Fairhurst S, Malapani C, Horvitz JC, Balsam PD. Effects of dopamine antagonists on the timing of two intervals. Pharmacol Biochem Behav. 2003;75(1):9–15. doi: 10.1016/s0091-3057(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27(29):7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droit-Volet S, Meck WH. How emotions colour our perception of time. Trends Cogn Sci. 2007;11(12):504–513. doi: 10.1016/j.tics.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Fetterman GJ, Killeen PR, Hall S. Watching the clock. Behavioural Processes. 1998;44(2):211–224. doi: 10.1016/S0376-6357(98)00050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisse P. Psychologie du temps. P.U.F.; Paris, France: 1957. [Google Scholar]
- Francois M. Contributions a l’etude du sens du temps: La temperature interne comme facteur de varuation de l’appreciation subjective des durees. Annee Psychologique. 1927;27:186–204. [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychological Review. 1977;84(3):279–325. [Google Scholar]
- Gibbon J, Church RM. Representation of time. Cognition. 1990;37(1-2):23–54. doi: 10.1016/0010-0277(90)90017-e. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Gooch CM, Wiener M, Portugal GS, Matell MS. Evidence for separate neural mechanisms for the timing of discrete and sustained responses. Brain Res. 2007;1156:139–151. doi: 10.1016/j.brainres.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Guilhardi P, Keen R, MacInnis ML, Church RM. How rats combine temporal cues. Behav Processes. 2005;69(2):189–205. doi: 10.1016/j.beproc.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, et al. Neural representation of interval encoding and decision making. Brain Res Cogn Brain Res. 2004;21(2):193–205. doi: 10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Hoagland H. The psychological control of judgements of duration: Evidence for a chemical clock. Journal of General Psychology. 1933;9:267–287. [Google Scholar]
- Killeen PR, Fetterman JG. A behavioral theory of timing. Psychological Review. 1988;95(2):274–295. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K. Packet theory of conditioning and timing. Behav Processes. 2002;57(2-3):89–106. doi: 10.1016/s0376-6357(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Church RM. Tracking of the expected time to reinforcement in temporal conditioning procedures. Learn Behav. 2003;31(1):3–21. doi: 10.3758/bf03195967. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- Machado A. Learning the temporal dynamics of behavior. Psychological Review. 1997;104(2):241–265. doi: 10.1037/0033-295x.104.2.241. [DOI] [PubMed] [Google Scholar]
- MacInnis MLM. Do rats time filled and empty intervals of equal duration differently? Behavioural Processes. 2007;75(2):182–187. doi: 10.1016/j.beproc.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Matell MS, Bateson M, Meck WH. Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology. 2006;188(2):201–212. doi: 10.1007/s00213-006-0489-x. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Reinforcement-induced within trial resetting of an internal clock. Behavioral Processes. 1999;45:157–171. doi: 10.1016/s0376-6357(99)00016-9. [DOI] [PubMed] [Google Scholar]
- Matell MS, Portugal GS. Impulsive responding on the peak-interval procedure. Behav Processes. 2007;74(2):198–208. doi: 10.1016/j.beproc.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behavioral Neuroscience. 1984;98(1):3–22. doi: 10.1037//0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- Roberts S. Isolation of an internal clock. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:242–268. [PubMed] [Google Scholar]
- Roberts S, Church RM. Control of an internal clock. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:318–337. [Google Scholar]
- Roberts WA, Cheng K, Cohen JS. Timing light and tone signals in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:23–35. [PubMed] [Google Scholar]
- Thorpe CM, Petrovic V, Wilkie DM. How rats process spatiotemporal information in the face of distraction. Behavioral Processes. 2002;58:79–90. doi: 10.1016/s0376-6357(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Treisman M. Temporal discrimination and the indifference interval. Implications for a model of the “internal clock”. Psychological Monograph. 1963;77(13):1–31. doi: 10.1037/h0093864. [DOI] [PubMed] [Google Scholar]
- Wiener M, Magaro CM, Matell MS. Accurate timing but increased impulsivity following excitotoxic lesions of the subthalamic nucleus. Neurosci Lett. 2008;440(2):176–180. doi: 10.1016/j.neulet.2008.05.071. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Cheng RK, Etchegaray M, Meck WH. “Speed” warps time: methamphetamine’s interactive roles in drug abuse, habit formation, and the biological clocks of circadian and interval timing. Curr Drug Abuse Rev. 2008;1(2):203–212. doi: 10.2174/1874473710801020203. [DOI] [PubMed] [Google Scholar]
- Woodrow H. The reproduction of temporal intervals. Journal of Experimental Psychology. 1930;13:473–499. [Google Scholar]