Abstract
Sphingosine-1-phosphate (S1P) is a sphingolipid signaling molecule crucial for cell survival and proliferation. S1P-mediated signaling is largely controlled through its biosynthesis and degradation, and S1P lyase (S1PL) is the only known enzyme, which irreversibly degrades sphingoid base-1-phosphates to phosphoethanolamine and the corresponding fatty aldehydes. S1PL-mediated degradation of S1P results in the formation of (2E)-hexadecenal, while hexadecanal is the product of dihydrosphingosine-1-phosphate (DHS1P) degradation. Fatty aldehydes can undergo biotransformation to fatty acids and/or alcohols, which makes them elusive and renders the task of fatty aldehyde quantitation challenging. We have developed a simple, highly sensitive, and high-throughput protocol for (2E)-hexadecenal quantitation as a semicarbazone derivative by liquid chromatography-electrospray ionization-tandem mass spectrometry. The approach was applied to determining S1PL activity in vitro, with the ability to use as low as 0.25 µg microsomal protein per assay. The method is also applicable to the use of total tissue homogenate as the source of S1PL. A correction for (2E)-hexadecenal disappearance due to its biotransformation during enzymatic reaction is required, especially at higher protein concentrations. The method was applied to confirm FTY720 as the inhibitor of S1PL with the IC50 of 52.4 µM.
Keywords: S1P lyase, Sphingosine-1-phosphate, (2E)-Hexadecenal, (2E)-Hexadecenal semicarbazone, FTY720
Introduction
Sphingosine-1-phosphate (S1P) is a sphingolipid signaling molecule crucial for cell survival and proliferation [1,2]. S1P-mediated signaling is largely controlled through its biosynthesis and degradation, and S1P lyase (S1PL) is the key enzyme in the regulation of S1P and sphingolipid homeostasis. The importance of S1PL for mammalian physiology has been revealed only recently. It was shown that the abnormal expression of S1PL is associated with cancer development, with the resistance to anticancer therapies, and with the developmental pathologies [3,4]. In the immune system, by regulating circulatory S1P level, S1PL contributes to the control of lymphocyte egress from lymph nodes [5], and is now considered as a promising target to treat inflammatory disorders [3].
S1PL is the only enzyme that irreversibly degrades sphingoid base-1-phosphates to phosphoethanolamine and the corresponding fatty aldehydes, thus eliminating sphingoid bases from the total pool of sphingolipids [6]. By degrading S1P, S1PL controls S1P-mediated signaling and also generates metabolites that may elicit potent physiological responses [7]. S1PL degrades both S1P and its endogenous analog dihydro-S1P (DHS1P). S1PL-mediated cleavage of S1P results in the formation of (2E)-hexadecenal while hexadecanal is the product of DHS1P degradation. Fatty aldehydes are reactive and can undergo further biotransformation to fatty acids and/or alcohols [8], making them elusive and rendering the task of fatty aldehyde quantitation and characterization of S1PL activity challenging. Two approaches have been used for characterization of S1PL activity in biological specimens. One is based on radiometric quantitation of the radioactive products of [4,5-3H]DHS1P degradation after separation by TLC [8]. The other approach is based on the use of fluorescently labeled (BODIPY- or NBD-) S1P, HPLC separation, and quantitation of the corresponding BODIPY- or NBD-labeled aldehydes [9,10]. There are notable disadvantages of each method, such as the use of a radioactive substrate or the complicated HPLC profile of fluorescently labeled products of the S1PL reaction; furthermore, neither method allows monitoring of the endogenous levels of (2E)-hexadecenal. Most importantly, neither method allows exhaustive correction for the ongoing degradation/bioconversion of the (2E)-hexadecenal formed during the reaction. In the current study, we take advantage of the high sensitivity of liquid chromatography-electrospray ionization-tandem mass spectrometery (ESI-LC/MS/MS) and use of commercially available internal standard for (2E)-hexadecenal quantitation, d5-labeled (2E)-hexadecenal, to develop a simple, sensitive, and high-throughput protocol for characterization of S1PL activity in vitro based on isotope dilution quantitation of (2E)-hexadecenal as a semicarbazone derivative.
Materials and methods
Reagents
Methanol, water, and acetonitrile (LC/MS grade) were purchased from Fisher (Pittsburgh, PA, USA). (2S,3R)-1,3-Dihydroxy-2-amino-4E-octadecene-1-phosphate (S1P) and (2E)-hexadecanal-(15,15,16,16,16-d5) were obtained from Avanti Polar Lipids (Alabaster, AL, USA). (2E)-Hexadecenal was made by Horner-Wadsworth-Emmons olefination of myristaldehyde with triethyl phosphonoacetate, followed by alane reduction of the α,β-unsaturated ester [11] and oxidation of the resulting primary alcohol with pyridinium chlorochromate in dichloromethane. FTY720 and (S)-FTY720-phosphate (FTY720-P) were synthesized as described in [12]. cis-11-Hexadecenal was purchased from Sigma (St. Louis, MO, USA).
Preparation of fatty aldehyde semicarbazone derivatives
Hexadecanal, cis-11-hexadecenal, and (2E)-hexadecenal, pure or as part of the total lipid extract, were converted to the corresponding semicarbazone derivatives by heating with 0.2 ml of 5 mM semicarbazide hydrochloride in methanol containing 5% formic acid at 40°C for 2 h. The derivatives were directly subjected to MS/MS or LC/MS/MS analyses. To study the effect of acid concentration on the effectiveness of derivatization, the reactions were performed in the presence of varying (0–5%) concentration of formic acid in methanol.
Hydrogenation of unsaturated aldehydes
(2E)-Hexadecenal and cis-11-hexadecenal were converted to hexadecanal by hydrogenation over ~1 mg platinum oxide in 1 ml of methanol for 1 h. After the catalyst was removed by centrifugation, hexadecanal was analyzed as its semicarbazone derivative by MS. The completeness of hydrogenation was confirmed by ESI-MS/MS by the absence of the 2- or 11-hexadecenal semicarbazone [M+H]+ ion.
ESI-LC/MS/MS analysis of fatty aldehyde semicarbazone derivatives
An API-4000 Q-trap triple quadrupole ion trap mass spectrometer (Applied Biosystems, Foster City, CA, USA) interfaced with an Agilent 1100 liquid chromatograph with autosampler (Agilent Technologies, Wilmington, DE, USA) was employed for ESI-LC/MS/MS analysis of the aldehyde semicarbazone derivatives. Positive ion ESI-LC/MS/MS was employed for detection of the fatty aldehyde semicarbazone derivatives. The ion source conditions and gas settings for positive ESI-LC/MS/MS analysis were as follows: ion spray voltage = 5500V, ion source heater temperature = 550°C, collision gas setting = 4, ion source gases 1 and 2 settings = 50, curtain gas setting = 30. Three MRM transitions were monitored for detection of (2E)-hexadecenal semicarbazone and cis-11-hexadecenal semicarbazone - m/z 296/279, m/z 296/253, and m/z 296/97. The monitored MRM transitions for hexadecanal semicarbazone derivative were m/z 298/281, m/z 298/255, and 298/238. The MRM transitions for the internal standard d5-(2E)-hexadecenal semicarbazone were m/z 301/284, 301/258, and 301/79. The optimized parameters for (2E)-hexadecenal semicarbazone and d5-(2E)-hexadecenal semicarbazone positive ion ESI LC/MS/MS analysis were as follows: declustering potential = 90V, collision energy (CE) for the m/z 296/279 and 301/284 transitions = 23V with collision cell exit potential (CXP) = 8V; CE for m/z 296/253 and 301/258 transitions = 21V with CXP = 8V; CE for m/z 296/79 and m/z 301/79 transitions = 33V with CXP = 6V. Chromatographic separation of semicarbazone derivatives of (2E)-hexadecenal, cis-11-hexadecenal, and hexadecanal was achieved by using a Discovery C18 column (50 × 2.1 mm, 5 µm particle size) from Supelco (Bellefonte, PA, USA) and a gradient elution program in which methanol, water, and formic acid (60:40:0.5, v/v) containing 5 mM ammonium formate was used as solvent A, and acetonitrile:chloroform:water:formic acid (90:10:0.5:0.5, v/v) containing 5 mM ammonium formate was used as solvent B. The elution protocol was composed of a 2-min column equilibration with 100% solvent A at a flow rate of 0.5 ml/min, followed by sample injection in methanol, a 0.5 min period with 100% solvent A at a flow rate of 0.5 ml/min, a 2.5-min linear gradient to 100% solvent B and 0.425 ml/min flow rate, a 3-min period with 100% solvent B at a flow rate of 0.425 ml/min, and a 0.75-min linear gradient to 100% solvent A and a flow rate of 0.5 ml/min. The program included three cyclic needle washes consisting of duplicate needle washes per cycle prior to sample injection.
Tissue homogenization and S1P lyase reaction
Frozen liver tissue was homogenized on ice in the tissue lysis buffer consisting of 5 mM MOPS, 1 mM EDTA, 0.25 M sucrose, 1 mM PMSF, 10% (v/v) glycerol, pH 7.4. Cell debris was removed by low speed (1,000 × g for 5 min) centrifugation, and the supernatant was used as a total tissue homogenate. To isolate the microsomal fraction, the total tissue homogenate was centrifuged at 10,000 × g for 15 min, and the supernatant was subjected to 100,000 × g centrifugation for 1 h. The resulting supernatant was used as a cytosolic fraction. The membrane fraction was gently dispersed in the lysis buffer using tip sonication and stored at −80°C until use.
Protein concentrations were determined using the Pierce bicinchoninic acid (BCA) reagent (ThermoFisher Scientific, Rockford, IL) and adjusted to 0.025 mg/ml with tissue lysis buffer (up to 0.25 mg/ml in some experiments). The S1PL reaction was initiated by mixing 0.025 ml of 0.4 mM S1P in 1% Triton X-100 in water, 0.175 ml of reaction buffer (35 mM potassium phosphate buffer, pH 7.4, 0.6 mM EDTA, 70 mM sucrose, 36 mM sodium fluoride, 0.57 mM pyridoxal-5’-phosphate (P5P), and 0.05 ml of protein preparation in lysis buffer (5 µg protein in the standard reaction, and up to 50 µg protein in experiments where the rate of (2E)-hexadecenal biotransformation was determined) in 8-ml glass screw-capped tubes. The reaction was performed for 20 min at 37°C, and was stopped by the addition of 2 ml of methanol. The internal standard ((2E)-d5-hexadecenal, 20 pmol) and chloroform (2 ml) were added, the tubes were vortexed, and phase separation was initiated by the addition of 1.55 ml of 0.9% KCl in water. After the tubes were extensively vortexed, the chloroform phase was separated by centrifugation, the solvent was evaporated by a stream of nitrogen, and aldehyde semicarbazone derivatives were obtained as described above.
Results and discussion
ESI-MS/MS analysis of fatty aldehydes as semicarbazone derivatives
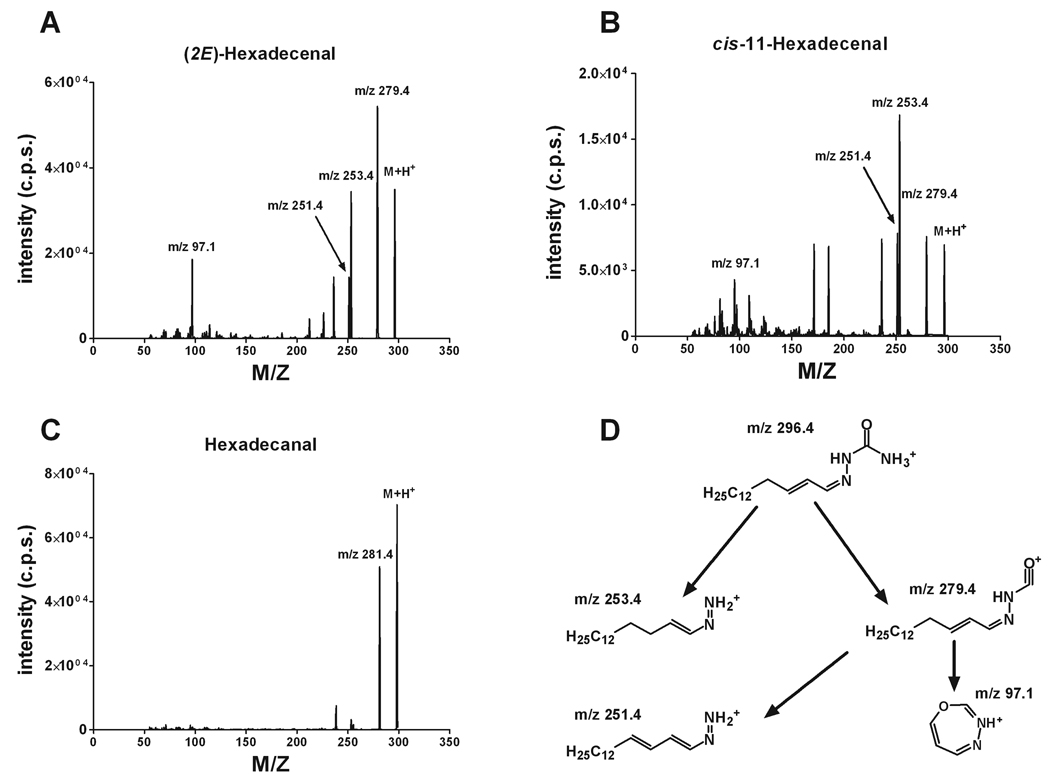
Fatty aldehydes are poorly ionized by themselves but can be easily modified into derivatives with strong ionization properties. Semicarbazide is one of several possible reagents that will provide a derivative with a strong molecular ion in positive ion mode during ESI (Fig. 1). The semicarbazones of (2E)-hexadecenal, cis-11-hexadecenal, and hexadecanal produced strong molecular ions at m/z 296, m/z 296, and m/z 298, respectively. The study of the product ion formation during collision-induced decomposition (CID) revealed a substantial difference in the breakdown pattern of these aldehyde derivatives (Figure 2A–C). Thus, both (2E)-hexadecenal and cis-11-hexadecenal derivatives produced major products at m/z 279, m/z 253, m/z 251, and m/z 97. However, (2E)-hexadecenal semicarbazone gave ions at m/z 279, m/z 253, and m/z 97 with almost equal output when CID conditions were optimized for the maximal generation of each product ion, whereas the cis-11-hexadecenal semicarbazone produced a minimal amount of the ion at m/z 97. Hexadecanal semicarbazone had the only major product ion at m/z 281 and did not form a product ion at m/z 97 (Figure 2C). The study of the ion precursor-product relationships for (2E)-hexadecenal semicarbazone revealed a direct relationship between ions at m/z 97 and m/z 279; the latter also produced the ion at m/z 251 while the ion at m/z 253 was formed directly from the molecular ion. Suggested ion structures and their relationships are shown in the Figure 2D. The fact that cis-11-hexadecenal semicarbazone also produced a minimal amount of the ion with m/z 97 indicates that this ion formation is a function of the presence and position of the double bond in the aliphatic chain, with a Δ2-position providing maximal ion output.
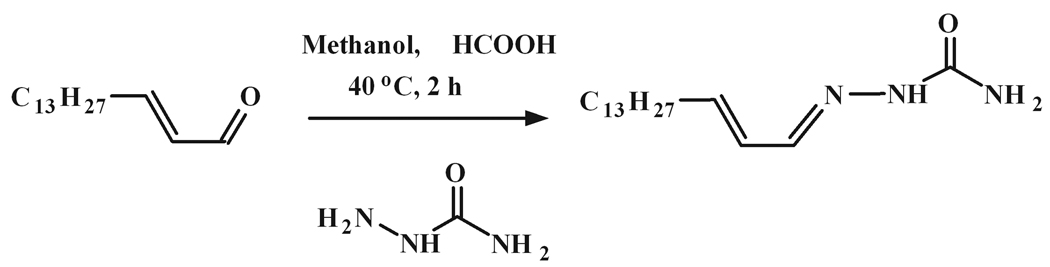
Figure 1.
Chemical synthesis of (2E)-hexadecenal semicarbazone.
Figure 2.
Positive product ion mass spectra of fatty aldehyde semicarbazone derivatives. (A) (2E)-Hexadecenal semicarbazone product ions. (B) cis-11-Hexadecenal semicarbazone product ions. (C) Hexadecanal semicarbazone product ions. (D) Proposed pathways for product ion formation during (2E)-hexadecenal semicarbazone collision-induced decomposition. Refer to text for the parameters of declustering, collision-induced decomposition, and collision cell exit potentials.
ESI-LC/MS/MS analysis of fatty aldehydes as semicarbazone derivatives
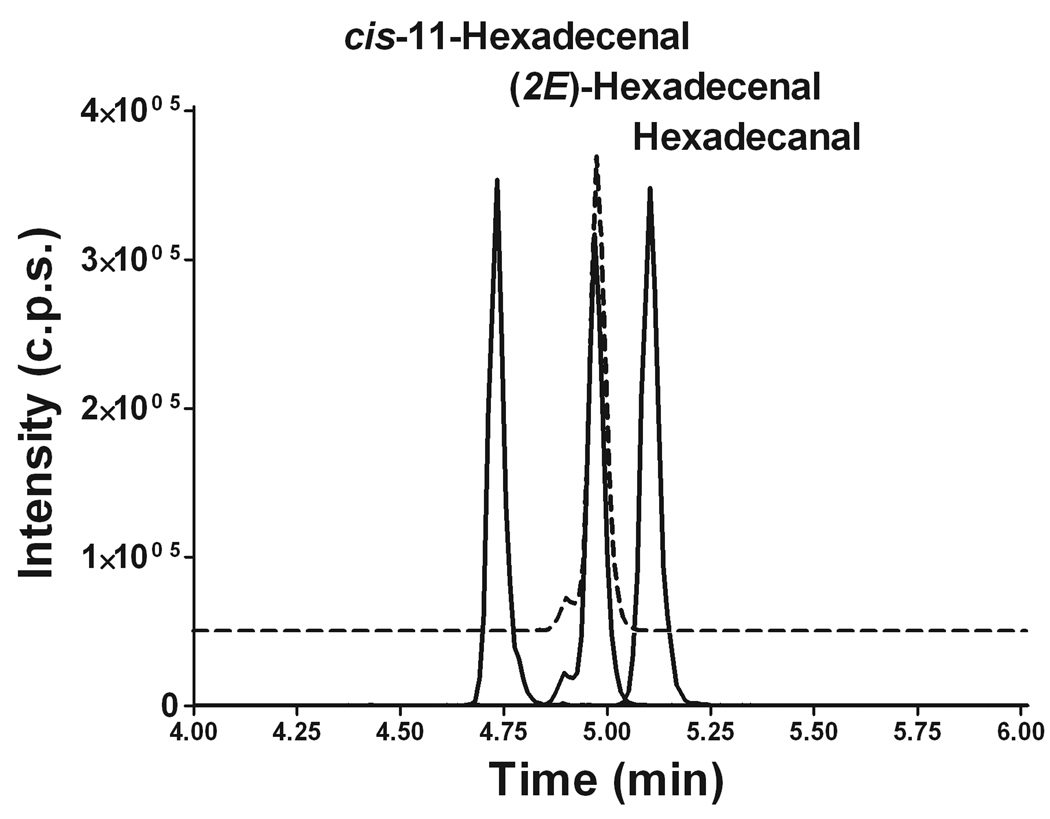
Next we developed a LC/MS/MS protocol to separate aldehyde semicarbazone products and to provide an additional degree of selectivity for the entire analysis. We employed a Supelco Discovery C8 column (50 × 3.5 mm, 5 µm particle size) and a gradient from a methanol:water:formic acid system to an acetonitrile:chloroform:water:formic acid system. All three transitions ([M-17]+, [M-43]+, and [M-199]+) were monitored. Figure 3 demonstrates complete peak base separation of all three aldehydes tested (only one transition based on the loss of the amino group is shown). It is especially important to achieve complete peak base separation between the semicarbazone products of (2E)-hexadecenal and hexadecanal as palmitaldehyde is a common constituent of plasmalogens [13] and may interfere with the analysis by overlapping (from its [M+3] natural isotopic analog) with the signal from the stable isotope-labeled internal standard.
Figure 3.
Positive ion ESI-LC/MS/MS analysis of semicarbazone derivatives of cis-11-hexadecenal, (2E)-hexadecenal, (2E)-d5-hexadecenal, and hexadecanal. The MRM based on the [M-17]+ transition is shown for the simplicity. The MRM profile for (2E)-d5-hexadecenal semicarbazone is shown as a dotted line.
(2E)-Hexadecenal semicarbazone eluted as a major peak with Rt of 4.98 min having a small shoulder at Rt of 4.89 min (Fig. 3). (2E)-d5-Hexadecenal semicarbazone demonstrated the same LC behavior (Fig. 3). However, cis-11-hexadecenal and hexadecanal semicarbazones eluted as single peaks (Fig. 3). This suggests that the presence of the double bond at C2 allows the potential formation of two stereoisomers during the reaction with semicarbazide, which are partially resolved using our chromatographic conditions. When both (2E)-hexadecenal and (2E)-d5-hexadecenal were hydrogenated over platinum oxide, they both were fully converted to hexadecanal, and their semicarbazones had the same Rt without the shoulder peak characteristic for (2E)-hexadecenal semicarbazone (Fig. 3). Therefore, when quantifying the signal from (2E)-hexadecenal semicarbazone during ESI-LC/MS/MS analysis, we took the sum of the areas of the major peak and of its shoulder peak.
Optimization of derivatization conditions
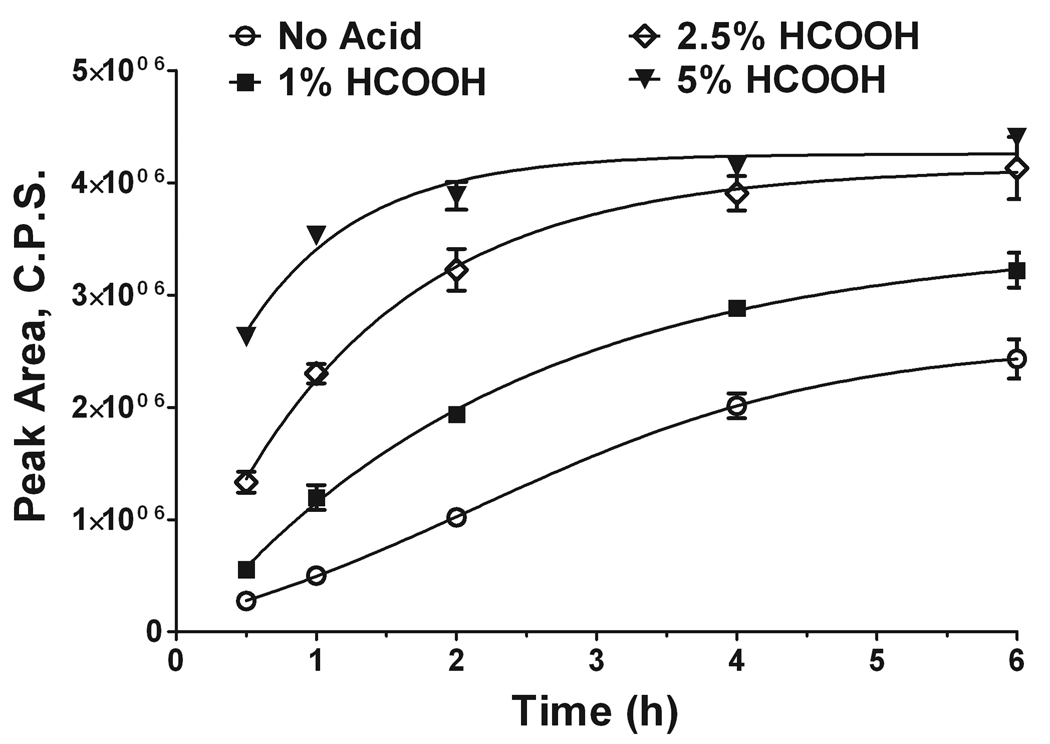
To prepare the semicarbazones, we initially used 5 mM semicarbazide in methanol with 1% formic acid in the reaction medium. Both methanol and formic acid are components of the solvent system used for column equilibration and initial chromatographic separation. Hence, injection of the methanol-based reaction mixture is the most compatible with the employed solvents and allows sample injection without additional steps of sample preparation before injection. Further, we explored the effect of the increasing acidity of the reaction mixture on the effectiveness of (2E)-hexadecenal derivatization with semicarbazide. Figure 4 shows that the increase in acidity has a direct effect on the rate of the semicarbazone formation performed at 40°C, with 5% formic acid in methanol giving almost complete conversion within 2 h. Based on these findings, all further derivatizations were performed with 5% formic acid in methanol and a 2-h derivatization time. These derivatization conditions ensured reliable detection of as low as 5 fmol (2E)-hexadecenal semicarbazone on-column (data not shown).
Figure 4.
Time- and pH-dependent formation of (2E)-hexadecenal semicarbazone. (2E)-hexadecenal (20 µM) was derivatized with 5 mM semicarbazide in methanol containing different concentration of formic acid for up to 6 h. Aliquots were withdrawn at the indicated time points and immediately analyzed by ESI-LC/MS/MS (10 pmol on-column for each data point). Data represent the average of the peak area corresponding to [M-17]+ transition ± S.E. of three independent measurements.
Optimization of S1PL reaction with liver homogenate
Rat [8] and mouse [9] liver homogenates were used previously to characterize S1PL activity with radioactive or fluorescently labeled substrates. We decided to use mouse liver as the source of the enzyme activity and employed the same lysis and reaction buffers as previously described [9] to optimize the assay conditions. However, our original attempts to detect S1P hydrolysis and the formation of (2E)-hexadecenal failed because of interference of buffer components with all targeted transitions chosen to detect both (2E)-hexadecenal and (2E)-d5-hexadecenal. Detailed investigation of the components of the reaction/lysis buffer(s) revealed that dithiothreitol (DTT) interferes in the reaction with semicarbazide. When DTT was omitted from the lysis buffer we detected the aldehydes as their semicarbazone derivatives.
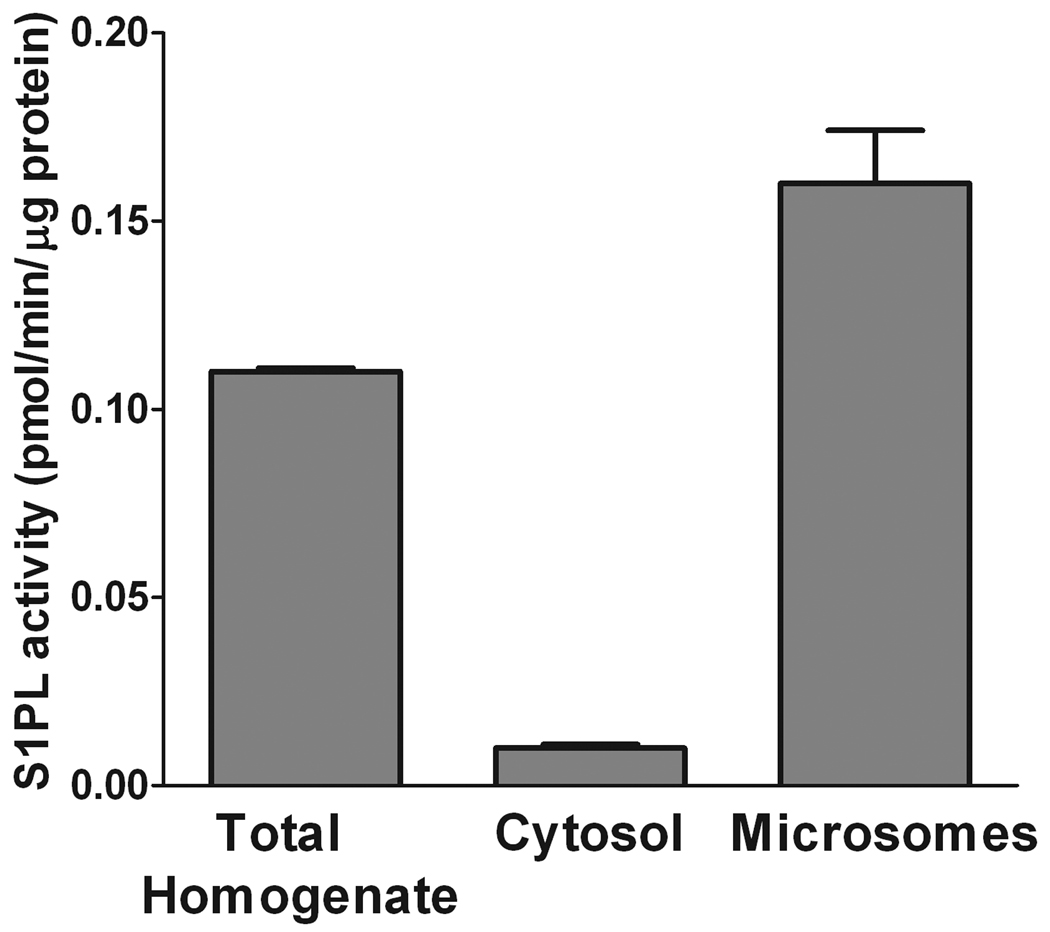
Another concern we had during the initial steps of method development was the reduction or oxidation of the aldehyde to 2-hexadecen-1-ol or 2-hexadecenoic acid after being formed from S1P upon degradation by S1PL [8,9]. One of the approaches to improve the assay conditions is to purify, even partially, the enzyme. S1PL is a microsomal enzyme with its active site exposed to the cytosol [8]. To diminish potential contributions of cytosolic proteins and cofactors to (2E)-hexadecenal biotransformations we compared S1PL activity in the cytosolic and membrane fractions with that of the total homogenate. Equal amounts of protein (5 µg) were taken into the reaction mixture. Figure 5 demonstrates that S1PL activity is mostly associated with the microsomal fraction. Hence, we continued characterization of S1PL activity using the microsomal fraction as the source of the enzyme. It is important to note that the 100,000 × g fraction prepared from mouse liver homogenized in the lysis buffer without DTT did not diminish S1PL activity for at least one month when stored at −80°C.
Figure 5.
Microsomal association of S1PL activity. Mouse liver microsomes were isolated from a total liver homogenate by ultracentrifugation. The S1PL reaction was performed for 20 min with 40 µM S1P and 5 µg total homogenate, cytosolic, or microsomal protein. Lipid extraction and derivatization was performed as described in the text. Data are presented as the mean ± S.E. of three independent experiments.
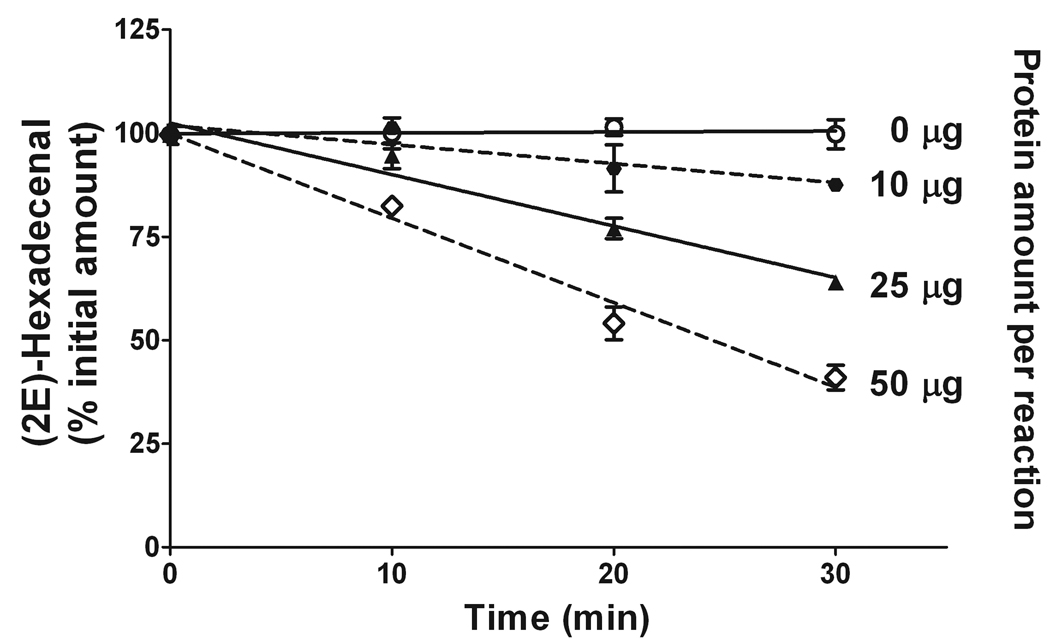
To investigate if (2E)-hexadecenal is degraded/converted during the S1PL enzymatic reaction performed in our conditions, we checked the disappearance of a standard (2E)-hexadecenal from the reaction mixture when all components of the reaction were present except for S1P. Figure 6 shows that the degree of (2E)-hexadecenal disappearance is directly related to the amount of the microsomal protein added to the reaction as well as to the duration of the incubation. Hence, to diminish or eliminate the interference with S1PL assay from the aldehyde biotransformation, it is best to minimize both the protein concentration and the reaction time. In the case of a mouse liver microsomal preparation, a reaction period of 20 min with 5 µg protein per reaction eliminated hexadecenal biotransformations. For better precision of S1PL-mediated S1P degradation and (2E)-hexadecenal quantitation we continued to determine the rate of (2E)-hexadecenal (20 pmol) biotransformation in each further experiment and incorporated the determined “disappearance” correction factor in our calculations. However, in the case of 5 µg mouse liver microsomal protein preparation per reaction and 20-min reaction time, the rate of (2E)-hexadecenal bioconversion was stably below 1%.
Figure 6.
Disappearance of (2E)-hexadecenal during enzymatic reaction as a function of time and protein concentration. (2E)-Hexadecenal (20 pmol) was incubated with the indicated amount of mouse liver microsomal protein in the reaction buffer. At the indicated time points, proteins were denatured by addition of methanol and chloroform (2:1, v/v). (2E)-d5-Hexadecenal (20 pmol) was added as the internal standard before lipid extraction and phase separation. Total lipid extracts were derivatized with 5 mM semicarbazide in 5% formic acid in methanol. Data represent the average ± S.E. of three different experiments.
Characterization of S1PL activity using liver microsomes
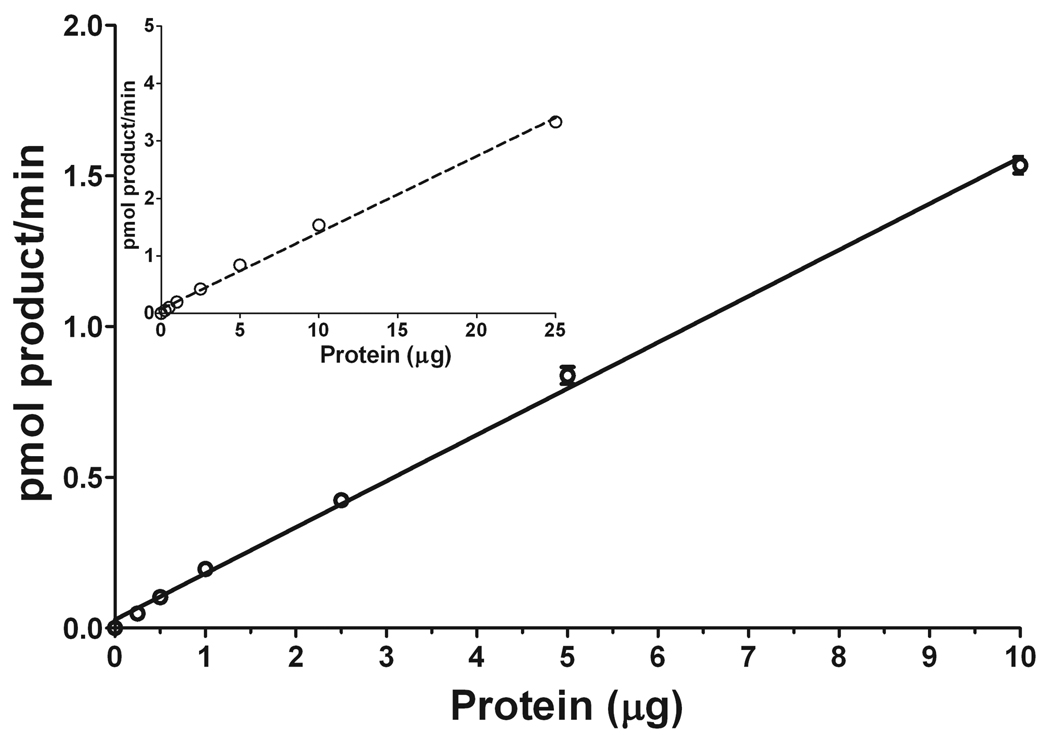
Having determined a method to correct for (2E)-hexadecenal biotransformations during the enzymatic reaction, we proceeded to further characterize S1PL activity. First, we determined the linearity of the S1PL reaction with 40 µM S1P as a function of microsomal protein concentration with a 20-min reaction time. Figure 7 shows that the S1PL reaction is linear within a broad range of protein concentration, with (2E)-hexadecenal biotransformation data correction required at protein concentration above 5 µg per reaction. It should be noted that the sensitivity of the LC/MS/MS approach allowed reliable quantitation of (2E)-hexadecenal when 2.5, 1, 0.5, and even 0.25 µg protein per reaction was used. Clearly, at these low protein concentrations there was no loss of (2E)-hexadecenal during the reaction because of its negligible rate of biotransformation. Badhuvula et al. [9] employed 5–50 µg total tissue or cell protein per assay from control or S1PL overexpressing cells, with 25 µg protein per assay in most experiments. They demonstrated (2E)-hexadecenal biotransformation under their experimental conditions; however, no approach to correct for the aldehyde loss was suggested. In the LC/MS/MS method we describe here, with a control for the (2E)-hexadecenal disappearance, a precise quantitation for S1PL-mediated S1P degradation is achieved even at conditions favorable for (2E)-hexadecenal biotransformation.
Figure 7.
The linearity of S1PL reaction. The reaction was performed for 20 min with 40 µM S1P as substrate and variable amounts of liver microsomal protein. The reaction was stopped by lipid extraction with the addition of 20 pmol (2E)-d5-hexadecenal as the internal standard. Total lipid extracts were derivatized with 5 mM semicarbazide in 5% formic acid in methanol for 2 h at 40°C and analyzed by ESI-LC/MS/MS. Data are presented as the mean ± S.E. of three independent experiments and were corrected for the disappearance of (2E)-hexadecenal during the enzymatic reaction.
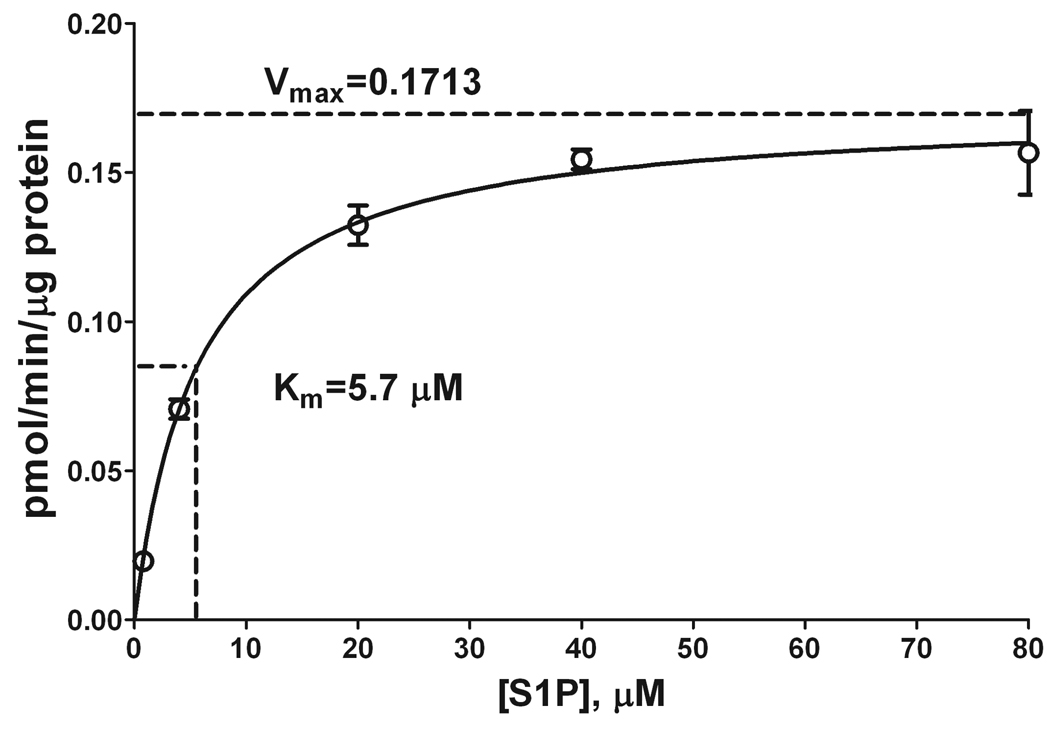
Next, we performed a kinetic analysis of the S1PL reaction by employing 5 µg microsomal protein per reaction, a 20-min reaction time, and various S1P concentrations. Figure 8 demonstrates that the S1PL activity was nearly maximal at 40 µM S1P, with a Km of 5.7 µM. This is by far the lowest published Km value for the S1PL reaction. Previous Km values of 9.0–20.1 µM were reported with DHS1P as the substrate [8–10]. Similar Km values were obtained with NBD-S1P (7–14.6 µM) [9] and BODIPY-S1P (35 µM) [10] as substrates. Our approach uses the natural and probably the best substrate for S1PL that results in a higher rate of enzymatic activity in comparison with all previously tested compounds. Also, by using S1P we avoid the limitations imposed by the use of radioactive substrate ([3H]-DHS1P), thus simplifying the entire procedure.
Figure 8.
S1PL activity as a function of substrate concentration. The S1PL reaction was performed for 20 min with variable amounts of S1P and 5 µg liver microsomal protein. The reaction was stopped by lipid extraction with the addition of 20 pmol (2E)-d5-hexadecenal as the internal standard. Total lipid extracts were derivatized with 5 mM semicarbazide in 5% formic acid in methanol for 2 h at 40°C and analyzed by ESI-LC/MS/MS. Data are presented as the mean ± S.E. of three independent experiments.
Inhibition of S1PL by FTY720
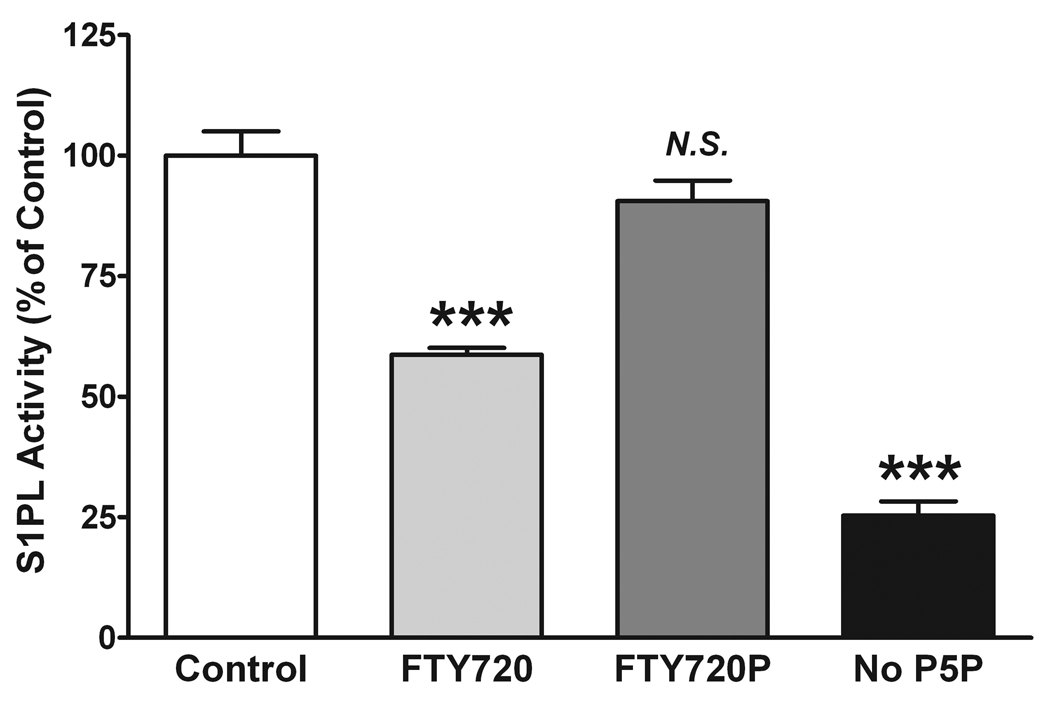
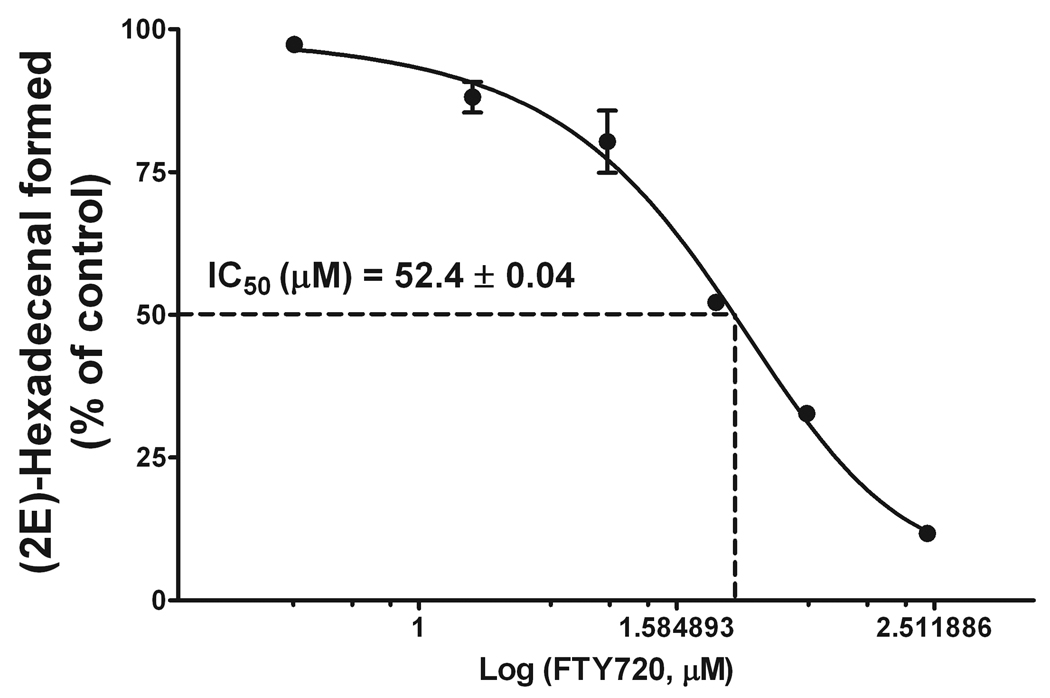
To test the feasibility of the developed LC/MS/MS approach to detect changes in S1PL activity, we performed an in vitro assay in the presence of a known inhibitor of S1PL, FTY720 [14]. We also tested the effect of (S)-FTY720-phosphate (FTY720P), which was previously shown not to inhibit S1PL [14]. FTY720 and FTY720P were solubilized in 1% Triton X-100 together with S1P and the reaction was performed with 5 µg mouse liver microsomal protein and 40 µM S1P, in the presence or absence of 30 µM FTY720 or FTY720P for 20 min. Consistent with the published data, we found that FTY720 but not FTY720P inhibited S1PL, with about 40% inhibition of S1PL activity by 30 µM FTY720 (Fig. 9). Further, to confirm that the described assay is applicable not only to a microsomal protein preparation but can be converted to a more practical setup with the use of total tissue homogenate, we evaluated the dose-dependent inhibition of S1PL activity with FTY720 using 25 µg total liver homogenate per reaction (Fig. 10). An IC50 value of 52.4 ± 0.04 µM was determined for the FTY720-dependent inhibition of S1PL activity. We also found almost identical inhibition when the reaction was performed using either the total tissue homogenate or the microsomal protein preparation (Figs. 9, 10). However, to achieve a comparable rate of (2E)-hexadecenal formation, we needed to increase the amount of protein per reaction five-fold when using total liver homogenate as the source of the enzyme.
Figure 9.
FTY720 but not FTY720P inhibits S1PL activity in vitro. The S1PL reaction was performed for 20 min with 40 µM S1P as substrate and 5 µg microsomal protein, in the presence or absence of 0.57 mM pyridoxal-5’-phosphate (P5P). FTY720 (30 µM) or FTY720P (30 µM) was added together with S1P. The reaction was stopped by lipid extraction with the addition of 20 pmol (2E)-d5-hexadecenal as the internal standard. Total lipid extracts were derivatized with 5 mM semicarbazide in 5% formic acid in methanol for 2 h at 40°C and analyzed by ESI-LC/ MS/MS. Data are presented as the mean ± S.E. of three independent experiments. *** -p<0.001; N.S. – not significant.
Figure 10.
Dose-dependent inhibition of S1PL activity by FTY720. The S1PL reaction was performed for 20 min with 40 µM S1P as a substrate and 25 µg total mouse liver homogenate protein, in the presence of 0.57 mM pyridoxal-5’-phosphate (P5P). FTY720 was added together with S1P. The reaction was stopped by lipid extraction with the addition of 20 pmol (2E)-d5-hexadecenal as the internal standard. Total lipid extracts were derivatized with 5 mM semicarbazide in 5% formic acid in methanol for 2 h at 40°C and analyzed by ESI-LC/MS/MS. Data are presented as the mean ± S.E.M. of three independent experiments. IC50 was calculated using GraphPad Prism 5.0 data analysis tool.
We also tested if S1P is degraded by S1PL when the co-factor for the reaction, P5P, is not added to the microsomal protein preparation. As expected, we found only minimal enzyme activity when P5P was omitted (about 20% of that at optimal conditions), which we attribute to a residual presence of P5P in the endogenous protein preparation. It is known that the endogenous P5P is bound to a multitude of apoenzymes but the P5P endogenous level is insufficient to saturate them [15]. This requires P5P supplementation when performing P5P-dependent in vitro assays [16].
In summary, we developed a sensitive assay to quantitate (2E)-hexadecenal as its semicarbazone derivative by ESI-LC/MS/MS using an isotope-dilution approach, and applied this method to characterize S1PL activity. Derivatization of (2E)-hexadecenal is performed as a one-step reaction, and the semicarbazone product is directly injected for LC/MS/MS analysis without any further purification. The method requires a correction for (2E)-hexadecenal biotransformation during the S1PL reaction at microsomal protein concentrations above 5 µg per reaction. The correction is performed by quantifying the disappearance of the standard (2E)-hexadecenal at the same experimental condition when the substrate (S1P) is absent. The described approach allows the use of total tissue homogenates as the source of the enzyme and demonstrates for the first time the IC50 of 52.4 µM for FTY720 as the inhibitor of S1PL. In overall, the method is ideal to search for novel S1PL inhibitors, to look for S1PL association with different pathologies, and to characterize the effect of xenobiotics or genetic manipulations on the enzyme activity.
Acknowledgments
This work was supported in part by the NIH grant HL-095440 and by the American Heart Association Science Development Grant 0930028N to E.B., by the NIH grant HL-083187 to R.B., by the NIH grant HL-079396 to V.N., and by The University of Chicago Department of Medicine through support to the Lipidomics facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell. Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Takabe K, Paugh WW, Milstien S, Spiegel S. "Inside-out" signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serra M, Saba JD. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv. Enzyme Regul. 2010;50:349–362. doi: 10.1016/j.advenzreg.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leong WI, Saba JD. S1P metabolism in cancer and other pathological conditions. Biochimie. 2010;92:716–723. doi: 10.1016/j.biochi.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 6.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ. Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 7.Kariya Y, Kihara A, Ikeda M, Kikuchi F, Nakamura S, Hashimoto S, Choi CH, Lee YM, Igarashi Y. Products by the sphingosine kinase/sphingosine 1-phosphate (S1P) lyase pathway but not S1P stimulate mitogenesis. Genes Cells. 2005;10:605–615. doi: 10.1111/j.1365-2443.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Veldhoven PP, Mannaerts GP. Subcellular localization and membrane topology of sphingosine-1-phosphate lyase in rat liver. J. Biol. Chem. 1991;266:12502–12507. [PubMed] [Google Scholar]
- 9.Bandhuvula P, Fyrst H, Saba JD. A rapid fluorescence assay for sphingosine-1-phosphate lyase enzyme activity. J. Lipid Res. 2007;48:2769–2778. doi: 10.1194/jlr.D700010-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Bandhuvula P, Li Z, Bittman R, Saba JD. Sphingosine 1-phosphate lyase enzyme assay using a BODIPY-labeled substrate. Biochem. Biophys. Res. Commun. 2009;380:366–370. doi: 10.1016/j.bbrc.2009.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Gong Y, Byun H-S, Bittman R. An improved two-step synthetic route to primary allylic alcohols from aldehydes. New J. Chem. 2010;34:470–475. [Google Scholar]
- 12.Lu X, Bittman R. Enantioselective synthesis of the phosphate esters of the immunosuppressive lipid FTY720. Tetrahedron Lett. 2006;47:825–827. [Google Scholar]
- 13.Lessig J, Fuchs B. Plasmalogens in biological systems: their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Curr. Med. Chem. 2009;16:2021–2041. doi: 10.2174/092986709788682164. [DOI] [PubMed] [Google Scholar]
- 14.Bandhuvula P, Tam YY, Oskouian B, Saba JD. The immune modulator FTY720 inhibits sphingosine-1-phosphate lyase activity. J. Biol. Chem. 2005;280:33697–33700. doi: 10.1074/jbc.C500294200. [DOI] [PubMed] [Google Scholar]
- 15.Rej R. Review: the role of coenzymes in clinical enzymology. Ann. Clin. Lab. Sci. 1977;7:455–468. [PubMed] [Google Scholar]
- 16.Stokol T, Erb H. The apo-enzyme content of aminotransferases in healthy and diseased domestic animals. Vet. Clin. Pathol. 1998;27:71–78. doi: 10.1111/j.1939-165x.1998.tb01022.x. [DOI] [PubMed] [Google Scholar]