Abstract
Background and Aims
Matrix metalloproteinase-2 (MMP-2), a type IV collagenase secreted by activated hepatic stellate cells (HSCs), is upregulated in chronic liver disease and is considered a profibrotic mediator due to its proliferative effect on cultured HSCs and ability to degrade normal liver matrix. Although associative studies and cell culture findings suggest that MMP-2 promotes hepatic fibrogenesis, no in vivo model has definitively established a pathologic role for MMP-2 in the development and progression of liver fibrosis. We therefore examined the impact of MMP-2 deficiency on liver fibrosis development during chronic CCl4 liver injury and explored the effect of MMP-2 deficiency and overexpression on collagen I expression.
Methods
Following chronic CCl4 administration, liver fibrosis was analyzed using Sirius Red staining with quantitative morphometry and real-time polymerase chain reaction (PCR) in MMP-2−/− mice and age-matched MMP-2+/+ controls. These studies were complemented by analyses of cultured human stellate cells.
Results
MMP-2−/− mice demonstrated an almost twofold increase in fibrosis which was not secondary to significant differences in hepatocellular injury, HSC activation or type I collagenase activity; however, type I collagen messenger RNA (mRNA) expression was increased threefold in the MMP-2−/− group by real-time PCR. Furthermore, targeted reduction of MMP-2 in cultured HSCs using RNA interference significantly increased collagen I mRNA and protein, while overexpression of MMP-2 resulted in decreased collagen I mRNA.
Conclusions
These findings suggest that increased MMP-2 during the progression of liver fibrosis may be an important mechanism for inhibiting type I collagen synthesis by activated HSCs, thereby providing a protective rather than pathologic role.
Keywords: Cirrhosis, Fibrogenesis, Type I collagen, Hepatic stellate cell, Matrix metalloproteinase-2
Introduction
Hepatic fibrosis, the predominant pathologic feature of end-stage liver disease, is a deleterious wound healing response characterized by progressive replacement of normal, type IV collagen-rich extracellular matrix (ECM) with interstitial “scar” matrix composed primarily of type I collagen. A central mediator of fibrosis is the hepatic stellate cell (HSC), a pericyte that resides in the perisinusoidal space and stores vitamin A in its quiescent state [1]. During liver injury, the activated HSC adopts a myofibroblastic phenotype and is capable of vigorous proliferation, matrix protein synthesis, and scar formation. In addition, the activated HSC produces several matrix metalloproteinases which can degrade the extracellular matrix. Thus, stellate cells may play a major role in tissue remodeling and repair after both acute and chronic injury [1].
Matrix metalloproteinase-2 (MMP-2), also known as gelatinase A, is a 72-kDa zinc-dependent type IV collagenase that is secreted as a proenzyme by multiple cell types, including activated HSCs [2], and can thereby modify the extracellular environment [3]. MMP-2 expression and activity are increased in rodent models of toxin-induced chronic liver injury [4]. In addition, MMP-2 mRNA levels are increased three- to fourfold in human fibrotic liver samples and collocalizes with activated HSCs [5]. MMP-2 upregulation in chronic liver disease has traditionally been considered profibrotic, in part by hastening the replacement of type IV collagen with type I collagen [6]. In cultured HSCs, MMP-2 may act as an autocrine proliferative signal, thereby contributing to the fibrotic response by increasing the numbers of activated HSCs after liver injury [7].
Although these associative studies and cell culture findings suggest that MMP-2 promotes hepatic fibrogenesis, no in vivo model has definitively established a pathologic role for MMP-2 in the development and/or progression of liver fibrosis. Moreover, some evidence suggests that MMP-2 may be antifibrotic in liver disease. In a rodent model of toxin-induced chronic liver injury, MMP-2 levels are increased during spontaneous recovery from micronodular cirrhosis [8], while levels of MMP-13, the classical rodent type I collagenase, are unchanged. Since this resolution of liver fibrosis requires a net increase in type I collagenase activity [9] and MMP-2 is capable of cleaving type I collagen in vitro [1], MMP-2 may function as an interstitial collagenase in this setting, although it is less potent than matrix metalloproteinase-1. Furthermore, HSC apoptosis, another potential mechanism for resolution of liver fibrosis, is associated with MMP-2 activation [10], suggesting that MMP-2 may play a role in limiting HSC activity after liver injury and thereby inhibit hepatic fibrosis.
No studies have examined the effect of MMP-2 on the development of hepatic fibrosis in an in vivo model of chronic liver injury. Given the importance of identifying therapeutic targets for the development of effective antifibrotic strategies, and to clarify the role of MMP-2 in chronic liver injury, we examined the impact of genetic MMP-2 deficiency in a model of CCl4-induced liver injury.
Materials and Methods
Animals
The MMP-2−/− mice (C57BL/6 background; 12–16 weeks of age) used in these studies were originally generated and described by Ito et al. [11]. Gelatin zymography revealed a complete lack of baseline MMP-2 activity in the MMP-2−/− mice in all tissues tested, including liver. MMP-2−/− mice develop normally and are fertile but show a slower growth rate and are smaller as compared with wild type. A more recent report suggests that MMP-2−/− mice have a bone phenotype characterized by progressive loss of bone mineral density, articular cartilage destruction, and abnormal long bone and craniofacial development [12]. MMP-2−/− and MMP-2+/+ mice used in this study were derived from MMP-2+/− crossings. Mice were used with the approval of the Institutional Animal Care and Use Committee (IACUC) and received care according to National Institutes of Health (NIH) guidelines.
Toxin-Induced Models of Acute and Chronic Liver Injury and Fibrosis
Carbon tetrachloride (CCl4, Sigma) was used to induce hepatic fibrosis. For acute injury studies, MMP-2+/+ and MMP-2−/− mice (n = 8 per group) received one intra-peritoneal (IP) injection of 50% CCl4 (diluted in corn oil) at a dose of 2 µl/g body weight. Under ketamine/xylazine anesthesia, animals were sacrificed 48 h later, and serum was collected and analyzed for biochemistries. In the chronic injury model, MMP-2+/+ and MMP-2−/− mice (n = 4 per group) received IP injections of 10% CCl4 (diluted in corn oil) at a dose of 5 µl/g body weight twice per week for 6 weeks. Two days after the final dose of CCl4, animals were sacrificed under ketamine/xylazine anesthesia. Given the presence of a bone phenotype in MMP-2−/− mice [12], baseline fibrosis was compared between MMP-2+/+ and MMP-2−/− (n = 4 per group) and increases in fibrosis with toxin-induced injury compared with the respective baseline/untreated cohort. Serum was collected and analyzed for biochemistries. Livers were harvested and processed for RNA, protein, and histology.
Histologic Assessment and Quantification of Hepatic Fibrosis
At time of sacrifice, the posterior one-third of the liver was harvested and fixed in 10% formalin for 24 h and embedded in paraffin. Five-micron sections were stained for collagen with Sirius Red (0.1% solution, diluted in picric acid, both from Sigma). Relative fibrosis area was assessed based on 36 fields from four Sirius-Red-stained liver sections per animal in a blinded fashion. As previously described [13], each field was acquired at 40× magnification and analyzed using a computerized Bioquant® morphometry system. Overall fibrosis was assessed by intensity of Sirius Red staining divided by total field area, multiplied by 100. Fold change was calculated to demonstrate increases in fibrosis from baseline control animals and to compare differences in Sirius Red staining between MMP-2−/− and MMP-2+/+ after chronic CCl4.
Immunoblots
Immunoblot analysis was performed as previously described [14] using whole liver extracts from untreated control (0 h) and fibrotic livers from MMP-2+/+ and MMP-2−/− mice. Protein samples (100 µg/sample) were separated in a sodium dodecyl sulfate (SDS)–polyacrylamide gel, transferred to a nitrocellulose membrane (Bio-Rad), and probed for latent and active MMP-9 (Chemicon; 1:1,000 dilution), α-smooth muscle actin (α-SMA) (Sigma; 1:1,000 dilution), membrane type 1-matrix metalloprotease (MT1-MMP) (Chemicon; 1:1,000 dilution), tissue inhibitor of metalloproteinases (TIMP)-1 (Chemicon; 1:1,000 dilution), TIMP-2 (Chemicon; 1:1,000 dilution), collagen I (Rockland; 1:1,000 dilution), and β-actin (Sigma; 1:1,000 dilution) as a loading control. Proteins were detected by chemiluminescence (Amersham Biosciences) and results were quantified by scanning densitometry.
Cell Culture and Transfection
LX2 cells, a human stellate cell line resembling an activated HSC phenotype [15], and passage 3 primary human hepatic stellate cells were used for all cell culture experiments. Primary stellate cells were isolated from wedge sections of normal human liver in patients undergoing hepatic resection for primary benign tumors or single metastasis from colon cancer as described previously [16]. The liver was washed and portal venules cannulated for in situ digestion with pronase and collagenase. Hepatic stellate cells were isolated by density centrifugation and plated on plastic. For MMP-2 overexpression, 70% confluent LX2 cells were washed twice with phosphate-buffered saline (PBS) and serum-starved for 24 h prior to transfection with full-length MMP-2 complementary DNA (cDNA) (WT-MMP-2), amplified from normal human fibroblasts, and cloned into a pcDNA3.1/V5-His-TOPO expression vector (Invitrogen), at concentration of 7.5 mg DNA per 10 cm dish using Lipofectamine 2000 (Invitrogen). Transfection of empty pcDNA3.1/V5-His-TOPO expression vector served as a control. RNA was extracted 24 h post transfection and used for semiquantitative real-time PCR analysis. For MMP-2 gene silencing, 70% confluent cells (LX2 cells and primary HSCs) were washed twice with PBS and serum-starved for 24 h prior to transfection with a pool of four small interfering RNAs (siRNAs) targeted against MMP-2 (siMMP-2; Dharmacon, catalog #M-005959-02) at final concentration of 100 nm using Lipofectamine 2000 (Invitrogen). Transfection of siControl nontargeting siRNA (Dharmacon, catalog #D-001206-13-20) was used as a control to account for any off-target effects of introducing siRNA into this cell line. In the LX2 cell line experiments, RNA was extracted 12, 24, 36, 48, 72, and 96 h after transfection and used for real-time PCR analysis. In the primary HSC experiments, RNA was extracted 12, 24, and 36 h after transfection and used for real-time PCR analysis. In addition, primary HSC culture supernatant was collected to measure secreted collagen I protein by immunoblot analysis. Briefly, protein was precipitated from supernatant by adding 8 ml acetone to 1 ml extracellular medium. Samples were kept at −20°C overnight and centrifuged at 10,000 rpm for 20 min at 4°C. Supernatant was then aspirated and the pellet was allowed to dry. Pellet was then resuspended in 250 ml radio-immuno precipitation (RIPA) buffer and stored at −20°C until immunoblot analysis was performed.
Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNA was extracted using the RNAeasy Mini Kit (Qiagen) from samples treated with RNAlater RNA stabilization reagent (Qiagen) and promptly stored at −70°C. RNA concentration and purity were measured by ultraviolet (UV) absorbance. RNA (1 µg) was reverse-transcribed using first-strand complementary DNA synthesis with random primers (AMV Reverse Transcriptase, Promega). Reverse-transcriptase products were diluted 20-fold in nuclease-free H2O, and 5 µl of each diluted sample was loaded into a 384-well plate for real-time PCR analysis using an ABI PRISM 7900 HT sequence detection system (Applied Biosystems). Amplification reactions including platinum Taq polymerase buffer and polymerase (Invitrogen), SYBR Green fluorescent DNA-binding dye, and forward and reverse oligonucleotide primers for each target gene or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene (Table 1) were performed as per the manufacturer’s recommendation. All samples were analyzed in triplicate and expression normalized to GAPDH. Fluorescence signals were analyzed during each of 40 cycles (denaturation, 15 s at 95°C; annealing, 15 s at 56°C; and extension, 40 s at 72°C). Denaturation curves of target genes and GAPDH confirmed the fidelity of the PCR amplification and the absence of primer dimers. Relative quantification was calculated using the comparative threshold cycle (CT) method, as described in User Bulletin #2, ABI PRISM 7700 Sequence Detection System. CT indicates the fractional cycle number at which the amount of amplified target genes reaches a fixed threshold within the linear phase of gene amplification and is inversely related to the abundance of mRNA transcripts in the initial sample. Median CT of triplicate measurements (or mean CT of duplicate measurements if outlier value greater than 0.3 cycles difference) was used to calculate ΔCT as the difference in CT for target gene and reference (GAPDH). ΔCT values for each sample were compared with the corresponding mean control ΔCT and expressed as ΔΔCT. Relative quantification was expressed as fold change of the gene of interest compared with the control according to the formula . Target gene expression between experimental and control groups were then compared using mean fold changes. ΔCT values were used to determine statistical significance in the animal models. Fold change values were used to determine statistical significance in the cell culture systems.
Table 1.
Real-time PCR primer sequences
| Gene | Forward sequence (5′-3′) | Reverse sequence (5′-3′) |
|---|---|---|
| Mouse MMP-2 | ACC CAG ATG TGG CCA ACT AC | TAC TTT TAA GGC CCG AGC AA |
| Mouse α-SMA | TCC TCC CTG GAG AAG AGC TAC | TAT AGG TGG TTT CGT GGA TGC |
| Mouse TGFβ-1 | TGC GCT TGC AGA GAT TAA AA | CTG CCG TAC AAC TCC AGT GA |
| Mouse α1(I) collagen | GTC CCT GAA GTC AGC TGC ATA | TGG GAC AGT CCA GTT CTT CAT |
| Mouse TIMP-1 | ACGAGACCACCTTATACCAGCG | GCGGTTCTGGCACTTGTGGGC |
| Mouse TIMP-2 | GCCAAAGCAGTGAGCGAGAAG | CACACTGCTGAAGAGGGGGC |
| Mouse MTI-MMP | GGC CTG GAA CAT TCT AAC GA | CTT TGT GGG TGA CCC TGA CT |
| Mouse MMP-13 | CCT GGA CCA AAC TAT GGT GGG | AAG CTC ATG GGC AGC AAC AA |
| Mouse TGFβ-1 receptor | CCA CTT GCG ACA ACC AGA AGT C | GTC GTT CTT CCT CCA CAC GG |
| Mouse PDGF-β receptor | CTT TGT GCC AGA TCC CAC CA | TCA CTC GGC ACG GAA TTG TC |
| Human MMP-2 | CAA CCC AGA TGT GGC CAA CT | GGT CCA GAT CAG GTG TGT AGC C |
| Human α1(I) collagen | GGC TTC CCT GGT CTT CCT GG | CCA GGG GGT CCA GCC AAT |
| Mouse/human GAPDH | CAA TGA CCC CTT CAT TGA CC | GAT CTC GCT CCT GGA AGA TG |
TGF-β1 transforming growth factor-beta 1
Gelatin Zymography
As described previously [17], protein samples (50 µg/ sample) from whole liver extracts (derived from fibrotic livers of MMP-2+/+ and MMP-2−/− mice) were separated in a 10% polyacrylamide gel containing 1 mg/ml bovine skin gelatin (Sigma). Gels were washed twice for 30 min in 2.5% Triton X-100, then once for 10 min in 0.1 M Tris (pH 7.4). Gels were then incubated for 18 h at 37°C in a solution of 0.1 M Tris (pH 7.4), 10 mM CaCl2, and 5 mM ZnCl2. Staining with 0.5% Coomassie Blue followed by subsequent destaining was then performed as described previously [18].
Type I Collagenase Activity Assay
Type I collagenase activity was assayed in whole liver extract protein samples (5 µg/sample and 10 µg/sample) using the Type I Collagenase Activity Assay Kit (Chemicon). A standard curve was generated using purified MMP-1 provided by the manufacturer. Protein samples were mixed with biotinylated bovine collagen and incubated at 37°C for 2 h. The resultant biotinylated fragments were transferred into biotin-binding wells and detected using a streptavidin-enzyme complex. The addition of substrate solution results in colored products. A microplate reader was used to measure optical density at wavelength of 450 nm. p-Aminophenylmercuric acetate (APMA)-activated MMP-1 was used as a positive control. References and samples were not activated prior to incubation. Measurements were performed in triplicate.
Statistics
Comparisons between groups were performed using an independent Student’s t-test. Statistics were performed using the SPSS statistical software package. All values are reported as mean ± standard error of the mean (SEM). Levene’s test was used to determine equality of variances. P-values < 0.05 were considered to be statistically significant.
Results
Liver Fibrosis is Significantly Increased in MMP-−2/−Mice Following Chronic CCl4 Administration
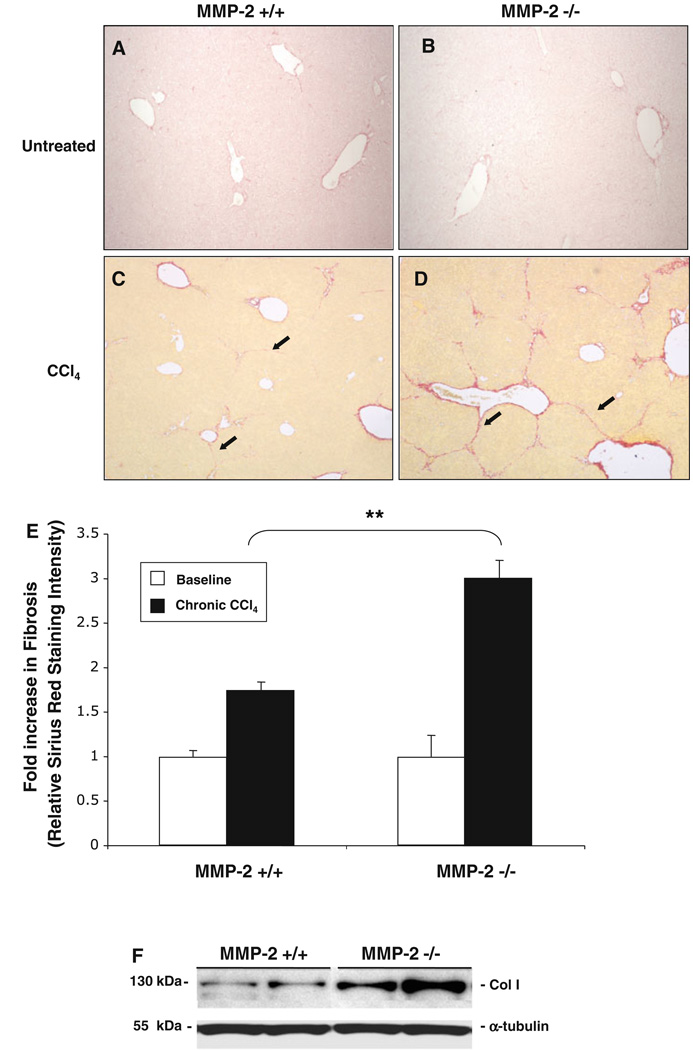
In order to evaluate MMP-2’s role in fibrosis following chronic liver injury, we compared the fibrotic response of 12–16-week-old MMP-2−/− mice against age-matched MMP-2+/+ mice after chronic exposure to CCl4. There was no difference in baseline fibrosis between MMP-2−/− and MMP-2+/+ mouse livers (12–16 weeks; n = 4 in each group) based on Sirius Red staining (Fig. 1a, b, e). In response to CCl4-induced chronic liver injury, MMP-2+/+ mice demonstrated a 1.7-fold increase in liver fibrosis while MMP-2−/− mice demonstrated a 3-fold increase in liver fibrosis from their respective baseline (Fig. 1e). Overall, MMP-2−/− mice demonstrated an almost twofold increase in liver fibrosis compared with MMP-2+/+ mice (P < 0.001). Since baseline Sirius Red staining in the MMP-2−/− mice was not significantly increased as compared with MMP-2+/+ animals, the increased liver fibrosis in the MMP-2-deficient mice represents increased net collagen deposition in response to progressive chronic injury. Representative Western blot confirms increased collagen I protein in MMP-2−/− fibrotic livers (Fig. 1f).
Fig. 1.
MMP-2−/− mice develop increased hepatic fibrosis in response to chronic liver injury. MMP-2+/+ and MMP-2−/− livers were harvested and sectioned, and degree of fibrosis assessed with Sirius Red staining at baseline and following chronic CCl4 administration. Percentage overall fibrosis was subsequently calculated via histomorphometric bioquant analysis of 36 images per animal (n = 4) in a blinded fashion. Representative baseline Sirius Red staining on MMP-2+/+ (a) and MMP-2−/− (b) livers and examples of bridging fibrosis after chronic CCl4 in MMP-2+/+ (c) and MMP-2−/− (d) mouse livers shown. Results of histomorphometric bioquant demonstrates a 1.7-and 3-fold increase in fibrosis in MMP-2+/+ and MMP-2−/− livers from untreated baseline, respectively. An almost twofold increase in fibrosis was observed in the MMP-2−/− livers as compared with the MMP-2+/+ livers after chronic CCl4 administration (e). Representative immunoblot using whole liver protein extracted from MMP-2+/+ and MMP-2−/− mice after chronic CCl4 administration confirms increase in collagen I expression (f). Original magnification 40×. Arrows point to areas of bridging fibrosis. Data represent means ± SEM; ** P < 0.001
Loss of MMP-2 Does Not Significantly Affect Degree of Hepatocellular Damage Following CCl4 Administration
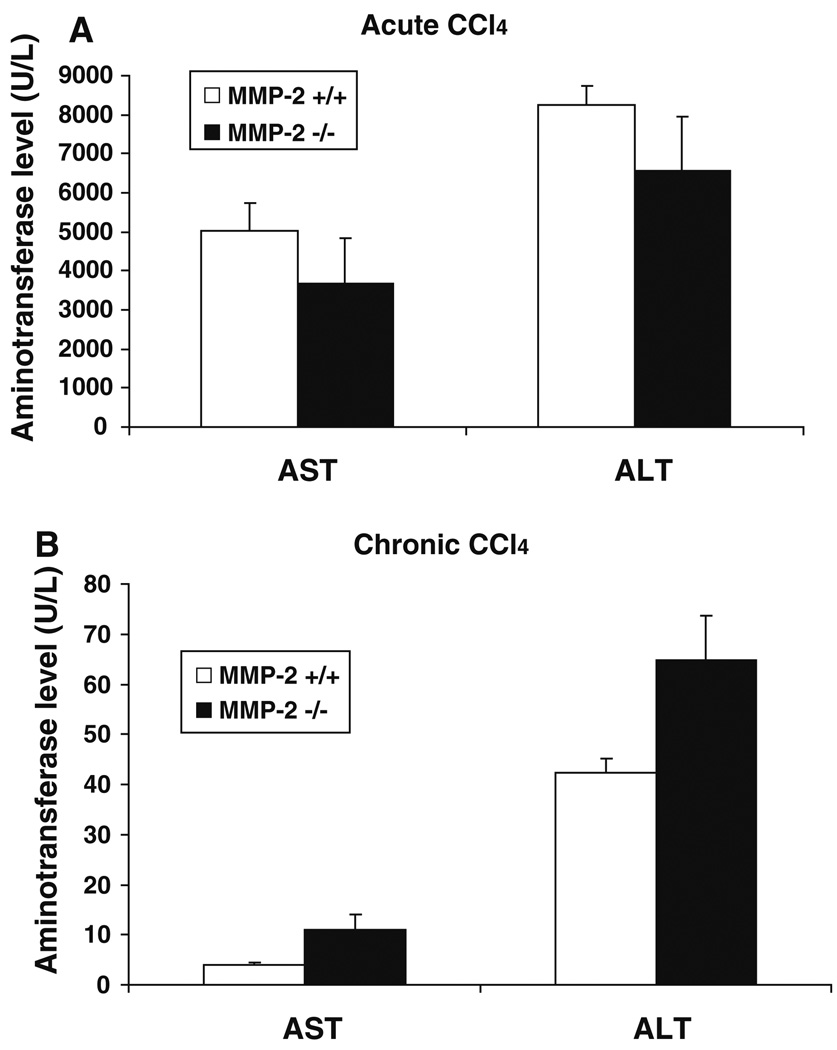
Liver fibrosis occurs in response to hepatocellular damage after injury; therefore, based on an earlier study in which MMP-2 inhibition decreased hepatocellular apoptosis and necrosis in a murine model of tumor necrosis factor-alpha (TNF-α)/d-galactosamine (Galn)-induced acute fulminant hepatitis, one might expect MMP-2-deficient mice to develop less fibrosis after chronic injury due to an overall decreased level of hepatocellular damage [19]. However, in our model of chronic liver injury, MMP-2-deficient mice developed increased fibrosis after CCl4 administration, suggesting that they may in fact have sustained a greater degree of hepatocellular injury. To distinguish between any effect of MMP-2 deficiency on hepatocellular injury versus a direct effect on hepatic fibrosis, serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured in both a chronic CCl4 injury model as well as an acute CCl4 injury model. As shown in Fig. 2, there were no significant differences in serum AST and ALT levels after either acute (AST: 5,032 U/L ± 714 versus 3,669 U/L ± 1,167, P < 0.35; ALT: 8,257 U/L ± 492 versus 6,596 U/L ± 1,393, P < 0.30) or chronic liver injury (AST: 4 U/L ± 0.4 versus 11 U/L ± 2.94, P < 0.07; ALT: 42.25 U/L ± 2.87 versus 64.75 U/L ± 8.82, P < 0.06) between the MMP-2+/+ and MMP-2−/− mice, respectively. While a trend towards increased AST/ALT was noted in the MMP-2−/− mice after chronic CCl4, no difference in AST/ALT was noted after acute injury. Taken together, these findings suggest that the increased fibrosis observed in the MMP-2-deficient mice is largely independent of an effect on hepatocellular damage.
Fig. 2.
MMP-2−/− mice do not sustain significantly increased hepatocellular damage after liver injury. Serum levels of markers of hepatocellular injury, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), measured in MMP-2+/+ and MMP-2−/− mice 48 h after one dose of CCl4 (a) (n = 8 per group) and 48 h after last dose of CCl4 in chronic CCl4 administration (b) (n = 4 per group). No statistically significant difference in AST or ALT was noted after either acute or chronic CCl4 injury, though an increasing trend was observed in the chronic injury model. Data represent means ± SEM
Type I Collagenase Activity in MMP-2−/− Mice and MMP-2+/+ Mice is Similar Following Chronic CCl4 Administration
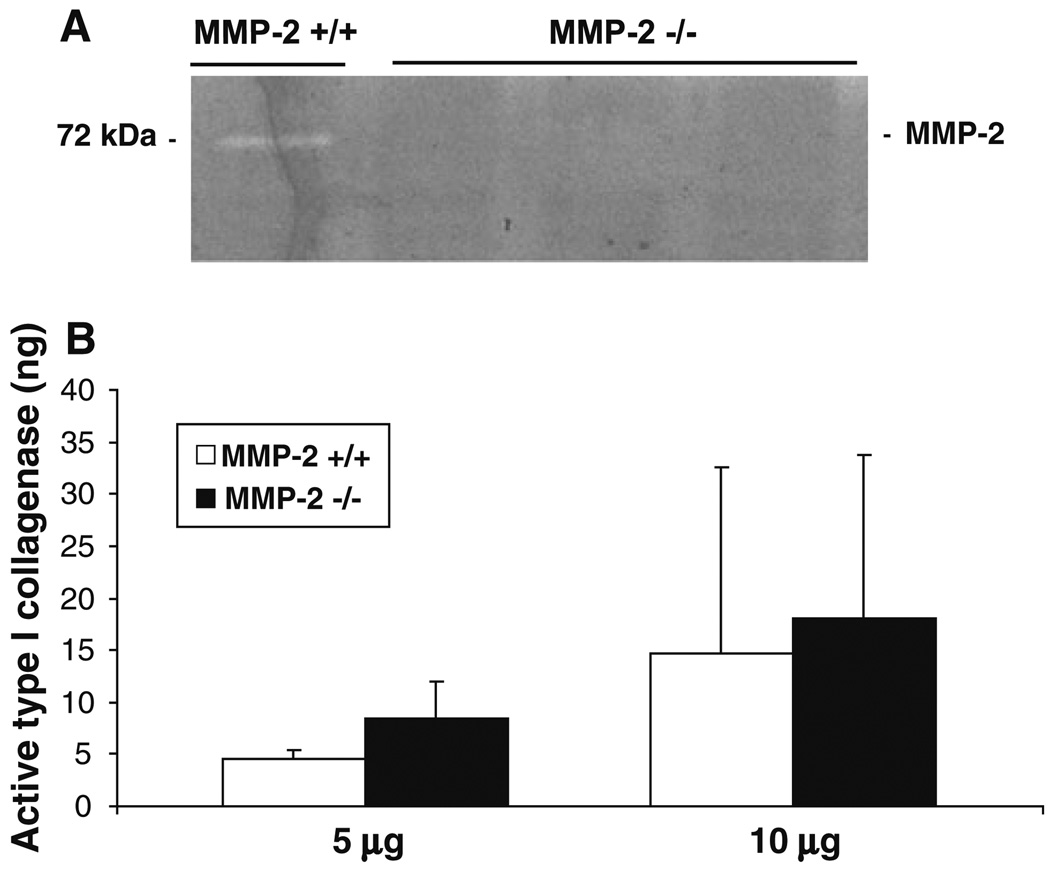
To confirm loss of MMP-2 activity in MMP-2−/− mice in the setting of toxin-induced liver injury, gelatin zymography was performed using whole liver extracts from both MMP-2−/− and MMP-2+/+ mice after chronic CCl4 administration (6 weeks after CCl4). As expected, no MMP-2 activity was observed in MMP-2−/− livers but was present in MMP-2+/+ livers (Fig. 3a). Since the observed increased collagen content in MMP-2−/− livers may be due to increased synthesis or decreased degradation, we explored the possibility that MMP-2, which demonstrates type I collagenase in vitro [1], acts as an interstitial collagenase after chronic liver injury; thus, in MMP-2’s absence, type I collagen degradation might be attenuated. Using a commercial type I collagenase activity assay, we measured collagenase activity in both 5 and 10 µg protein samples from MMP-2+/+ and MMP-2−/− whole liver extracts after 6 weeks of CCl4 administration (Fig. 3b). In contrast to the expected finding, type I collagenase activity was similar in MMP-2−/− and MMP-2+/+ mouse livers after chronic liver injury (5 µg samples: 4.50 ± 0.84 ng versus 8.46 ± 3.50 ng, P < 0.16; 10 µg samples: 14.69 ± 17.95 ng versus 18.02 ± 15.68 ng, P < 0.86). These data indicate that MMP-2 is unlikely to be a vital interstitial collagenase during progression of CCl4-induced liver fibrosis but does not rule out its potential role in fibrosis resolution.
Fig. 3.
MMP-2−/− mice do not have decreased levels of active type I collagenase after chronic liver injury. Gelatin zymography performed on whole liver extracts from MMP-2+/+ and MMP-2−/− mice after 6 weeks of CCl4 administration confirmed lack of MMP-2 activity in MMP-2−/− livers but presence in MMP-2+/+ livers (a). Active type I collagenase was measured using 5 and 10 µg samples of whole liver protein extract from MMP-2+/+ and MMP-2−/− mice after 6 weeks of CCl4 administration (n = 4) by mixing samples with biotinylated bovine collagen. The resultant biotinylated fragments were then transferred into biotin-binding wells and detected using a streptavidin-enzyme complex and a microplate reader. Purified MMP-1 was used to generate standard curves. MMP-2−/− mice did not have significantly decreased amounts of hepatic type I collagenase activity as compared with MMP-2+/+ mice (b). Analysis performed in triplicate for each sample. Data represent means ± SEM
Increased Fibrosis in MMP-2−/− Livers is Not Due to Differences in Stellate Cell Activation
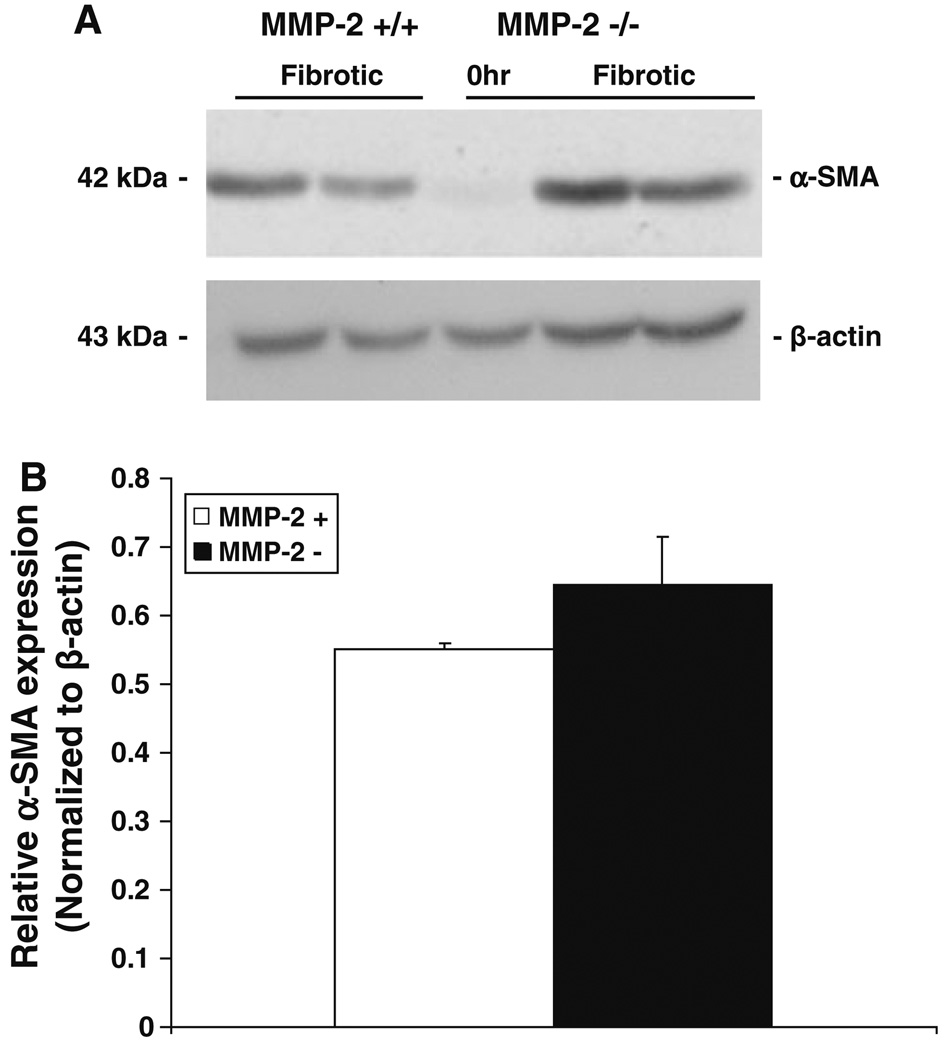
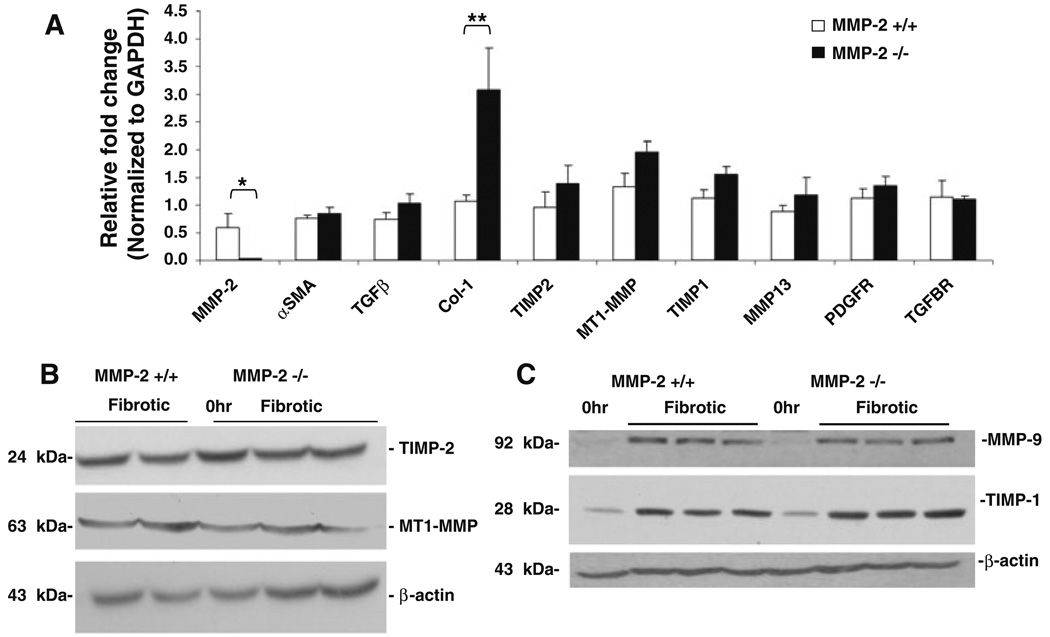
Hepatic stellate cell activation is viewed as a two-stage process consisting of initiation and perpetuation [20]. Initiation of HSC activation is mediated by a variety of paracrine stimuli, including reactive oxygen intermediates [21] and transforming growth factor-beta 1 (TGF-β1) [22, 23], a well-known profibrotic cytokine released by damaged hepatocytes, endothelial cells, Kupffer cells, and platelets [20]. Previous in vitro studies have demonstrated increased MMP-2 activity after HSC exposure to TGF-β1 [24] and decreased HSC proliferation in the presence of MMP-2 inhibition [7]. These data have suggested a role for MMP-2 in the initiation and/or perpetuation of HSC activation. To determine the effect of in vivo loss of MMP-2 on HSC activation in chronic liver injury, we examined the expression of α-smooth muscle actin (α-SMA) expression, a marker of activated stellate cells, by both immunoblot and real-time PCR analysis (Figs. 4, 5a, respectively) in MMP-2−/− livers compared with wild-type controls. No significant differences in α-SMA expression were observed between the two groups, suggesting that increased fibrosis in MMP-2−/− mice is not secondary to increased HSC activation. In addition, α-SMA staining and quantification of number of activated HSC/per 20 high-power fields (HPFs) performed on both MMP-2−/− and MMP-2+/+ fibrotic livers confirmed no significant difference in the number of activated HSCs between the two groups (data not shown).
Fig. 4.
α-Smooth muscle actin (α-SMA) expression is not decreased in MMP-2−/− mouse livers after chronic liver injury. Immunoblot analysis for α-SMA performed on protein (100 µg) extracted from MMP-2−/− and MMP-2+/+ livers following chronic CCl4. β-Actin used to normalize protein loading (a). Scanning densitometry comparing relative α-SMA expression, normalized to β-actin, in whole liver extract from MMP-2+/+ and MMP-2−/− mice (b) demonstrated no decrease in MMP-2−/− mice as compared with wild type (n = 3). Data represent means ± SEM
Fig. 5.
Increased levels of α1(I) collagen mRNA in MMP-2−/− mice after chronic liver injury. Following chronic CCl4 administration, total RNA extracted from MMP-2+/+ and MMP-2−/− mouse livers (n = 4) was reverse-transcribed and real-time PCR analysis was performed to compare expression of “profibrotic genes” as well as genes relevant to MMP-2 activity and function (a). Of all the genes examined only α1(I) collagen mRNA expression was significantly increased (approximately threefold) in the MMP-2−/− group as compared with wild-type control. Results normalized to GAPDH. Data represent means ± SEM; * P < 0.05, ** P < 0.02. b–c Expression of TIMP-1, TIMP-2, MT1-MMP, and MMP-9 examined by immunoblot using whole liver protein extract collected from MMP-2+/+ and MMP-2−/− mice after chronic CCl4 administration. No significant difference in protein expression of molecular regulators of MMP-2 activity (b), MMP-9, a type IV collagenase with substrate specificity similar to MMP-2, or TIMP-1, tissue inhibitor of type I collagenase activity (c) was noted. Expression normalized to β-actin. Representative immunoblots shown
α1(I) Collagen mRNA Expression is Significantly Upregulated in MMP-2−/− Mice Following Chronic CCl4 Administration
Because neither differences in hepatocyte injury, type I collagenase activity nor HSC activation entirely accounted for the increased collagen deposition observed in the MMP-2−/− mice with chronic CCl4-induced liver, we examined the expression of a panel of “profibrotic genes” as well as those relevant to MMP-2 activity and function. Genes examined included: (1) transforming growth factor β1 (TGF-β1) and TGF-β1 receptor, as TGF-β1 is considered the most potent profibrogenic cytokine in chronic liver disease; (2) platelet-derived growth factor receptor (PDGFR), a marker of HSC proliferation; (3) TIMP-2 and MT1-MMP, molecular regulators of MMP-2 activity; (4) the alpha-1 (α1) chain of type I collagen, the predominant ECM component in the cirrhotic liver; and (5) TIMP-1, MT1-MMP, and MMP-13, known regulators of type I collagenase activity. As shown in Fig. 5a, expression of α1(I) collagen mRNA was significantly upregulated in the MMP-2−/− mouse livers compared with MMP-2+/+ livers (fold change: 3.07 ± 0.766 versus 1.07 ± 0.110, P < 0.02). Lack of any significant differences in the expression of several proteins regulating MMP-2 activation and inhibition (e.g., TIMP-1, TIMP-2, and MT1-MMP) was confirmed by immunoblot (Fig. 5b). Of note, matrix metalloproteinase-9 (MMP-9), also known as gelatinase B, shares similar in vitro substrate specificity as MMP-2, including type IV collagen, and is also upregulated after liver injury [25]. Thus, we explored the possibility that, in response to injury, MMP-2−/− mice expressed increased MMP-9 activity to compensate for their lack of MMP-2. As shown in Fig. 5c, MMP-9 expression was similar between MMP-2−/− and MMP-2+/+ mice by immunoblot. Given no significant difference in type I collagenase activity (Fig. 3b), the significant upregulation of α1(I) collagen mRNA by real-time PCR suggests that the increased collagen deposition observed in the MMP-2−/− mice may be secondary to increased type I collagen expression.
MMP-2 Regulates α1(I) Collagen mRNA Expression in Cultured Human Stellate Cells
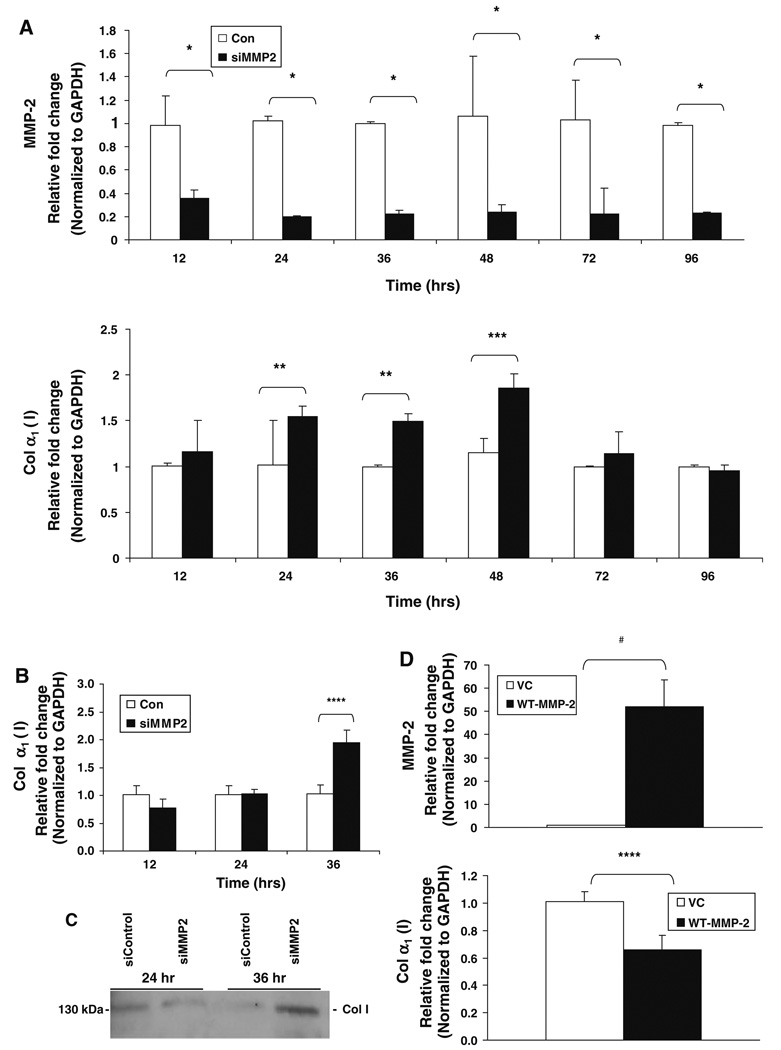
The increased expression of α1(I) collagen mRNA observed in the MMP-2−/− mice by real-time PCR analysis raised the possibility that MMP-2 regulates type I collagen expression. Because HSCs are the primary source of both type I collagen and MMP-2 in the liver, we studied the effect of MMP-2 on α1(I) collagen mRNA expression in LX2 cells, a human hepatic stellate cell line with a phenotype that resembles activated HSCs, including the expression of MMP-2 and type I collagen [15]. As shown in Fig. 6a, targeted reduction of MMP-2 in LX2 cells using a pool of four siRNA constructs specifically targeted against MMP-2 led to a significant increase in the expression of type I collagen mRNA at several time points (24 h fold change: 1.55 ± 0.003 versus 1.02 ± 0.046, P < 0.006; 36 h fold change: 1.49 ± 0.037 versus 1.0 ± 0.012, P < 0.006; 48 h fold change: 1.86 ± 0.19 versus 1.15 ± 0.26, P < 0.05). These results were confirmed in passage 3 primary human hepatic stellate cells in which MMP-2 knockdown with siRNA significantly increased both collagen α1(I) mRNA (fold change: 1.95 ± 0.217 versus 1.03 ± 0.162, P < 0.03) and collagen I protein secretion, as assessed by immunoblot analysis of primary HSC cell culture supernatants 36 h after transfection (Fig. 6b, c, respectively). Furthermore, overexpression of MMP-2 in LX2 cells by cDNA transfection decreased the expression of α1(I) collagen mRNA by 35% at 24 h (Fig. 6d, fold change: 0.66 ± 0.11 versus 1.01 ± 0.07, P < 0.03).
Fig. 6.
Type I collagen expression is regulated by MMP-2 in cell culture. Real-time PCR analysis for MMP-2 and collagen α1(I) mRNA was performed using RNA extracted from LX2 cells, an immortalized human hepatic stellate cell line, after transient transfection with either MMP-2-targeted siRNA (siMMP-2) or nontargeting control siRNA (siControl). MMP-2 is significantly downregulated in LX2 cells at multiple time points after transfection with siMMP-2, as compared with transfection with siControl (a). MMP-2 inhibition with siRNA results in a significant increase in α1(I) collagen mRNA expression at 24, 36, and 48 h (a). Real-time PCR analysis and immunoblot analysis for collagen I expression was performed after transient transfection with siMMP-2 or siControl in passage 3 primary human hepatic stellate cells. A twofold increase in α1(I) collagen mRNA levels was observed 36 h post transfection (b). Culture supernatant was collected and protein was precipitated with acetone. Thirty-five micrograms of protein was loaded per sample. An increased amount of extracellular collagen was observed 36 h after transfection. A representative immunoblot of three independent experiments is shown (c). Real-time PCR analysis for MMP-2 and α1(I) collagen mRNA was performed using RNA extracted from LX2 cells 24 h after transfection with either full-length wild-type MMP-2 (WT-MMP-2) or empty vector control (VC). Overexpression of MMP-2 was associated with a significant decrease in α1(I) collagen mRNA expression (d). All PCR results normalized to GAPDH. Data represent means ± SEM; * P < 0.002, ** P < 0.006, *** P < 0.05, **** P < 0.03, # P < 0.003
Discussion
In this study, we have demonstrated that MMP-2−/− mice developed increased liver fibrosis compared with MMP-2+/+ mice after chronic exposure to CCl4, a widely used model of toxic parenchymal liver injury. This increased fibrosis was not associated with a significantly altered level of hepatocellular damage. Although prior studies correlating changes in MMP-2 expression with the development and resolution of hepatic fibrosis [5, 26, 27] have suggested a profibrotic role for elevated MMP-2 in chronic liver injury, our data suggest that increased expression of MMP-2 may have some antifibrotic effects. This conclusion is supported by previous in vivo studies showing persistent overexpression of MMP-2 during both experimental treatment and spontaneous regression of liver fibrosis [28, 29].
The upregulation of collagen I expression by MMP-2 inhibition represents a previously unsuspected pathway by which this molecule may exert antifibrotic activity in liver injury. A profibrotic role is underscored by the marked upregulation of α1(I) collagen mRNA expression by real-time PCR in the MMP-2−/− livers, combined with a similar, significant increase in α1(I) collagen expression by hepatic stellate cells in vitro after MMP-2 inhibition with siRNA and further substantiated by the suppression of α1(I) collagen mRNA expression in the setting of MMP-2 overexpression. In contrast, the putative antifibrotic activity of MMP-2 via its action as a type I collagenase was not apparent, as type I collagenase activity was not significantly decreased in the MMP-2−/− mice as compared with MMP-2+/+ mice during progressive CCl4-induced liver injury. Whether MMP-2 may play an important role as an interstitial collagenase during the regression of liver fibrosis was not addressed by these studies. In addition, based on expression of α-SMA, a marker of HSC activation, there was no significant difference in hepatic stellate cell activation to account for the increased fibrosis in the MMP-2−/− group.
While the precise mechanism underlying the effect of MMP-2 on collagen I gene expression requires further study, our results suggest a novel role for MMP-2 in the regulation of fibrogenesis by activated HSCs. Potential mechanisms by which MMP-2 may modulate downstream signaling pathways and thereby inhibit α1(I) collagen mRNA expression include the enzyme’s ability to cleave cytokines [30] and cell surface receptors [31] and release cytokines and growth factors bound within the fibrotic liver’s extracellular matrix [32, 33]. In line with our observations, it has also recently been shown that reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy [34]. While the precise mechanism(s) may differ, this study underscores the potential protective effect of MMP-2 in tissue fibrosis.
Recent evidence has revealed several alternative substrates and novel biologic roles for matrix metalloproteinases [35]. Although MMP-2 is traditionally viewed as an extracellular protease, a potential “nontraditional” role as an intracellular protease has also been suggested; for example, MMP-2 collocalizes with and degrades troponin-I, a regulatory protein of the actin–myosin contractile apparatus [36]. Furthermore, MMP-2 is present in the nucleus of cardiac myocytes and is capable of cleaving poly(ADP-ribose) polymerase (PARP) in vitro [37]. Therefore, further studies are needed to explore the potential role of intracellular MMP-2 in regulating gene expression within stellate cells.
While a role only in toxin-induced injury cannot be excluded, replication of the key findings in cultured stellate cells argues against a CCl4-specific effect. Furthermore, while we intentionally focused our cell culture experiments on the effect of MMP-2 inhibition on HSCs, MMP-2 may also be expressed by other resident cells within the liver, including hepatocytes [38] and sinusoidal endothelial cells [39]. It is unclear whether the loss of MMP-2 expression by these cells, or possibly even infiltrating leukocytes and macrophages, may have contributed to the increased fibrosis observed in the MMP-2−/− group.
In conclusion, these findings indicate that MMP-2 plays a protective role in chronic liver injury and attenuates the development of hepatic fibrosis. While the precise mechanism requires further clarification, our study suggests that MMP-2 limits α1(I) collagen expression by activated hepatic stellate cells and thereby attenuates type I collagen deposition, pointing to a novel role for MMP-2 in tissue fibrosis.
Acknowledgments
Supported by National Institutes of Health (NIH) T32 DK07792 (B.D.R.); NIH DK6047402 and DK071745 (M.B.B.); NIH DK56621 (S.L.F.); AGA Shirley and Miles Fiterman Basic Research Award (M.B.B.), The Feld Fibrosis Program.
Contributor Information
Brian D. Radbill, Division of Nephrology, Department of Medicine, Mount Sinai School of Medicine, New York, NY 10029, USA
Ritu Gupta, Division of Liver Diseases, Department of Medicine, Mount Sinai School of Medicine, 1425 Madison Avenue, Room 11-70, Box 1123, New York, NY 10029, USA.
Maria Celeste M. Ramirez, Departments of Medicine and Genetics and Genomic Sciences, Mount Sinai School of Medicine, New York, NY, USA
Analisa DiFeo, Departments of Medicine and Genetics and Genomic Sciences, Mount Sinai School of Medicine, New York, NY, USA.
John A. Martignetti, Departments of Medicine and Genetics and Genomic Sciences, Mount Sinai School of Medicine, New York, NY, USA
Carlos E. Alvarez, Division of Liver Diseases, Department of Medicine, Mount Sinai School of Medicine, 1425 Madison Avenue, Room 11-70, Box 1123, New York, NY 10029, USA
Scott L. Friedman, Division of Liver Diseases, Department of Medicine, Mount Sinai School of Medicine, 1425 Madison Avenue, Room 11-70, Box 1123, New York, NY 10029, USA
Goutham Narla, Departments of Medicine and Genetics and Genomic Sciences, Mount Sinai School of Medicine, New York, NY, USA.
Raluca Vrabie, Division of Liver Diseases, Department of Medicine, Mount Sinai School of Medicine, 1425 Madison Avenue, Room 11-70, Box 1123, New York, NY 10029, USA.
Robert Bowles, Division of Liver Diseases, Department of Medicine, Mount Sinai School of Medicine, 1425 Madison Avenue, Room 11-70, Box 1123, New York, NY 10029, USA.
Yedidya Saiman, Division of Liver Diseases, Department of Medicine, Mount Sinai School of Medicine, 1425 Madison Avenue, Room 11-70, Box 1123, New York, NY 10029, USA.
Meena B. Bansal, Division of Liver Diseases, Department of Medicine, Mount Sinai School of Medicine, 1425 Madison Avenue, Room 11-70, Box 1123, New York, NY 10029, USA meena.bansal@mssm.edu
References
- 1.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type i collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 2.Arthur MJ, Friedman SL, Roll FJ, Bissell DM. Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type iv) collagen. J Clin Invest. 1989;84:1076–1085. doi: 10.1172/JCI114270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tournier JM, Polette M, Hinnrasky J, Beck J, et al. Expression of gelatinase a, a mediator of extracellular matrix remodeling, by tracheal gland serous cells in culture and in vivo. J Biol Chem. 1994;269:25454–25464. [PubMed] [Google Scholar]
- 4.Takahara T, Furui K, Funaki J, Nakayama Y, et al. Increased expression of matrix metalloproteinase-ii in experimental liver fibrosis in rats. Hepatology. 1995;21:787–795. [PubMed] [Google Scholar]
- 5.Milani S, Herbst H, Schuppan D, Grappone C, et al. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994;144:528–537. [PMC free article] [PubMed] [Google Scholar]
- 6.Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373–384. doi: 10.1055/s-2001-17552. [DOI] [PubMed] [Google Scholar]
- 7.Benyon RC, Hovell CJ, Da Gaca M, Jones EH, et al. Progelatinase a is produced and activated by rat hepatic stellate cells and promotes their proliferation. Hepatology. 1999;30:977–986. doi: 10.1002/hep.510300431. [DOI] [PubMed] [Google Scholar]
- 8.Issa R, Zhou X, Constandinou CM, Fallowfield J, et al. Spontaneous recovery from micronodular cirrhosis: Evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Iredale JP, Benyon RC, Pickering J, McCullen M, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preaux AM, D’Ortho MP, Bralet MP, Laperche Y, Mavier P. Apoptosis of human hepatic myofibroblasts promotes activation of matrix metalloproteinase-2. Hepatology. 2002;36:615–622. doi: 10.1053/jhep.2002.35279. [DOI] [PubMed] [Google Scholar]
- 11.Itoh T, Ikeda T, Gomi H, Nakao S, et al. Unaltered secretion of beta-amyloid precursor protein in gelatinase a (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 12.Mosig RA, Dowling O, DiFeo A, Ramirez MC, et al. Loss of mmp-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum Mol Genet. 2007;16:1113–1123. doi: 10.1093/hmg/ddm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safadi R, Ohta M, Alvarez CE, Fiel MI, et al. Immune stimulation of hepatic fibrogenesis by cd8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870–882. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 14.Kovalovich K, DeAngelis RA, Li W, Furth EE, et al. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149–159. doi: 10.1002/hep.510310123. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Hui AY, Albanis E, Arthur MJ, et al. Human hepatic stellate cell lines, LX-1 and LX-2: New tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong F, Tuyama A, Lee T, Loke J, et al. Hepatic stellate cells express functional CXCR4: Role in stromal cell-derived factor - 1α -mediated stellate cell activation. Hepatology. 2009;49:2055–2067. doi: 10.1002/hep.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansal MB, Kovalovich K, Gupta R, Li W, et al. Interleukin-6 protects hepatocytes from CCl4-mediated necrosis and apoptosis in mice by reducing MMP-2 expression. J Hepatol. 2005;42:548–556. doi: 10.1016/j.jhep.2004.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le J, Dauchot P, Perrot JL, Cambazard F, et al. Quantitative zymography of matrix metalloproteinases by measuring hydroxyproline: Application to gelatinases a and b. Electrophoresis. 1999;20:2824–2829. doi: 10.1002/(SICI)1522-2683(19991001)20:14<2824::AID-ELPS2824>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Wielockx B, Lannoy K, Shapiro SD, Itoh T, et al. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nat Med. 2001;7:1202–1208. doi: 10.1038/nm1101-1202. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: New insights and prospects for therapy. J Gastroenterol Hepatol. 1999;14:618–633. doi: 10.1046/j.1440-1746.1999.01928.x. [DOI] [PubMed] [Google Scholar]
- 21.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 22.Friedman SL. Seminars in medicine of the beth Israel hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 23.Gressner AM. Liver fibrosis: Perspectives in pathobiochemical research and clinical outlook. Eur J Clin Chem Clin Biochem. 1991;29:293–311. [PubMed] [Google Scholar]
- 24.Yang C, Zeisberg M, Mosterman B, Sudhakar A, et al. Liver fibrosis: Insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 25.Knittel T, Mehde M, Grundmann A, Saile B, et al. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443–453. doi: 10.1007/s004180000150. [DOI] [PubMed] [Google Scholar]
- 26.Lichtinghagen R, Michels D, Haberkorn CI, Arndt B, et al. Matrix metalloproteinase (mmp)-2, mmp-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis c. J Hepatol. 2001;34:239–247. doi: 10.1016/s0168-8278(00)00037-4. [DOI] [PubMed] [Google Scholar]
- 27.Ebrahimkhani MR, Kiani S, Oakley F, Kendall T, et al. Naltrexone, an opioid receptor antagonist, attenuates liver fibrosis in bile duct ligated rats. Gut. 2006 doi: 10.1136/gut.2005.076778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reif S, Aeed H, Shilo Y, Reich R, et al. Treatment of thioacetamide-induced liver cirrhosis by the ras antagonist, farnesylthiosalicylic acid. J Hepatol. 2004;41:235–241. doi: 10.1016/j.jhep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Lee HS, Huang GT, Miau LH, Chiou LL, et al. Expression of matrix metalloproteinases in spontaneous regression of liver fibrosis. Hepato-Gastroenterology. 2001;48:1114–1117. [PubMed] [Google Scholar]
- 30.Gearing AJ, Beckett P, Christodoulou M, Churchill M, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 31.Levi E, Fridman R, Miao HQ, Ma YS, et al. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci USA. 1996;93:7069–7074. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 33.Dallas SL, Rosser JL, Mundy GR, Bonewald LF. Proteolysis of latent transforming growth factor-beta (tgf-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of tgf-beta from bone matrix. J Biol Chem. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- 34.Van Linthout S, Seeland U, Riad A, Eckhardt O, et al. Reduced mmp-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2008;103:319–327. doi: 10.1007/s00395-008-0715-2. [DOI] [PubMed] [Google Scholar]
- 35.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, et al. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106:1543–1549. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- 37.Kwan JA, Schulze CJ, Wang W, Leon H, et al. Matrix metallo-proteinase-2 (mmp-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (adp-ribose) polymerase (parp) in vitro. FASEB J. 2004;18:690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 38.Garciade Leon Mdel C, Montfort I, Tello Montes E, Lopez Vancell R, et al. Hepatocyte production of modulators of extracellular liver matrix in normal and cirrhotic rat liver. Exp Mol Pathol. 2006;80:97–108. doi: 10.1016/j.yexmp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Upadhya AG, Harvey RP, Howard TK, Lowell JA, et al. Evidence of a role for matrix metalloproteinases in cold preservation injury of the liver in humans and in the rat. Hepatology. 1997;26:922–928. doi: 10.1002/hep.510260418. [DOI] [PubMed] [Google Scholar]