Abstract
Patients with recurrent or refractory Epstein Barr virus (EBV)-positive nasopharyngeal carcinoma (NPC) continue to have poor outcomes. Our earlier Phase I dose escalation clinical study of 10 NPC patients showed that infusion of EBV-specific cytotoxic T cells (EBV-CTLs) was safe and had antitumor activity. To better define the overall response rate and discover whether disease status, EBV-antigen specificity, and/or in vivo expansion of infused EBV-CTLs predicted outcome, we treated 13 additional NPC patients with EBV-CTLs in a fixed-dose, Phase II component of the study. We assessed toxicity, efficacy, specificity and expansion of infused CTLs for all 23 recurrent/refractory NPC patients treated on this Phase I/II clinical study. At the time of CTL infusion, 8 relapsed NPC patients were in remission and 15 had active disease. No significant toxicity was observed. Of the relapsed patients treated in their second or subsequent remission, 62% (5/8) remain disease free (at 17-75 months), while 48.7% (7/15) of those with active disease had a CR/CRu (33.3%) or PR (15.4%). In contrast to locoregional disease, metastatic disease was associated with an increased risk of disease progression (HR: 3.91, p=0.015) and decreased overall survival (HR: 5.55, p=0.022). Neither the specificity of the infused CTLs for particular EBV antigens nor their measurable in vivo expansion discernibly influenced outcome. In conclusion, treatment of patients with relapsed/refractory EBV-positive NPC with EBV-CTLs is safe and can be associated with significant, long-term clinical benefit, particularly for patients with locoregional disease.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) arises from the epithelial cells of the nasopharynx and almost all non-keratinizing and undifferentiated forms of the tumor are associated with Epstein Barr virus (EBV).1;2 NPC patients with limited local disease have a good prognosis when treated with chemotherapy and intensity-modulated radiation therapy, but patients with bulky locoregional or metastatic disease often relapse or have refractory disease.1;3;4 Historical 5-year overall survival rates for those with advanced stage III or IV disease are reported between 54 – 76% and 29 – 56%, respectively.1;3;5;6 Patients who survive experience severe short and long term morbidity, with an associated profound impact on their quality of life.7;8 Hence, there is a need for novel therapies that both improve disease-free survival and reduce treatment-related complications.
One promising approach is therapy with autologous EBV-specific cytotoxic T lymphocytes (EBV-CTLs) targeted to the EBV antigens EBNA1, latent membrane protein (LMP) 1 and LMP2 expressed by most NPC tumors.1;2 Although adoptive immunotherapy with EBV-CTLs for NPC has shown evidence of clinical activity in Phase I dose escalation studies,9;10 at present the overall response rate and most appropriate patient population (i.e. those with locoregional as compared to metastatic, relapsed/refractory disease) is unclear. It has also been unclear whether antitumor efficacy is influenced by the particular EBV antigens the infused lines are primarily directed toward, or by the degree of in vivo expansion measured within the peripheral blood, as observed for other T-cell therapeutics.11;12 This information is critical for developing further CTL applications and improving clinical progress.
We therefore conducted a Phase II extension of our previously reported Phase I clinical study. Thirteen additional NPC patients were treated with EBV-CTLs, and we have included all 23 NPC patients treated at our institution in this analysis. The overall response rate was 48.7% and although disease status predicted outcome, the EBV-antigen specificity of the CTL product and the degree of in vivo CTL expansion did not.
MATERIAL AND METHODS
Subjects
This study was approved by the Institutional Review Board at Baylor College of Medicine and The Methodist Hospital, and by the Food and Drug Administration. Patients were eligible for the study if they had been treated for either advanced stage [stage III or IV disease according to the American Joint Committee on Cancer (AJCC) staging system] or had relapsed/refractory EBV-positive NPC. EBV-positivity was determined by in situ hybridization or polymerase chain reaction (PCR) amplification for EBV-encoded RNAs (EBER). Patients had to have received no therapy or experimental agents for at least 4 weeks prior to study entry, and recovered from treatment related acute adverse effects. Patients had to have a life expectancy > 6 weeks, Karnofsy score ≥ 50, and no evidence of pregnancy or intercurrent infection. Laboratory parameters included bilirubin and creatinine values < 2 times normal, aspartate aminotransferase levels < 3 times normal, and a hemoglobin level > 8.0 g/dL. All patients were treated between July of 2002 and August of 2007.
Generation of EBV-transformed lymphoblastoid cell lines and EBV-CTLs
Autologous lymphoblastoid cell lines (LCL) and EBV-CTLs were generated according to current Good Manufacturing Practice (cGMP) guidelines as previously described 9;13;14. After expansion, EBV-CTLs were tested for sterility, human leukocyte antigen (HLA)-identity, immunophenotype, and EBV-specificity at the time of cryopreservation. Specificity was tested in a 4-hour 51Chromium release cytotoxicity assay.
Study description
All patients had imaging with magnetic resonance imaging (MRI) and/or fluorodeoxyglucose positron emission tomography (PET) to assess overall disease burden prior to CTL infusion. Patients treated on the Phase I portion of the study were treated on one of 3 dose levels and received either 2 doses of 2×107 CTL/m2 (dose level 1), 1 dose of 2×107 and 1 dose of 1×108 CTL/m2 (dose level 2), or 1 dose of 1×108 and 1 dose of 2×108 CTL/m2 (dose level 3). All patients treated on the Phase II extension were treated at the highest dose level. CTL infusions were given 2 weeks apart. Peripheral blood samples were obtained prior to CTL infusion and at pre-determined time points after infusion to evaluate for toxicity and EBV immunity. Clinical response to CTL was assessed by radiographic imaging 8 weeks after the last infusion date (or earlier if indicated). Patients with either a partial response (PR) or stable disease (SD) at the time of re-imaging were eligible to receive additional CTL infusions. Long-term follow up continued until July 2009. While initial data from ten patients treated on the Phase I portion of this clinical trial has been previously published,9 updated information related to clinical status and CTL product analysis is included within the results data detailed below. All imaging studies were re-reviewed, one patient (815), who was previously reported as having no disease, is now reported as having imaging findings of unknown significance.
Clinical response criteria
Clinical response to CTL infusion was evaluated by comparing disease identified by MRI and/or FDG-PET radiographs obtained pre-infusion to those obtained 8 weeks post-infusion, or as clinically indicated. FDG-PET imaging was used as additional conformation of response, since many patients have residual masses after standard therapy that may not represent active disease. Furthermore, as this study was open to children, re-biopsy of residual masses was not mandatory for study participation. Patients in remission at the time of infusion were defined as having no evidence of disease (NED) if subsequent scans had no new lesions. Patients with abnormalities of unknown significance (i.e. infection vs. disease) whose subsequent scans had no radiographic evidence of abnormal lesions were considered a complete response undetermined (CRu). All other responses, including CR, PR, SD, and PD, were determined using Response Evaluation Criteria in Solid Tumors (RECIST).15
Enzyme-linked immunospot (ELIspot) assay
The frequency of EBV-, LMP1- and LMP2-specific T cells in the CTL product and peripheral blood of patients was measured using interferon-γ (IFN-γ) ELIspot assays as previously described.16;17 CTL or PBMCs were stimulated with HLA-restricted peptides derived from EBV antigens (Genemed Synthesis, San Antonio, TX) or overlapping peptide mixes for BZLF1, EBNA1, EBNA3A, EBNA3B, EBNA3C, LMP1, and LMP2 were used in ELIspot assays.18-20 Peptide mixes contained 15 amino-acid peptides covering the entire length of the corresponding protein with an 11 amino-acid overlap (JPT Peptide Technologies, Berlin, Germany). The frequency of EBV-, LMP1-, and LMP2-specific T cells in the peripheral blood of patients was determined by using autologous LCL or LMP1 and LMP2 peptide mixes. For cytomegalovirus (CMV)-seropositive patients, the frequency of CMV pp65 protein (CMVpp65)-specific T cells was determined using a CMVpp65 peptide mix (JPT Peptide Technologies).
Flow cytometry
A FACScalibur instrument (Becton Dickinson, San Jose, CA) and CellQuest software (Becton Dickinson) was used for flow cytometric analysis. Monoclonal antibodies were obtained from Becton Dickinson and included anti-CD3, -CD4, -CD8, -CD16, -CD19, -CD27, -CD28, -CD45RO, CD45RA, -CD56, -TCRα/β, and -TCRγ/δ. Negative controls included isotype antibodies.
Statistical analysis
The Phase I/II study was designed to be a dose escalation investigation leading to a fixed dose Phase II study. Four patients were treated on dose level 1 (one compassionate care), three patients on dose level 2, and 16 patients on (fixed) dose level 3. No dose-limiting toxicity was observed, and all patients were included in the analysis. Descriptive statistics were used to evaluate standard demographic and clinical variables. Biological responses by EBV-, LMP2-, and CMV-specific T cells were analyzed for their changes from the baseline (CTL infusion) to 2 weeks and 6 weeks post infusion, respectively, using the nonparametric Wilcoxon signed-rank test. Survival curves were constructed using the Kaplan Meier method and compared using the weighted log-rank test. Patient data were censored at the time of death or if enrolled on another therapeutic/experimental trial, or if lost to follow-up. We used univariate Cox regression to determine clinical factors significantly associated with survival. A p-value less than 0.05 was considered statistically significant.
RESULTS
Patient characteristics
The clinical and disease specific characteristics of the 23 patients are listed in Tables 1 and 2. The majority of patients were male, Caucasian, and had either AJCC Stage III or IV disease at the time of initial diagnosis. At the first CTL infusion, the mean patient age was 29.2 years, and most patients had failed 1 or more previous chemotherapy protocols. Approximately 65% had either active disease or diagnostic scans of unknown significance.
Table 1.
Characteristics of enrolled study population
| Gender (%) | |
| Male | 17 (73.9) |
| Female | 6 (26.1) |
| Mean age at enrollment, years (range) | 29.2 (11 - 63) |
| Ethnic origin (%) | |
| African-American | 6 (26.1) |
| Asian | 4 (17.4) |
| Caucasian | 11 47.8) |
| Hispanic | 1 (4.4) |
| Native American | 1 (4.4) |
| Disease status at 1st CTL infusion (%) | |
| Remission | 8 (34.8) |
| Local-Regional | 3 (13.0) |
| Unknown | 2 (8.7) |
| Metastatic | 10 (43.5) |
| Mean number of chemotherapy regimens prior to study enrollment (range) | 2.6 (1-6) |
Table 2.
Detailed patient characteristics and outcome
| P# | Age and Gender at Infusion | Stage at Diagnosis | Prior Therapy | Site of Disease at Infusion | CTL* (Dose Level) | Response to Therapy (Duration in Months) | ||
|---|---|---|---|---|---|---|---|---|
| CT | XRT | |||||||
| 729 | 50 | F | IV: T4N3bM0 | 1 | 1 | NED | 2 (1) | Remains in remission (>82 mths); alive |
| 606 | 59 | F | III: T3N2M0 | 1 | 1 | NED | 2 (1) | Recurrent disease (60 mths); alive |
| 697 | 11 | F | III: T1N2M0 | 1 | 1 | NED | 2 (1) | Remains in remission (>80 mths); alive |
| 1642 | 11 | M | IV: TXNXM1 | 3 | 1 | NED | 2 (3) | Remains in remission (>42 mths); alive |
| 1946 | 63 | F | III: T3N1M0 | 1 | 1 | NED | 2 (3) | Remains in remission (>28 mths); alive |
| 1969 | 38 | M | III: T3N0M0 | 2 | 2 | NED | 2 (3) | Recurrent disease (14 mths); died at 27 mths |
| 2024 | 60 | M | III: T2N2M0 | 1 | 1 | NED | 2 (3) | Recurrent disease (2 mths); alive |
| 2047 | 16 | M | II: T2N1M0 | 3 | 3 | NED | 2 (3) | Remains in remission (>25 mths); alive |
| 894 | 36 | M | III: T3N0M0 | 3 | 2 | Primary site | 2 (2) | CR (44 mths); died at 65 mths |
| 389 | 17 | F | IV: T4N2M0 | 2 | 1 | Primary site | 5 (2) | CR (>53 mths); alive |
| 918 | 16 | M | IV: T4N2M0 | 1 | 1 | Primary site | 2 (2) | PR (12 mths); died at 20 mths |
| 815 | 19 | M | IV: T4N3M0 | 1 | 1 | Primary site (unknown significance) | 2 (3) | CRu (>46 mths); alive |
| 845 | 11 | M | IV: T4N2M0 | 6 | 2 | Primary site, LN | 1 (1) | PD; died at 12 mths |
| 1042 | 46 | F | II: T2N1M0 | 2 | 2 | Bone | 2 (3) | CR (>68mths); alive |
| 1046 | 16 | M | IV: T2bN3M0 | 4 | 1 | LN, bone | 2 (3) | SD (2 mths); died at 3 mths |
| 1241 | 18 | M | IV: T4N1M0 | 3 | 2 | Lung (unknown significance) | 2 (3) | CRu (36 mths); alive |
| 1902 | 17 | M | III: T3N1M0 | 2 | 1 | Primary site, bone, lung | 2 (3) | PD; died at 14 mths |
| 1968 | 34 | M | IV: T4N1M0 | 4 | 2 | Primary site, lung, CNS | 2 (3) | PD; died at 11 mths |
| 2019 | 17 | M | IV: T4N3M0 | 3 | 3 | Primary site, bone | 2 (3) | SD (4 mths); died at 7 mths |
| 2061 | 32 | M | IV: T0N3M1 | 4 | 1 | Primary, LN, bone, lung | 2 (3) | PD; died at 16 mths |
| 2078 | 16 | M | IV: T2N3aM1 | 4 | 2 | LN, bone, soft tissue | 2 (3) | PD; died at 7 mths |
| 1668 | 21 | M | IV: T4N2aM0 | 4 | 4 | LN, bone | 3 (3) | PR (7 mths); alive |
| 1976 | 48 | M | I: T1N0M0 | 3 | 1 | Lung | 6 (3) | SD (12 mths); alive |
P#: patient identification number; CT: chemotherapy regimens; XRT: radiotherapy regimens; NED: no evidence of disease; LN: lymph node; CNS: central nervous system; CTL*: number of CTL infusions; Mths: months; CR: complete response; PR: partial response; CRu: complete response undetermined; SD: stable disease; PD: progressive disease
Characteristics of EBV-CTL lines
EBV-CTL lines were generated successfully for all 23 study participants. Patients’ CTL lines contained a high percentage of CD3-positive T cells [mean, 96.2%; standard deviation (sd), 4.0%]; predominantly CD8 [mean, 83.2%; sd, 13.9%] with a small CD4 component [mean, 7.6%; sd 14.4%]. Flow cytometric analysis of memory markers revealed mixed populations of CD45RAneg CD27pos CD28pos T-cells [mean 34.2%; sd 14.5%] and CD45RAneg CD27neg CD28neg T-cells [mean 21.1%; sd 12.3%]. Besides T cells, natural killer (NK) cells were present in low numbers [mean, 5.7%; sd, 3.8%]. In 4-hour 51Chromium release cytotoxicity assays, lines from all patients produced significantly greater killing of autologous LCL compared to mismatched LCL, NK cell targets (HSB-2 or K562), or autologous PHA blasts at an effector to target ratio of 20:1 (for all targets p=<0.001).
To determine which EBV antigens were recognized by the patients’ CTL lines, we used IFN-γ ELIspot assays to measure responses to HLA restricted peptides derived from EBV antigens. For patients in whom no HLA restricted peptides were available, we used peptide mixes that contained 15 amino acid peptides scanning the entire length of EBV antigens. T cells specific for immunodominant EBV antigens (lytic or EBNAs 3A, B, C) were present in all cell lines tested (supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/JIT/A73). EBNA1-specific T cells were present in 5 out of 16 cell lines tested. LMP2-specific T cells were detected in 17 cell lines, but the frequency was less than 0.1% in 9 out of the 17. T cells specific for LMP1 were detected at a low frequency in 7 cell lines.
Safety of EBV-CTL infusions
Infusion of autologous EBV-CTL products was not associated with long-term toxicity, although as previously reported, 1 patient with bulky disease and preexisting facial swelling required a tracheostomy for airway protection after his facial swelling increased 2 days following the first CTL infusion.9 No inflammatory cells were detected in the needle biopsy of his mass, but CTL administration could not be excluded as a contributing factor to the swelling.
Immune responses after EBV-CTL Infusion
We measured the overall precursor frequency of EBV-specific T cells in patients’ peripheral blood using autologous LCL as stimulators in IFN-γ ELIspot assays. There was no significant increase 2 and 6 weeks after the 1st CTL infusion (Figure 1A). Since LCL preferentially activate T cells specific for immunodominant EBV proteins which are absent on NPC tumors, we next determined the frequency of T cells specific for LMP2, a subdominant EBV antigen expressed by NPC. Seventeen patients received CTL lines which contained LMP2-specific T cells and we were able to serially determine the frequency of LMP2-specific CTL in the peripheral blood of 14 using LMP2 peptide ELIspot assays. We found a trend towards increased LMP2-specific CTL at 2 weeks post infusion (p=0.081), which returned to baseline by week 6 (Figure 1B). As a control, we sequentially measured the frequency of CMVpp65-specific T cells and noted no detectible changes (Figure 1C). No correlation was observed between disease stage at CTL infusion and the frequency of EBV-, LMP2-, and CMV-specific T cells. Therefore, infusion of EBV-specific CTL may transiently increase the frequency of LMP2-specific T cells in peripheral blood.
Figure 1. Transient increase of LMP2-specific T-cell frequency after EBV-CTL infusion.
The frequency of (A) EBV-, (B) LMP2-, and (C) CMV-specific T cells was determined using ELIspot assays. There was a trend towards increased LMP2-specific T cells (p=0.081) at 2 weeks after CTL infusion (- -: individual patients; –: median; NED: no evidence of disease).
Clinical responses and subgroup analysis after EBV-CTL infusion
We measured clinical responses by comparing imaging studies pre- and post-CTL infusion as detailed in the methods section. These data are summarized in Table 3. Of the 8 patients treated in remission, 5 remained disease-free 25 – 82 months post-infusion. Of the remaining three, one had recurrent, biopsy proven, EBV-positive lymph node disease 2 months post CTL infusion. This patient is still alive with indolent disease 34 months post CTL infusion having received no additional therapy. Two patients had recurrent metastatic disease, 14 and 60 months post CTL infusion, respectively. Of three patients treated with local recurrent disease, 2 have had CRs for > 44 and > 53 months. The 3rd patient had a partial response that persisted for 12 months. A fourth patient had imaging findings of unknown significance prior to CTL infusion, and these resolved post infusion. He remains without evidence of disease (CRu) at > 46 months.
Table 3.
Univariate Cox regression analysis
| Variable | PFS | OS | ||
|---|---|---|---|---|
| Hazard Ratio | P value | Hazard Ratio | P value | |
| Age at 1st CTL infusion | 0.996 | 0.787 | 0.972 | 0.209 |
| Ethnic Origin | ||||
| African-American | 0.581 | 0.531 | 0.860 | 0.869 |
| Caucasian | 1.492 | 0.515 | 0.858 | 0.841 |
| Other | 1 | 1 | ||
| Stage at Diagnosis | ||||
| Stage I-III | 1 | 1 | ||
| Stage IV | 1.325 | 0.606 | 2.225 | 0.263 |
| Disease status at 1st CTL infusion | ||||
| Remission | ||||
| Yes | 0.343 | 0.103 | 0.154 | 0.080 |
| No | 1 | 1 | ||
| Local-Regional | ||||
| Yes | 0.740 | 0.695 | 1.388 | 0.687 |
| No | 1 | 1 | ||
| Unknown | ||||
| Yes | 0.643 | 0.674 | NA* | |
| No | 1 | |||
| Metastatic Disease | ||||
| Yes | 3.908 | 0.015 | 5.551 | 0.022 |
| No | 1 | 1 | ||
no death event occurred
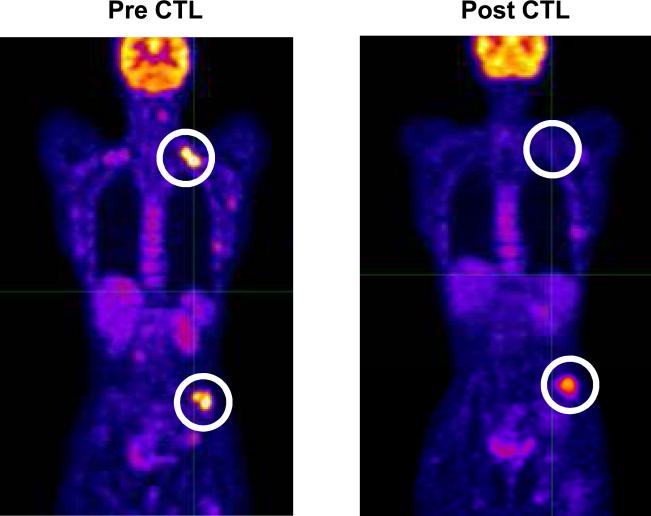
In the eleven patients with metastatic disease, one patient (1042) has had a durable response for > 68 months. This patient was initially reported at having stable disease > 14 months;9 starting 26 months post CTL she had no evidence of disease and remains disease free >68 months post CTL infusion. In this analysis she is therefore reported as having a CR. Patient 1241 had a CRu for 36 months. The latter then had a local, EBV-negative recurrence. One patient had a significant PR with resolution of PET positive lymph nodes and a significant decrease in a metastatic bone lesion (Figure 2). Two other patients had SD for 2 and 12 months respectively. Six patients had progressive disease.
Figure 2. Clinical response after EBV-CTL infusion.
PET images of patient (P1668) before and 8 weeks after EBV-specific CTL infusion.
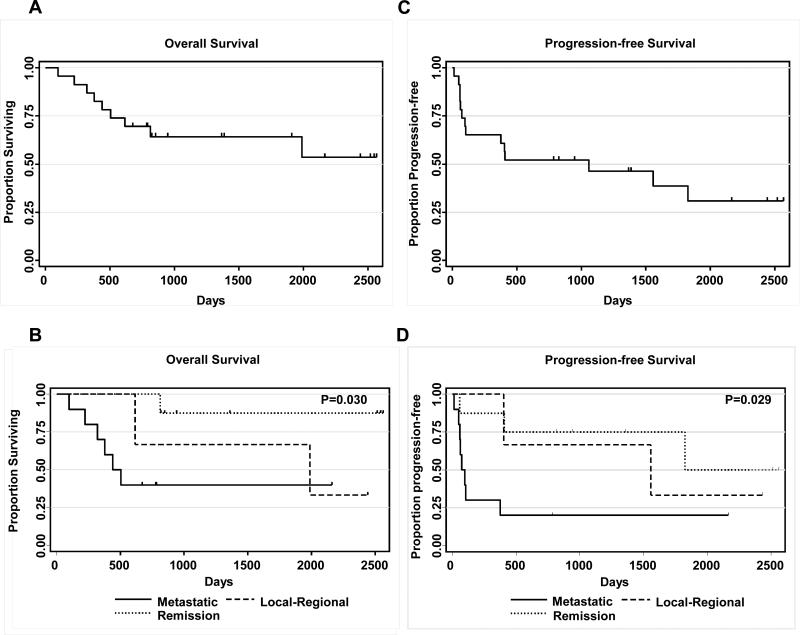
For the entire study cohort, the median time to progression was 1059 days with progression free survival (PFS) rates of 65% at 1-year and 52% at 2-years (Figure 3). The overall survival (OS) was 87% at 1-year and 70% at 2-years. Metastatic disease was associated with an increased risk of disease progression (HR: 3.91, p=0.015) and decreased overall survival (HR: 5.55, p=0.022). No significant association was observed for patients with locoregional disease. PFS and OS did not correlate with ethnicity, stage at diagnosis (Table 3), or CTL-specificity (supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/JIT/A74).
Figure 3. Overall and progression-free survival after EBV-CTL infusion.
A) Overall survival, B) Overall survival by disease status at 1st CTL infusion, C) Progression-free survival, D) Progression by disease status at 1st CTL infusion (p-value: metastatic disease vs. remission).
DISCUSSION
In this study we evaluated the safety and efficacy of EBV-CTL in patients with EBV-positive NPC. Twenty three NPC patients were treated with EBV-specific CTLs and followed long-term. We found that EBV-CTLs had potent antitumor activity in patients with locoregional disease, while similar effects were limited in patients with metastatic disease. Nonetheless, progression free survival was 65% at 1-year and 52% at 2-years, while overall survival was 87% at 1-year and 70% at 2-years. In this limited cohort of patients, clinical outcome did not correlate with specificity or expansion of adoptively transferred CTL lines.
Although patients with non-bulky, recurrent, locoregional NPC have a long-term survival rate greater than 60% with chemoradiation,21;22 these therapies are often mutilating and associated with significant acute and long-term complications profoundly diminishing quality of life. Our results suggest that EBV-CTLs may have comparable efficacy and greatly reduced toxicities. More extensive studies will be required to validate the therapeutic advantages of autologous EBV-CTLs in this subset of patients.
The outcome for patients with recurrent, metastatic disease remains poor. Currently, there is no accepted standard second-line therapy for this group of patients. Since 2002, there have been at least 7 Phase II clinical studies in which patients with relapsed and/or metastatic NPC were treated with systemic chemotherapy.23-29 Four of the regimens used single agents (irinotecan, capecitabine, gemcitabine, or gefitinib) while the remainder gave two-drug, platinum-based therapy. The overall CR and PR rates in these studies were only were 4.4% (0-20.5%) and 27% (14.3-78%) respectively. The median time to progression was variable (81-318 days), most likely due to selection bias of study subjects. Unfortunately, our EBV-CTLs had an equally limited antitumor activity in patients with metastatic disease (CR rate 10%; PR rate 10%), with a median progression free survival of 74 days. One of patients with recurrent disease had an EBV-negative tumor. Antigen-negative recurrences have been reported after the infusion of T-cell clones.30 We have also seen EBV-negative recurrences in 2 patients treated on our ongoing protocols with polyclonal EBV-specific CTLs for EBV-positive lymphomas (unpublished). In addition, we previously reported a patient with EBV-positive lymphoproliferative disease, who had failed EBV-specific T-cell Therapy, in which the tumor virus had deleted immunodominant EBV epitopes.31 Thus immune escape can occur even in instances when polyclonal antigen-specific T cells are infused.
NPC is classified as an EBV Type II latency-associated tumor as it expresses only a limited range of EBV-associated antigens.2 We anticipated that the greatest antitumor activity would therefore be associated with those lines expressing the highest frequency of effector cells specific for the three EBV antigens expressed by these tumors: LMP1, LMP2 or EBNA 1. Although this cohort of patients was small, we found no positive correlation between CTL specificity and antitumor activity. Activity was even seen after the infusion of lines with an absence of reactivity with LMP1 and EBNA1. While the majority of T cell lines contained CTLs specific for LMP2, their overall frequency was low and there was no correlation between the levels seen and the antitumor activity observed. Nonetheless it seems likely that one requirement for increasing the effectiveness of autologous EBV-CTLs in metastatic NPC will be to augment the frequency of EBNA1-, LMP1- and/or LMP2-specific T cells in the CTL product.17;32-34 Such benefits are certainly observed when LMP1/LMP2 specific CTLs are used to treat EBV-positive lymphomas, which are also predominantly Type II latency tumors.35 An alternative strategy for augmenting activity in metastatic disease is to induce tumor expression of more immunodominant EBV antigens by using chemotherapeutic agents, histone deacetylase or proteasome inhibitors.36;37 Pre-clinical data have shown benefit from this type of approach, but clinical experience is thus far limited.38;39
We observed limited in vivo expansion of EBV-CTLs in peripheral blood of patients after infusion. IFN-γ ELIspot assays showed only transient two-fold increases in LMP2-specific T cells. Lymphodepletion prior to T-cell infusion appears to enhance the expansion and persistence of infused cells, and may also be a valuable approach for NPC patients.16;40 The NPC patients enrolled on this study were not lymphopenic (mean absolute lymphocyte count: 829/μl; sd 373/μl), and thus lympodepletion would be an option to improve in vivo, post-infusion expansion. In several patients we observed a slight, non-significant decrease in the precursor frequency of EBV-CTL. This most likely reflects physiological fluctuations, which we have observed in our previous studies.16;35
In summary, treatment of patients with relapsed or refractory EBV-positive NPC with EBV-specific CTL is safe and can be associated with significant, long-term clinical benefit, primarily in patients with locoregional disease. For patients with metastatic disease, further modifications to this cellular therapeutic approach are warranted.
Supplementary Material
Acknowledgements
We thank S. Hardwick, V. Torrano, D. Copper-Havlik, and C. Perera for help with coordinating patients’ follow up and collecting patients’ data; A. Durett for expert technical assistance; and the staff of the GMP facility for assisting in CTL line production.
Financial support
The authors were supported by NIH grants PO1 CA94237, NIDDK T32 DK64717, NCI K12 CA09043306 and the GCRC at Baylor College of Medicine (RR00188). C.M.B. was supported by an award from the Gillson Longenbaugh Foundation. S.G. was the recipient of a Doris Duke Clinical Scientist Development Award. H.E.H was the recipient of a Doris Duke Distinguished Clinical Scientist Award.
Footnotes
Financial Disclosure: All authors have declared there are no conflicts of interest in regards to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol. 2002;13:1007–15. doi: 10.1093/annonc/mdf179. [DOI] [PubMed] [Google Scholar]
- 2.Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431–41. doi: 10.1016/s1044579x0200086x. [DOI] [PubMed] [Google Scholar]
- 3.Mould RF, Tai TH. Nasopharyngeal carcinoma: treatments and outcomes in the 20th century. Br J Radiol. 2002;75:307–39. doi: 10.1259/bjr.75.892.750307. [DOI] [PubMed] [Google Scholar]
- 4.Ayan I, Kaytan E, Ayan N. Childhood nasopharyngeal carcinoma: from biology to treatment. Lancet Oncol. 2003;4:13–21. doi: 10.1016/s1470-2045(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 5.Lee CC, Huang TT, Lee MS, et al. Clinical application of tumor volume in advanced nasopharyngeal carcinoma to predict outcome. Radiat Oncol. 2010;5:20. doi: 10.1186/1748-717X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper JS. Current and future therapy of nasopharyngeal cancer. Int J Radiat Oncol Biol Phys. 1997;37:973–4. doi: 10.1016/s0360-3016(97)00020-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang CC, Chen ML, Hsu KH, et al. Second malignant tumors in patients with nasopharyngeal carcinoma and their association with Epstein-Barr virus. Int J Cancer. 2000;87:228–31. doi: 10.1002/1097-0215(20000715)87:2<228::aid-ijc12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Louis CU, Paulino AC, Gottschalk S, et al. A Single Institution Experience With Pediatric Nasopharyngeal Carcinoma: High Incidence of Toxicity Associated With Platinum-based Chemotherapy Plus IMRT. J Pediatr Hematol Oncol. 2007;29:500–5. doi: 10.1097/MPH.0b013e3180959af4. [DOI] [PubMed] [Google Scholar]
- 9.Straathof KC, Bollard CM, Popat U, et al. Treatment of Nasopharyngeal Carcinoma with Epstein-Barr Virus-specific T Lymphocytes. Blood. 2005;105:1898–904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 10.Comoli P, Pedrazzoli P, Maccario R, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–9. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rooney CM, Smith CA, Ng CYC, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55. [PubMed] [Google Scholar]
- 14.Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T Lymphocyte Therapy for Epstein-Barr Virus+ Hodgkin's Disease. J Exp Med. 2004;200:1623–33. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Louis CU, Straathof K, Bollard CM, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009;113:2442–50. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottschalk S, Edwards OL, Sili U, et al. Generating CTL against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive Immunotherapy of EBV-associated malignancies. Blood. 2003;101:1905–12. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 18.Straathof KC, Leen AM, Buza EL, et al. Characterization of latent membrane protein 2 specificity in CTL lines from patients with EBV-positive nasopharyngeal carcinoma and lymphoma. J Immunol. 2005;175:4137–47. doi: 10.4049/jimmunol.175.6.4137. [DOI] [PubMed] [Google Scholar]
- 19.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 20.Khanna R, Burrows SR. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu Rev Microbiol. 2000;54:19–48. doi: 10.1146/annurev.micro.54.1.19. [DOI] [PubMed] [Google Scholar]
- 21.Chua DT, Sham JS, Leung LH, Au GK. Re-irradiation of nasopharyngeal carcinoma with intensity-modulated radiotherapy. Radiother Oncol. 2005;77:290–4. doi: 10.1016/j.radonc.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Chua DT, Sham JS, Au GK. Induction chemotherapy with cisplatin and gemcitabine followed by reirradiation for locally recurrent nasopharyngeal carcinoma. Am J Clin Oncol. 2005;28:464–71. doi: 10.1097/01.coc.0000180389.86104.68. [DOI] [PubMed] [Google Scholar]
- 23.Ngan RK, Yiu HH, Lau WH, et al. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol. 2002;13:1252–8. doi: 10.1093/annonc/mdf200. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy JS, Tannock IF, Degendorfer P, Panzarella T, Furlan M, Siu LL. A Phase II trial of docetaxel and cisplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. Oral Oncol. 2002;38:686–90. doi: 10.1016/s1368-8375(01)00134-8. [DOI] [PubMed] [Google Scholar]
- 25.Chua DT, Sham JS, Au GK. A phase II study of capecitabine in patients with recurrent and metastatic nasopharyngeal carcinoma pretreated with platinum-based chemotherapy. Oral Oncol. 2003;39:361–6. doi: 10.1016/s1368-8375(02)00120-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Zhang Y, Huang PY, Xu F, Peng PJ, Guan ZZ. Phase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum-based chemotherapy. Cancer Chemother Pharmacol. 2008;61:33–8. doi: 10.1007/s00280-007-0441-8. [DOI] [PubMed] [Google Scholar]
- 27.Poon D, Chowbay B, Cheung YB, Leong SS, Tan EH. Phase II study of irinotecan (CPT-11) as salvage therapy for advanced nasopharyngeal carcinoma. Cancer. 2005;103:576–81. doi: 10.1002/cncr.20802. [DOI] [PubMed] [Google Scholar]
- 28.Chan AT, Hsu MM, Goh BC, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005;23:3568–76. doi: 10.1200/JCO.2005.02.147. [DOI] [PubMed] [Google Scholar]
- 29.Chua DT, Wei WI, Wong MP, Sham JS, Nicholls J, Au GK. Phase II study of gefitinib for the treatment of recurrent and metastatic nasopharyngeal carcinoma. Head Neck. 2008;30:863–7. doi: 10.1002/hed.20792. [DOI] [PubMed] [Google Scholar]
- 30.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottschalk S, Ng CYC, Smith CA, et al. An Epstein-Barr virus deletion mutant that causes fatal lymphoproliferative disease unresponsive to virus-specific T cell therapy. Blood. 2001;97:835–43. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 32.Fogg MH, Wirth LJ, Posner M, Wang F. Decreased EBNA-1-specific CD8+ T cells in patients with Epstein-Barr virus-associated nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 2009;106:3318–23. doi: 10.1073/pnas.0813320106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bollard CM, Straathof KC, Huls MH, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother. 2004;27:317–27. doi: 10.1097/00002371-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Lutzky VP, Corban M, Heslop L, et al. A novel approach to the formulation of an EBV antigen-based nasopharyngeal carcinoma vaccine. J Virol. 2009 doi: 10.1128/JVI.01303-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110::2838–45. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng WH, Israel B, Raab-Traub N, Busson P, Kenney SC. Chemotherapy induces lytic EBV replication and confers ganciclovir susceptibility to EBV-positive epithelial cell tumors. Cancer Res. 2002;62:1920–6. [PubMed] [Google Scholar]
- 37.Feng WH, Kenney SC. Valproic acid enhances the efficacy of chemotherapy in EBV-positive tumors by increasing lytic viral gene expression. Cancer Res. 2006;66:8762–9. doi: 10.1158/0008-5472.CAN-06-1006. [DOI] [PubMed] [Google Scholar]
- 38.Chan AT, Tao Q, Robertson KD, et al. Azacitidine induces demethylation of the Epstein-Barr virus genome in tumors. J Clin Oncol. 2004;22:1373–81. doi: 10.1200/JCO.2004.04.185. [DOI] [PubMed] [Google Scholar]
- 39.Perrine SP, Hermine O, Small T, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood. 2007;109:2571–8. doi: 10.1182/blood-2006-01-024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.