Abstract
Freezing of gait (FOG) is one of the most disabling symptoms that affect patients with Parkinson's disease (PD). While the patho-physiology underlying FOG largely remains an enigma, several lines of evidence suggest that the autonomic nervous system might be involved. To this end, we tested the hypothesis that heart rate (HR) increases during FOG and, further, that HR increases just prior to FOG. To evaluate these hypotheses, fifteen healthy older adults, ten patients with PD who experienced FOG and ten patients who did not were studied. Patients with PD were tested during their “off” medication state. HR and HR variability were measured as subjects carried out tasks that frequently provoke FOG. 120 FOG episodes were evaluated. During FOG, HR increased (p=0.001), by an average of 1.8 bpm, as compared to HR measured before the beginning of FOG. HR also increased just prior to FOG, by 1 bpm (p<0.0001). In contrast, during sudden stops and 180° turns, HR decreased by almost 2 bpm (p<0.0001). HR variability was not associated with FOG. To our knowledge, these findings are the first to document the association of FOG to autonomic system activation, as manifested by HR dynamics. One explanation is that the changes in HR before and during FOG may be a sympathetic response, secondary to limbic activation, that contributes to the development of freezing. While further studies are needed to evaluate these associations, the present results provide experimental evidence linking impaired motor blockades to autonomic nervous system function among patients with PD.
Keywords: Parkinson's disease, gait, heart rate, autonomic nervous system, freezing of gait
INTRODUCTION
Freezing of gait (FOG) is a disabling, episodic gait disturbance that is common among patients with advanced Parkinson's disease (PD). FOG typically lasts a few seconds during which time the patient feels as if his or her feet are glued to the ground1-5. FOG is associated with falls and functional dependence1,3. For example, in a 12 month, prospective study, Latt et.al observed 2,160 falls among 113 patients with PD and reported that most occurred as a result of FOG6. 538 (25%) of the falls were injurious, highlighting the debilitating nature and the clinical importance of this symptom.
The pathogenesis underlying FOG has yet to be fully explained. Traditionally, FOG has been viewed as another motor symptom related to disease severity7-10. Indeed, a number of studies have identified changes in the gait patterns of patients with FOG that have been associated with its pathogenesis11-19. Evidence from several lines of reasoning suggests, however, that non-motor systems may be involved with FOG. In particular, one possibility is that the autonomic nervous system (ANS) may be activated during and perhaps just prior to FOG, reflecting a sympathetic response that exacerbates the risk of FOG or occurs in conjunction with FOG. The possibility of ANS involvement in FOG is consistent with several observations. While not all investigations have reported consistent results, a few studies have demonstrated that FOG can be alleviated with the administration of L-threo-DOPS20. This is a pre-cursor of norepineprhine, a neurotransmitter which has a major role in the sympathetic control over heart rate and blood pressure, among other things. Stress, anxiety, depression and cognitively challenging situations have been associated with FOG21-24. An increased frequency of FOG is also commonly seen during gait initiation, during locomotor transitions, and when patients hurry to pick up the phone, enter an elevator or door or cross the street1,9,21,22,24,25. Consistent with this, anecdotal reports1,3,22,26,27 have suggested that FOG may be related to anxiety, arousal, and the emotional state. One interpretation of all of these findings is the possibility that FOG may be related to ANS activation. Nonetheless, the nature of the relationship between FOG and the ANS has yet to be fully elucidated.
Given the putative associations between FOG, stress and the emotional state, we hypothesized that heart rate (HR), one of the most widely used markers of ANS activation, would increase during FOG episodes, i.e., as a reflection of the aroused state or secondary to sympathetic activation. We further speculated that HR would increase just prior to FOG. Theoretically, an increase in HR during FOG can be explained by sympathetic activation due to stress or increased anxiety, while an increase in HR before FOG is consistent with a possible role of the arousal system or the preparatory system in the pathogenesis of FOG. Such a finding would support of the hypothesis that sympathetic activation is one of the triggers that contribute to the occurrence of FOG. The goal of the present work was, therefore, to investigate whether FOG is indeed associated with changes in HR dynamics.
METHODS
Participants
Twenty patients with PD, as defined by the UK Brain Bank criteria, were recruited from the outpatient clinic of the Movement Disorders Unit of the Tel Aviv Sourasky Medical Center. Ten patients reported a history of FOG episodes (PD+FOG) and scored 2 or above on question 3 of the FOG-Questionnaire28,29 and 10 patients had no or few reported FOG episodes (PD-FOG). i.e., they answered 0 or 1 on question number 3 on the FOG-Questionnaire. All patients were tested in the “off” state, at least 12 hours after their last anti-parkinsonian medications intake. The control group included 15 healthy age-matched individuals. The healthy controls and the PD-FOG groups were included to serve as references for comparison of HR response to challenging locomotion conditions (e.g., sudden stops and turns, see below) and to evaluate whether observed HR changes are simply an expression of the generalized cardiovascular impairment seen in PD.
Participants were included if they were between 50-80 years of age, able to walk unaided during the “off” medication state, and had no history of other neurological orthopedic or musculoskeletal disorders that would likely impact their gait. Subjects were excluded if they scored less than 24 on the Mini-Mental State Examination (MMSE)30 or if they received any medication known to affect the ANS (e.g., anticholinergics, benzodiazepines, β blockers). To characterize the subjects, the following clinical measures were assessed: the Unified Parkinson Disease Rating Scale (UPDRS)31, disease duration, Hoehn & Yahr staging32, the FOG questionnaire (FOG-Q)28,29 and gait speed in comfortable and fast walking. The study was approved by the human studies committee of the Tel-Aviv Sourasky Medical Center. Informed written consent was obtained from all study participants.
Methods and Materials
An ambulatory monitor was used to record 2 electrocardiogram (ECG) leads at a frequency of 256Hz (Mobi8-2b6a, Twente Medical Systems International). Gait was measured using insoles with four pressure-sensitive sensors, inserted in each shoe and with a 3-D accelerometer that was attached to the lower back. The walking protocol (see below) was also recorded using videotape which was synchronized with the Mobi system to facilitate observational analysis of FOG.
An episode of freezing was defined as an unintentional interruption of gait in which the patient failed to make a normal and effective step forward. The FOG episode was considered to be finished when the participant took at least two consecutive normal steps3,9,24. Each FOG episode was identified by an examiner with extensive experience in the assessment of FOG using the video recording. Time annotations obtained from the video were then corroborated by examining times in which the distinct changes in the acceleration signal obtained from the anterior- posterior axis of 3D accelerometer occurred (i.e.; an increase in the high frequency components, as compared to regular locomotion)33-35.
Heart rate and heart rate variability were monitored using the Mobi under 4 conditions: 1) during 1 minute of comfortable walking, 2) during 1 minute of fast walking, 3) during 1 minute of lying while supine and, 4) during 1 minute of standing. The Mobi recorded the ECG signals (two orthogonal leads) continuously. Off-line, the QRS, the electrical activity related to the contraction of the heart's ventricular muscles and typically manifested by a large change in the ECG waveform, with a high signal to noise ratio, were automatically identified. The time difference between the peak of the QRS, denoted by R in the waveform and the next R peak (i.e., the R-R interval) was used to assess heart rate, on a beat-to-beat basis, and heart rate variability. The R peak timings were extracted from the raw ECG using a QRS detector, and the RR intervals were defined as the difference between the R peak timings. HR was defined as 60/RR intervals and HRV was defined as the coefficient of variation of the measured heart rate. All measures of HR were determined “blinded” to the determination of FOG.
Protocol
Subjects were assessed in the morning during the patient's “off” state. Participants were fitted with the monitoring system and then familiarized with the walking protocol. The protocol was designed to provoke FOG3,9,24 by including fast walks, turns (90°, 180°) and sudden stops in five different walking tasks: A) Walk for 1-minute at a self-selected pace along an 18 meter, straight corridor, B) Walk for 1-minute at a fast pace, as if they were trying to catch a bus, C) 5 walking trials at a self-selected pace that included 90° turns and 180° turns, with advanced knowledge of the requested action, and sudden instructed stops (total path length 200 meters). D) Same as in (C) but at a fast pace. E) Walk for 2 minutes in a circle (4 m diameter) while asked at random times, without advanced knowledge, to perform sharp, 180° turns in order to change the direction of the walk and 360° on the spot turns.
After the completion of the walking trials, the patients took their regular morning dose of levodopa. Once the patients reported that they reached their regular “on” state the full UPDRS31, MMSE30, and FOGQ28,29 were evaluated along with the evaluation of blood pressure while supine and after 2 minutes of standing.
Data Analysis
Heart rate measures were matched to the gait data collected during the gait conditions (A and B). Cardiovascular measures collected during the standing period were measured before starting the walking trials. The supine measurements were collected after the completion of the walking trials and after the patients took their regular morning dose of levodopa. Measurement duration while supine was 15 minutes, but the analysis was only performed on data from the 1 minute collected after 5 minutes of lying supine (i.e.; from minute 5 to 6) so that its duration would match that of the other conditions.
To control for different gait conditions (e.g., straight walking vs. turning) and since almost all of the FOG episodes occurred during turns, we included in this analysis only FOG episodes that occurred during turns. As there were no differences in the amount or type of FOG episodes during the comfortable vs. fast walks, the analysis of HR during FOG was collapsed across all walks without distinguishing between conditions. FOG episodes were divided depending on their duration; less than 3 seconds, 3-10 seconds and longer than 10 seconds, as per previous suggestions9,28. For each detected FOG episode, HR was examined in intervals of 3 and 10 seconds before and after the occurrence of FOG (see below). A 3 second window was used to allow for a more precise estimate of changes in HR, while allowing for a fairly robust estimate of HR (typically based on at least 3 beats). The interval of 10 seconds (localizing the FOG episodes in the middle of the interval) was used in order to also examine HRV. HR during FOG episodes that lasted more than 10 seconds were examined over the entire FOG duration.
In order to evaluate whether HR started to change prior to any FOG episode, we examined HR in the 3 seconds just prior to the initiation of each FOG episode and compared the measures obtained to the previous 3 seconds. The same analysis was done for intervals before sudden stops and 180° turns, i.e., control conditions without FOG, to assess how HR changes just prior to these conditions that share bio-mechanical properties (i.e., stopping) that are similar to FOG.
Statistical Analyses
General Linear Models (GLM) were used to investigate between and within group comparisons. Differences in HR between the four conditions (i.e., supine, standing, comfortable walking, fast walking) were examined within and between each group. Post hoc analysis was applied if significant between group differences were observed. HR before during and after FOG was compared using repeated measures ANOVA and post hoc, paired t-test was used for comparison of segments before, during and after FOG and before and during sudden stops and 180° turns. Results were considered significant if α = 0.05. Statistical analysis was performed using SPSS for Windows version 15.0.
RESULTS
As summarized in Table 1, no significant differences were found between the groups for demographic data (p>0.12), height and weight (not shown). Disease duration and total scores on the UPDRS tended to be higher in the PD+FOG patients, compared to PD-FOG patients, but UPDRS-motor scores and Hoehn and Yahr stage were similar in both PD groups. Gait speed was significantly lower in the patients with PD, compared to the healthy control group, during both comfortable and fast walking (p<0.01) but was not significantly different between the PD groups (with and without FOG) (p>0.214).
Table 1.
Characteristics of the three subject groups
| Statistical comparison (p-values) | |||||
|---|---|---|---|---|---|
| Control | PD-FOG | PD+FOG | Control vs. PD | PD-FOG vs. PD+FOG | |
| Age (yrs) | 68±9.4 | 67.3±6.5 | 65.9±6 | 0.597 | 0.623 |
| Gender (m/f) | 6/9 | 8/2 | 6/4 | 0.76 | 0.329 |
| MMSE | 29.5±0.5 | 28.8±1.2 | 28.1±1.2 | 0.005 | 0.245 |
| Disease duration | NA | 6.4±4.2 | 12.3±8.6 | NA | 0.069 |
| UPDRS-total | NA | 37.3±14.6 | 50.1±15.5 | NA | 0.074 |
| UPDRS-motor | NA | 18.9±8.2 | 19.5±7 | NA | 1.000 |
| Hoehn &Yahr Stage | NA | 2.7±0.5 | 3.0±0.6 | NA | 0.226 |
| FOG-Q Total | NA | 3.7±3.8 | 14.1±5.5 | NA | 0.001 |
| Comfortable Gait speed (m/s) | 1.01±0.04 | 0.86±0.03 | 0.83±0.07 | 0.009 | 0.777 |
| Fast Gait speed (m/s) | 1.48±0.05 | 1.23±0.07 | 1.09±0.07 | 0.0001 | 0.214 |
Entries are means ± SE; UPDRS: Unified Parkinson's Disease Rating Scale; FOG-Q: Freezing of Gait Questionnaire
Heart rate and Blood Pressure
As anticipated, differences were observed in HR measures in the four measurement conditions within all groups (p<0.0001), but there were no significant differences between the groups (see Table 2). Resting systolic and diastolic blood pressure (see Table 2) and changes in blood pressure in response to standing (data not shown) were not significantly different between the groups. Conversely, HRV was generally lower in the patients with PD, compared to the control group, during all four conditions (p<0.094), yet no differences were observed between PD-FOG and PD+FOG (p>0.728). HRV did not distinguish between the four conditions in any of the groups (p=0.980).
Table 2.
HR measurements in the three groups
| Statistical comparisons (p-values) | |||||
|---|---|---|---|---|---|
| Control | PD-FOG | PD+FOG | Control vs. PD | PD-FOG vs. PD+FOG | |
| HR Supine (bpm) | 67.8±2.7 | 63.6±3.3 | 75.4±3.9 | 0.671 | 0.071 |
| HR Standing (bpm) | 78.5±3 | 76.0±4.3 | 85.5±7.8 | 0.694 | 0.666 |
| HR comfortable walk (bpm) | 88.6±2.8 | 85.7±5.3 | 87.9±3.8 | 0.688 | 1.000 |
| HR fast walk (bpm) | 102.0±3.2 | 95.2±5.9 | 93.0±3.8 | 0.130 | 1.000 |
| HRV supine (%) | 5.9±1.5 | 2.3±0.5 | 2.6±0.6 | 0.019 | 1.000 |
| HRV standing (%) | 4.8±0.7 | 3.9±0.8 | 2.4±0.5 | 0.094 | 0.728 |
| HRV comfortable walk (%) | 4.2±0.4 | 2.6±0.4 | 3.4±1.0 | 0.078 | 1.000 |
| HRV fast walk (%) | 5.2±0.7 | 2.7±0.7 | 2.6±0.3 | 0.006 | 1.000 |
| Supine Systolic BP (mm Hg) | 130.6± 16.3 | 125.8± 18.11 | 128.2± 21.9 | 0.512 | 0.739 |
| Supine Diastolic BP (mm Hg) | 76.6± 9.1 | 76.8± 11.3 | 78.6± 9.7 | 0.788 | 0.638 |
Entries are means ± SE
FOG episodes
During the protocol, 120 FOG episodes were observed. 83% occurred during turns with 61% lasting 3 seconds or less. There was no difference in the number, the type or the duration of FOG episodes between usual and fast walking (p>0.403). It is important to note that most PD+FOG patients experienced at least 7 FOG episodes, i.e., the number of FOG episodes was fairly evenly distributed among the PD+FOG patients.
HR increase during and after FOG
Figure 1a shows an example of a FOG episode in which an increase in HR is apparent. This increase was a consistent finding, whether HR was examined over 10 second or 3 second intervals. In general, analysis of HR before, during and after a FOG episode demonstrated significant differences between the measurement periods (p=0.001) (see Figure 2). HR increased during FOG episodes, compared to HR before FOG (p=0.002). When averaging HR over a 10 second window, HR increased by an average of 1.8 ± 0.2 bpm (see Figure 2), compared to the HR before FOG (p=0.001). Analysis of 3 seconds intervals demonstrated a similar pattern; HR increased by an average of 0.8 ± 0.2 bpm, compared to HR before FOG (p=0.001). In 83% of the FOG episodes, HR increased during FOG. After FOG, HR tended to decrease, but it remained higher then HR measured prior to FOG (p<0.0001) (Figure 1). Conversely, HRV was not significantly different before, during or after FOG (p=0.722).
Figure 1a.
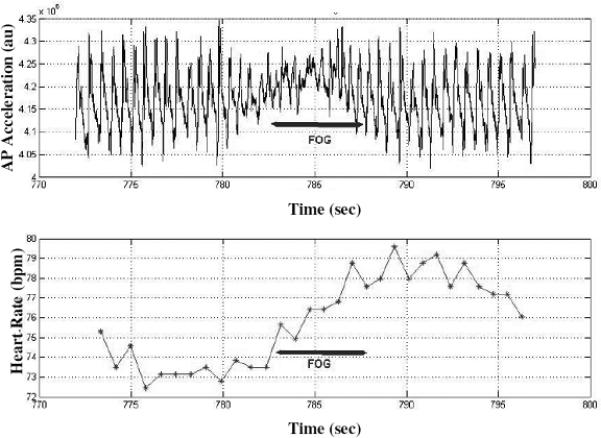
Data recorded from one patient with PD and FOG during half a minute of walking. The upper trace depicts an acceleration time series from the anterior-posterior axis of a 3 dimensional accelerometer. Between 782-787 seconds, the rhythmic pattern of the acceleration disappears and evidence for “freezing” can be seen; the time during which FOG was observed from the video are indicated by the horizontal arrows. In the lower trace, an increase in HR can be observed during FOG.
Figure 2.
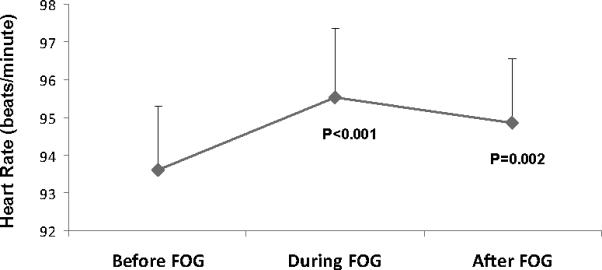
Heart rate (HR) before, during, and after FOG episodes. HR significantly increased during FOG, compared to HR before. The HR increase, relative to pre-FOG HR, was also seen immediately after the cessation of the FOG episodes. These results are based on the analysis of 10 second windows; similar results were obtained using 3 second windows.
HR changes during turns and stops
When the subjects (from all groups) were requested to suddenly stop walking (i.e., a sudden stop, in some ways similar bio-mechanically to FOG), there was a significant decrease in HR (p<0.0001), based on a 3 second analysis window. During sudden stops without FOG, HR decreased by 1.4±1.0 bpm (p=0.177) in the PD+FOG group, by 3.2±0.2 bpm (p<0.001) in the PD-FOG group, and by 2.0±0.8 bpm (p<0.017) in the healthy controls. Similarly, HR during 180° turns without FOG decreased in all three groups: by 0.6±0.2 bpm (p=0.044) in the PD+FOG group, by 3.2±0.2 bpm (p<0.0001) in the PD-FOG group, and by 2.7±0.2 bpm (p<0.0001) in the healthy controls. This finding is in contrast to the increase in HR observed during turns with FOG episodes (see Figure 3).
Figure 3.
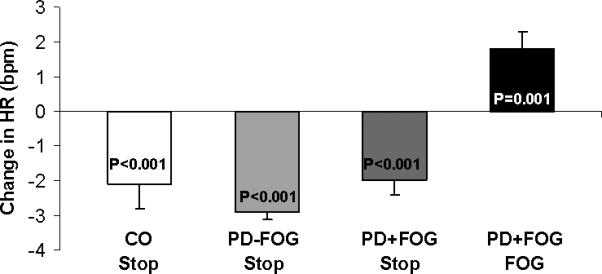
Change in heart rate average during FOG episodes and during sudden stops. In healthy controls (CO), in PD-FOG patients, and in PD+FOG patients, heart rate decreased during sudden stops; in each group, there was a mean decrease of 1 bpm during the stops. In contrast, during FOG, there was an increase of 1.8 bpm during FOG. These results are based on the analysis of 10 second windows.
HR Changes before FOG
Comparison between the two consecutive 3 second periods just prior to FOG demonstrated an increase of 0.7 ± 0.2 bpm in HR in the interval closer to the initiation of FOG, compared to the interval that occurred earlier (p<0.0001). In fact, an increase in HR was observed prior to 75% of the FOG episodes. Again, this result was generally observed in all of the PD+FOG patients. In contrast, the same analysis for the two consecutive 3 second periods just prior to instructed sudden stops and 180° turns demonstrated no change in HR between the two segments in any of groups (p>0.10). In other words, HR tended to increase before FOG, but not before instructed sudden stops or 180° when FOG did not occur.
DISCUSSION
The overall objective of the present study was to examine the possibility that the ANS is activated in conjunction with FOG in patients with PD. Specifically, we evaluated HR dynamics since this is a commonly used measure of stress and since an aroused state is known to affect cardiovascular reactivity by way of an increase in sympathetic cardiac control, a decrease in parasympathetic control, or both. HR increased by almost 2 bpm during FOG. In addition, we observed a small, but consistent increase in HR just prior to FOG. In other words, HR generally increased just before FOG and it increased further during FOG.
The magnitude of the observed increases in HR during FOG (about 2 bpm) is fairly small. Still, this small, but significant increase is very similar to that seen when patients with FOG go from standing to walking (recall Table 2). Thus, in some sense, the cardiovascular response to FOG is similar to that of the physiologic response to walking in these patients. Although significant group differences were not observed, it appears as if the cardiovascular reactivity in response to a physiologic challenge, at least to walking, is somewhat blunted in the PD+FOG patients, suggesting perhaps that the small change in HR during FOG may actually underestimate the underlying stress. In this regard, it is also important to keep in mind the contrast between the increase in HR during FOG and the decrease in HR seen when turning or when walking was interrupted not as a result of FOG, i.e., by spontaneous stops. Although the bio-mechanics and the motor function involved in stopping and FOG are not 100% identical, the comparison of FOG to non-FOG stops is a reasonable starting point for identifying whether heart rate typically changes when a patient with PD comes to a stop. The physiologic demands, as a first approximation, are similar in both conditions. During FOG, sudden stops, and turns, there is a decrease in gait velocity that should, from the perspective of energy costs, be accompanied by a decrease in HR. Indeed, this decrease was observed during sudden stops and turns in all subject groups, whereas HR increased during FOG. This contrast between the HR behavior during FOG, on the one hand, and during stops and turns, on the other, demonstrates that these events have different effects on reciprocal sympathetic activation and vagal withdrawal in patients with PD who suffer from FOG. Apparently, FOG is associated with some form of ANS activation (perhaps secondary to mental stress or arousal), rather than just changes in the neuro-muscular state.
The small increase in HR observed just prior to FOG, but not before sudden stops and 180° turns, also supports the less intuitive possibility that ANS activity might even contribute to and cause FOG and it is not merely a response to FOG. Still, this increase in HR could be explained in several ways. Several reports have documented that the gait pattern sometimes deteriorates just prior to the FOG episode12,13,18. Thus, the observed decrease in HR already before FOG onset might reflect the subtle changes in gait that are taking place before the complete motor blockade occurs. In other words, according to this possibility, the changes in HR are a reaction to these motor alterations and not driving them. The present study was not designed to fully address this question. Since most of the FOG episodes occurred during turning when the gait pattern has already deviated from that of steady state, straight line walking, we were not able to determine if the HR changes preceded any gait changes. Nonetheless, we can rule out the possibility that a festinating gait – sometimes viewed as an antecedent to FOG - preceded FOG events. Gait festination was never observed, perhaps because we focused on FOG that occurred during turns. Therefore, we would suggest that festination was not a cause of the changes in heart rate observed before FOG. In our view, the fact that the pre-FOG HR changes might coincide with the pre-FOG gait changes strengthens the notion that autonomic modulation is, to some extent, related to the occurrence of FOG and that changes in ANS might be contributing to and not just following the changes in the gait pattern.
As alluded to above, in general, the present findings can be interpreted in at least two ways. 1. The increase in HR is a response to the arousal and emotional change taking place during FOG, i.e., it is a reaction to the abnormal motor state. 2. The increase in HR reflects the potential role of the ANS in the pathogenesis of FOG. Of course, both possibilities could co-exist. Aspects of this response pattern manifested by an increase in HR during stress have been reported for numerous stressors including, for example, acute laboratory psychological/cognitive stressors such as mental arithmetic or speech and real-life acute stressors such as testing36. Perhaps, turning, time limits, cognitive loading and start initiation, events associated with FOG21,22,24, are interpreted as stressful situations that lead to a form of arousal or emotional stress, and hence sympathetic activation, among patients with FOG.
This latter possibility needs to be reconciled with one of the enigmas surrounding FOG: the fact that often patients who suffer from this symptom at-home do not experience it in the clinic or laboratory3,5, when patients are likely to be under somewhat increased stress (i.e., the white coat syndrome). One explanation that was proposed to explain the relative absence of FOG in the clinic is that patients utilize more attentional resources in the clinic and during experiments in which they have become the center of the attention3,22. Perhaps changes in the relationship between stress, attention and other factors might explain how stress can play a role in FOG, even though FOG is sometimes alleviated in stressful situations like the clinic. The changes in heart rate that we observed are consistent with other reports that have suggested that stress involves autonomic responses and stress may play a role in the initiation of freezing. Clearly, however, this is not the only factor.
A more intriguing understanding of the observed results is that HR changes just prior to FOG combine with the abnormal gait patterns previously associated with FOG11-18 to increase the likelihood that FOG will occur. This small, but highly consistent increase in HR several seconds prior to FOG suggests that this so-called sudden, episodic event actually evolves over a relatively long time (i.e., a few seconds). If we are able to more completely identify all of the processes that lead to FOG, perhaps we will be able to arrest or prevent this abnormal cessation of gait.
The specific relationships between the ANS and FOG remain to be clarified. It seems possible, however, that primary or secondary ANS activation might contribute to FOG, perhaps through higher neurobehavioral systems via the limbic system22,36-38. Although simple fear conditions can be established and maintained largely by subcortical structures such as the amygdale36, more generalized stress states entail an attentional focus on threat-related cues together with a response bias that likely depends on higher-level cortical/cognitive process39. Perhaps PD patients, who frequently experience FOG while turning or crossing the street, feel threatened by the situation. This fear condition may be initiated even before the onset of the turn or crossing the street. In this regard, one can speculate that cortical regions that have been implicated in anxiety and the areas that have been shown to have direct monosynaptic projections to brainstem autonomic centers and source nuclei, such as the medial prefrontal cortex39,40 may be activated just prior to and during the FOG episodes. This possibility is consistent with a recent patho-physiological model of FOG in PD in which limbic system alterations and their effect on basal ganglia circuitry is one of the keys to FOG occurrence41. Involvement of higher-level cortical/cognitive processes in altering autonomic branch activity and the involvement of limbic system in provoking FOG episodes might partially explain the role of different arousal and emotional states in causing FOG.
ANS activation appears to be associated with FOG in either a cause or effect (i.e., response) relationship. Thus, one can speculate that eliminating or minimizing emotional and arousal changes during conditions associated with FOG may reduce FOG intensity, frequency and duration. Perhaps if patients can learn to better cope with FOG or if they are trained with auditory or visual cues or psychological tools to deal with stress related to different situations, FOG propensity may be reduced. Alternatively, selective pharmacological blockades of the autonomic branches to reduce autonomic excitation, especially of higher neurobehavioral areas, may perhaps reduce the predisposition to FOG. Pharmacologic studies that test this idea will likely provide additional insight into the putative cause and effect relationship between ANS activation and FOG.
Additional studies are needed to further elucidate the origins of the changes in HR during and apparently just prior to FOG and the role of arousal and the emotional state. There are a number of different ways to interpret the present findings and future work is needed to definitively explain the observed results. Competing hypotheses could explain the observed HR dynamics in patients with FOG. Perhaps, future study of HR during upper extremity tasks or other manual tasks that are challenging to patients with PD or patients with freezing more specifically10 can be used to test these hypotheses. In addition, it might be helpful to measure gait speed, HR and FOG, and perhaps other measures of autonomic function, on a continuous basis to study how HR and ANS function changes with FOG with a higher degree of time resolution, perhaps in straight line situations where it will be easier to identify changes in the gait pattern just prior to FOG. In the mean time, as far as we know, this report is the first to quantitatively measure HR measured before and during FOG and to document changes associated with FOG. Thus, we anticipate that the present findings will motivate additional studies that can more fully address the questions that were raised and, in this way, it will help to move the field forward in a search for a more complete understanding of the role of ANS activation in an episodic motor phenomenon that has debilitating consequences for many patients with PD.
Figure 1b.
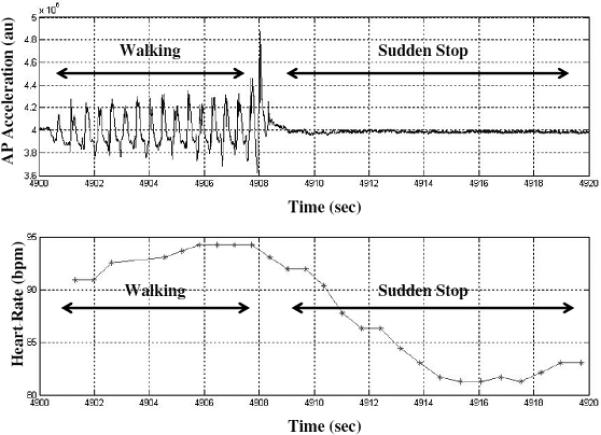
Data recorded from one patient with PD and FOG during an instructed sudden stop. As in Figure 1a, the upper trace depicts acceleration time series from the anterior-posterior axis of a 3 dimensional accelerometer. Between 4908-4920 seconds, the rhythmic pattern of the acceleration becomes flat, consistent with the absence of walking. In the lower trace, a decrease in HR can be observed during the sudden stop. Contrast this to Figure 1a.
ACKNOWLEDGEMENTS
We thank the participants for their time and effort.
Financial disclosure related to research covered in this article
This work was supported in part by the European Commission in the context of FP6 projects DAPHNet, fet-018474-2, and SENSACTION-AAL, infso-ist-045622 and by the National Parkinson Foundation. The funding agencies were not involved with data collection, data analysis, or manuscript preparation. The authors have no competing interests to disclose.
Full financial disclosure for the previous 12 months
I Maidan, M Plotnik, A Mirelman, A Wess and JM Hausdorff were supported in part by the European Commission in the context of FP6 projects DAPHNet, fet-018474-2, and SENSACTION-AAL, infso-ist-045622 and by the National Parkinson Foundation. JM Hausdorff and A Mirelman were supported in part by the Michael J Fox Foundation for Parkinson's Research; M Plotnik and JM Hausdorff were funded in part by the Israeli Ministry of Veteran Affairs; and JM Hausdorff was funded in part by the National Institutes on Health and the Israel Science Foundation.
REFERENCES
- 1.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 2.Giladi N, McDermott MP, Fahn S, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56:1712–1721. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwboer A, Giladi N. The challenge of evaluating freezing of gait in patients with Parkinson's disease. Br J Neurosurg. 2008;22(Suppl 1):S16–S18. doi: 10.1080/02688690802448376. [DOI] [PubMed] [Google Scholar]
- 4.Okuma Y. Freezing of gait in Parkinson's disease. J Neurol. 2006;253(Suppl 7):VII27–VII32. doi: 10.1007/s00415-006-7007-2. [DOI] [PubMed] [Google Scholar]
- 5.Okuma Y, Yanagisawa N. The clinical spectrum of freezing of gait in Parkinson's disease. Mov Disord. 2008;23(Suppl 2):S426–S430. doi: 10.1002/mds.21934. [DOI] [PubMed] [Google Scholar]
- 6.Latt MD, Lord SR, Morris JG, Fung VS. Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. Mov Disord. 2009;24:1280–1289. doi: 10.1002/mds.22561. [DOI] [PubMed] [Google Scholar]
- 7.Bartels AL, Balash Y, Gurevich T, et al. Relationship between freezing of gait (FOG) and other features of Parkinson's: FOG is not correlated with bradykinesia. J Clin Neurosci. 2003;10:584–588. doi: 10.1016/s0967-5868(03)00192-9. [DOI] [PubMed] [Google Scholar]
- 8.Giladi N, Treves TA, Simon ES, et al. Freezing of gait in patients with advanced Parkinson's disease. J Neural Transm. 2001;108:53–61. doi: 10.1007/s007020170096. [DOI] [PubMed] [Google Scholar]
- 9.Schaafsma JD, Balash Y, Gurevich T, et al. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease. Eur J Neurol. 2003;10:391–398. doi: 10.1046/j.1468-1331.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwboer A, Vercruysse S, Feys P, et al. Upper limb movement interruptions are correlated to freezing of gait in Parkinson's disease. Eur J Neurosci. 2009;29:1422–1430. doi: 10.1111/j.1460-9568.2009.06681.x. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorff JM, Schaafsma JD, Balash Y, et al. Impaired regulation of stride variability in Parkinson's disease subjects with freezing of gait 8. Exp Brain Res. 2003;149:187–194. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- 12.Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing in Parkinson's disease and the stride length sequence effect interaction. Brain. 2009;132:2151–2160. doi: 10.1093/brain/awp053. [DOI] [PubMed] [Google Scholar]
- 13.Nieuwboer A, Dom R, De WW, et al. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson's disease. Mov Disord. 2001;16:1066–1075. doi: 10.1002/mds.1206. [DOI] [PubMed] [Google Scholar]
- 14.Nieuwboer A, Dom R, De WW, et al. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson's disease. Brain. 2004;127:1650–1660. doi: 10.1093/brain/awh189. [DOI] [PubMed] [Google Scholar]
- 15.Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson's disease related to asymmetric motor function? Ann Neurol. 2005;57:656–663. doi: 10.1002/ana.20452. [DOI] [PubMed] [Google Scholar]
- 16.Plotnik M, Hausdorff JM. The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson's disease. Mov Disord. 2008;23(Suppl 2):S444–S450. doi: 10.1002/mds.21984. [DOI] [PubMed] [Google Scholar]
- 17.Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of walking and freezing of gait in Parkinson's disease. Eur J Neurosci. 2008;27:1999–2006. doi: 10.1111/j.1460-9568.2008.06167.x. [DOI] [PubMed] [Google Scholar]
- 18.Iansek R, Huxham F, McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord. 2006;21:1419–1424. doi: 10.1002/mds.20998. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oribe E, Kaufman H, Yahr M. Freezing phenomena in Parkinson's disease: Clinical features and effect of treatment with L-threo-DOPS. In: Narabayashi H, editor. Norepinephrine Deficiency and Its Treatment with L-threo DOPS in Parkinson's Disease and the Related Disorders. Publishing Group; New-York: 1993. pp. 89–96. eal. [Google Scholar]
- 21.Amboni M, Cozzolino A, Longo K, Picillo M, Barone P. Freezing of gait and executive functions in patients with Parkinson's disease. Mov Disord. 2008;23:395–400. doi: 10.1002/mds.21850. [DOI] [PubMed] [Google Scholar]
- 22.Giladi N, Hausdorff JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson's disease. J Neurol Sci. 2006;248:173–176. doi: 10.1016/j.jns.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Maruyamaa T, Yanagisawab N. Cognitive impact on freezing of gait in Parkinson's disease. Parkinsonism and Related Disorders. 2006;12:S77–S82. [Google Scholar]
- 24.Moreau C, Defebvre L, Bleuse S, et al. Externally provoked freezing of gait in open runways in advanced Parkinson's disease results from motor and mental collapse. J Neural Transm. 2008;115:1431–1436. doi: 10.1007/s00702-008-0099-3. [DOI] [PubMed] [Google Scholar]
- 25.Almeida QJ, Lebold CA. Freezing of gait in parkinson's disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry. 2009 doi: 10.1136/jnnp.2008.160580. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman A. Are freezing of gait (FOG) and panic related? J Neurol Sci. 2006;248:219–222. doi: 10.1016/j.jns.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. The factors that induce or overcome freezing of gait in Parkinson's disease. Behav Neurol. 2008;19:127–136. doi: 10.1155/2008/456298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giladi N, Shabtai H, Simon ES, et al. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000;6:165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 29.Giladi N, Tal J, Azulay T, et al. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov Disord. 2009;24:655–661. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Fahn S, Elton RL, Members of the UPDRS Development Committee Unified Parkinson's Disease Rating Scale. Macmillan Health Care Information. 1987;2:153–164. [Google Scholar]
- 32.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 33.Bachlin M, Plotnik M, Roggen D, et al. A wearable system to assist walking of Parkinson s disease patients. Methods Inf Med. 2010;49:88–95. doi: 10.3414/ME09-02-0003. [DOI] [PubMed] [Google Scholar]
- 34.Hausdorff JM, Balash Y, Giladi N. Time series analysis of leg movements during freezing of gait in Parkinson's disease: akinesia, rhyme or reason? Physica A-Statistical Mechanics and Its Applications. 2003;321:565–570. [Google Scholar]
- 35.Moore ST, MacDougall HG, Ondo WG. Ambulatory monitoring of freezing of gait in Parkinson's disease. J Neurosci Methods. 2008;167:340–348. doi: 10.1016/j.jneumeth.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Fechir M, Schlereth T, Purat T, et al. Patterns of sympathetic responses induced by different stress tasks. Open Neurol J. 2008;2:25–31. doi: 10.2174/1874205X00802010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchholz K, Schachinger H, Wagner M, Sharma AM, Deter HC. Reduced vagal activity in salt-sensitive subjects during mental challenge. Am J Hypertens. 2003;16:531–536. doi: 10.1016/s0895-7061(03)00905-1. [DOI] [PubMed] [Google Scholar]
- 38.Philippsen C, Hahn M, Schwabe L, et al. Cardiovascular reactivity to mental stress is not affected by alpha2-adrenoreceptor activation or inhibition. Psychopharmacology (Berl) 2007;190:181–188. doi: 10.1007/s00213-006-0597-7. [DOI] [PubMed] [Google Scholar]
- 39.Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav Brain Res. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 40.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 41.Lewis SJ, Barker RA. A pathophysiological model of freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. 2009;15:333–338. doi: 10.1016/j.parkreldis.2008.08.006. [DOI] [PubMed] [Google Scholar]


