Abstract
The targeted delivery of nanoparticles to solid tumors is one of the most important and challenging problems in cancer nanomedicine, but the detailed delivery mechanisms and design principles are still not well understood. Here we report quantitative tumor uptake studies for a class of elongated gold nanocrystals (called nanorods) that are covalently conjugated to tumor-targeting peptides. A major advantage in using gold as a “tracer” is that the accumulated gold in tumors and other organs can be quantitatively determined by elemental mass spectrometry (gold is not a natural element found in animals). Thus, colloidal gold nanorods are stabilized with a layer of polyethylene glycols (PEGs), and are conjugated to three different ligands: (i) a single-chain variable fragment (ScFv) peptide that recognizes the epidermal growth factor receptor (EGFR); (ii) an amino terminal fragment (ATF) peptide that recognizes the urokinase plasminogen activator receptor (uPAR); and (iii) a cyclic RGD peptide that recognizes the avb3 integrin receptor. Quantitative pharmacokinetic and biodistribution data show that these targeting ligands only marginally improve the total gold accumulation in xenograft tumor models in comparison with nontargeted controls, but their use could greatly alter the intracellular and extracellular nanoparticle distributions. When the gold nanorods are administered via intravenous injection, we also find that active molecular targeting of the tumor microenvironments (e.g., fibroblasts, macrophages, and vasculatures) does not significantly influence the tumor nanoparticle uptake. These results suggest that for photothermal cancer therapy, the preferred route of gold nanorod administration is intra-tumoral injection instead of intravenous injection.
Keywords: tumor targeting, nanoparticle delivery, gold nanorod, peptide, lung cancer, EGFR, uPAR, RGD
The targeted delivery of nanoparticles to solid tumors is a key task in the development of cancer nanomedicine for in-vivo molecular imaging and targeted therapy.1–8 Despite extensive research and significant progress in the last 10–15 years, there are still major fundamental and technical barriers that need to be understood and overcome.1–9 These problems include the complex interactions between naoparticles and biological systems in-vivo, rapid uptake and clearance of nanoparticles by the reticuloendothelial system (RES) organs (such as the liver and spleen), and very limited penetration of nanoparticles to poorly vascularized or necrotic tumor regions.2,10 Current methods for nanoparticle delivery are mainly based on an “active” mechanism and a “passive” mechanism.9,10 In the active mode, molecular ligands such as antibodies, peptides, or small molecules are used to recognize specific receptors on the tumor cell surface, often followed by receptor-mediated endocytosis and nanoparticle internalization. In the passive mode, nanoparticles without targeting ligands are accumulated and retained in the tumor interstitial space mainly through the enhanced permeability and retention (EPR) effect.11–13 In both mechanisms, a common feature is that nanoparticles in the blood stream must first move across the tumor blood vessels (usually leaky vasculatures) and extravasate into the tumor interstitium or the perivascular region.11
Under this general framework, however, there is still considerable debate about the relative contributions of such active and passive targeting mechanisms.14 Recent work by several groups has shown that the use of tumor-targeting ligands does not increase the total accumulation of nanoparticles in solid tumors, although it does function to increase receptor-mediated internalization and could thus improve therapeutic efficacy for cancer drugs that act on intracellular protein targets.14–17 In particular, Park and coworkers15 have shown that PEGylated liposomes linked with anti-HER2 antibodies are internalized into tumor cells, but their total tumor accumulation is not different from that of nontargeted particles. Davis and coworkers16,17 have also reported that transferrin targeting improves the intracellular localization of polymeric nanoparticles, but does not increase their total tumor uptake. In addition, theoretical modeling studies by Wittrup and coworkers18 reveal that the total tumor uptake of liposomes is not enhanced by conjugation to targeting ligands.
There is also a considerable amount of opposing data showing that the use of tumor targeting ligands is effective in delivering imaging and therapeutic agents into solid tumors.19–24 For example, tumor targeting studies using fluorescently and radioactively labeled antibodies have shown higher tumor uptake (measured on the basis of per gram of tumor mass) for macromolecules and nanoparticles than their nontargeted controls.25,26 Complicating this matter further, recent work has shown that that the use of targeting ligands might even be “detrimental” because the exposed ligands can accelerate nanoparticle opsonization (adsorption of blood proteins) and blood clearance, leading to an overall reduction in tumor nanoparticle uptake.27–29 Regarding the effects of nanoparticle structure and shape, Discher, Sailor, and their coworkers30,31 have shown that elongated nanoparticles (called nanofilaments and nanoworms) have longer blood circulation times and show improved accumulation in solid tumors in comparison with their spherical counterparts.
Here we report quantitative tumor uptake studies for elongated gold nanocrystals (called nanorods, NRs) that are covalently conjugated to targeting peptide ligands. A major advantage in using gold (Au) as a “tracer” is that the amount of accumulated Au can be quantitatively determined by inductively coupled plasmon - mass spectrometry (ICP-MS), a common procedure for elemental analysis.32 Also, Au NRs have novel optical and electronic properties, and have broad applications in cellular imaging,33–39 biosensing,40–42 surface-enhanced Raman scattering (SERS),43–44 and near-infrared photothermal therapy.33,45–49 By using animal models bearing A549 lung tumors, we have examined the pharmacokinetic and biodistribution properties of both targeted and nontargeted Au nanocrystals. The A549 xenograft tumors are especially well suited for nanoparticle targeting studies because of their high expressions of several extracellular receptors including the epidermal growth factor receptor (EGFR), the urokinase plasminogen activator receptor (uPAR), and the avb3 integrin receptor.50–54 Thus, we have used a single-chain variable fragment peptide (ScFv, 25 KD, Kd = 3.4 nM) to target EGFR; an amino-terminal fragment peptide (ATF, 15 KD, Kd = 0.1 nM) to target uPAR; and a cyclic RGD peptide (0.9 KD, Kd = 2 nM) to target the avβ3 receptor.
It is worth noting that the uPAR and avβ3 receptors are broadly expressed in the tumor stroma and/or on the surface of tumor blood vessels,55–57 so the use of ATF and RGD ligands could allow active targeting of both the tumor cells and the tumor microenvironments. In fact, we had hypothesized targeting tumor stroma and vasculatures could be more effective than targeting tumor cells because the circulating nanoparticles are expected to encounter the tumor vasculature and stroma before binding to tumor cells. However, our quantitative ICP-MS data show that active targeting of tumor cells or the tumor microenvironments only marginally improves the total tumor uptake of gold nanoparticles. The main differences caused by active targeting are observed in the distribution patterns of nanoparticles inside tumor cells and in the tumor microenvironments. These results suggest that nanoparticle delivery is likely limited by mass transport across the tumor vasculature, at least for large and rigid nanoparticles such as Au NRs. Thus, for photothermal cancer therapy using large nanorods, the preferred route of administration is direct tumor injection, not intravenous systemic injection.
Results and Discussion
Properties of Au NRs and Ligand Conjugation
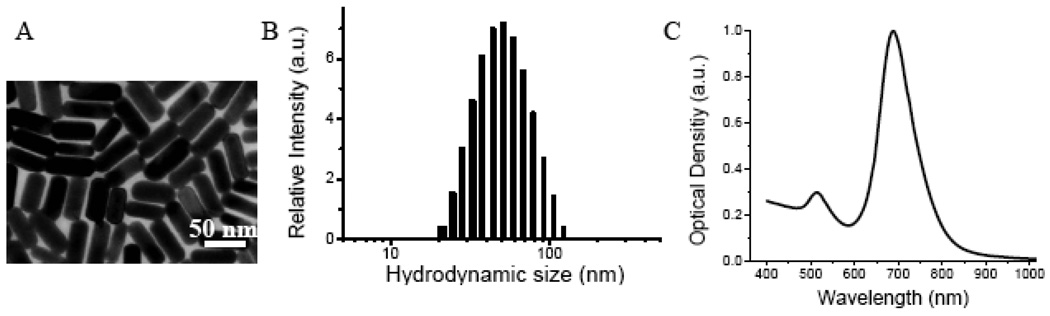
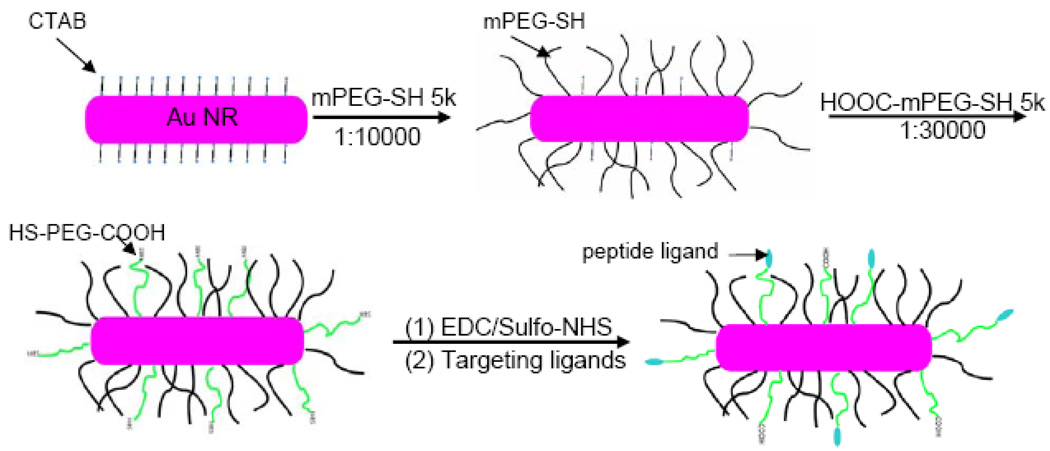
Au NRs were synthesized according to the seed-mediated growth method of Nikoobakht and El-Sayed.58 The “as-synthesized” Au NRs have an aspect ratio (length-to-width) of 3.0, an average hydrodynamic size (diameter or D) of 51 nm, a positive surface charge (zeta potential or ζ) of 40 mV, as well as two prominent surface plasmon resonance (SPR) peaks located at 520 nm and 680 nm (see Figure 1 and Table 1). Note that a cationic surfactant (cetyltrimethyl ammonium bromide or CTAB) is used to cap and stabilize the nanorods during synthesis, and this surfactant forms a bilayer structure on the nanorod surface, giving rise to the positive surface charge measured by dynamic light scattering (DLS). For colloidal stability, biocompatibility, and ligand conjugation, the Au NRs are coated with a mixed layer of two thiolated PEGs (methoxy-PEG or mPEG and carboxy-PEG or HOOC-PEG, both 5000 MW) in two separate steps. As illustrated in Figure 2, the NRs are first stabilized by using mPEG-SH (neutral) and then by HOOC-PEG-SH in a sequential manner. This process introduces functional –COOH groups for peptide conjugation, and the sequential procedure is necessary to avoid nanoparticle aggregation, a major problem when CTAB-capped NRs (positively charged) are exposed directly to HOOC-PEGs (negatively charged). Dynamic light scattering data reveal that the first mPEG coating step increases the particle hydrodynamic size by 18 nm and decreases the surface potential by 33 mV. The HOOC-PEG coating step further increases the hydrodynamic size by 10 nm, and decreases the zeta potential by 20 mV.
Figure 1. Structure and optical properties of as-synthesized Au NRs.
(A) TEM micrograph showing that the NRs have an aspect ratio of 3; (B) dynamic light scattering data showing an average hydrodynamic size of 51 nm; and (C) optical absorption spectrum showing two surface plasmon resonance peaks at 520 nm and 680 nm.
Table 1.
Properties of As-Synthesized, PEG-Coated, and Peptide-Conjugated Gold Nanorods.
| Surface Ligands | Hydrodynamic Diameter (nm) | Zeta Potential (mV) |
|---|---|---|
| CTAB | 51 ± 2 | +40 ± 5 |
| mPEG-SH 5k | 68 ± 2 | −5 ± 3 |
| mPEG-SH 5k/HS-PEG-COOH 5k | 78 ± 3 | −25 ± 2 |
| ScFv EGFR | 79 ± 3 | −15 ± 2 |
| ATF | 80 ± 3 | −19 ± 2 |
| c-RGD | 81 ± 2 | −16 ± 2 |
Abbreviations: CTAB = cetyltrimethyl ammonium bromide; mPEG-SH = thiolated methoxy-poly (ethylene) glycol; HS-PEG-COOH = thiolated carboxy-poly (ethylene) glycol; ScFv EGFR = single-chain variable fragment of anti-epidermal growth factor receptor; ATF = amino-terminal fragment of urokinase plasminogen activator; c-RGD = cyclic arginine-glycine-aspartate.
Figure 2. Stabilization and bioconjugation of Au NRs for cellular and in-vivo tumor targeting.
Surfactant-capped nanorods are first stabilizied by using methoxy-PEG-SH, followed by carboxy-PEG-SH. The functional carboxy (-COOH) groups are activatetd by using EDC/Sulfo-NHS, and are covalently conjugated to tumor targeting peptides via stable amide bonds. Approximately 400–500 peptide molecules are conjugated to each Au NR. See text for discussion.
To determine the number of attached PEG molecules on each nanorod, we have used an amine-terminated PEG (NH2-PEG-SH, MW 5000) to replace mPEG or HOOC-PEG for NR coating. The number of primary amine groups is determined by using N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP), a colorimetric agent that generates pyridine-2-thione and can be quantified by UV-Vis absorption.59 The results indicate that each Au NR is coated with approximately 7,500 mPEG molecules and 4,300 HOOC-PEG molecules. Note that these numbers are only rough estimates because we have not quantitatively determined the reproducibility and accuracy of the indirect colorimetric procedure. To avoid this uncertainty, we have used the same batch of pegylated gold nanorods for both control studies and for conjugation to the various targeting ligands. So, the control and targeted nanorods should have the same amounts of mPEG and HOOC-PEG. The final PEG-protected NRs are stable even under high-salt and strong acid/base conditions, allowing covalent conjugation to peptide ligands via amide bonds by using EDC/sulfo-NHS coupling reactions. Under our experimental conditions, the overall peptide conjugation efficiency is about 40–50 %,60 so starting with 1000 peptide molecules per rod in the reaction mixture, one can expect that each NR is conjugated to 400–500 peptide molecules, a value that is further confirmed by direct protein assays (Bio-Rad Protein Assay using Coomassie Blue G-250 dyes). . Dynamic light scattering data further reveal that this level of ligand conjugation only slightly changes the particle’s hydrodynamic size (an increase of 1–3 nm), but reduces the particle’s surface charge by as much as 6–10 mV (see Table 1). This is most likely caused by a decrease in free COOH groups after covalent conjugation and by positive charges on the peptide ligands.
Cellular Binding and Uptake
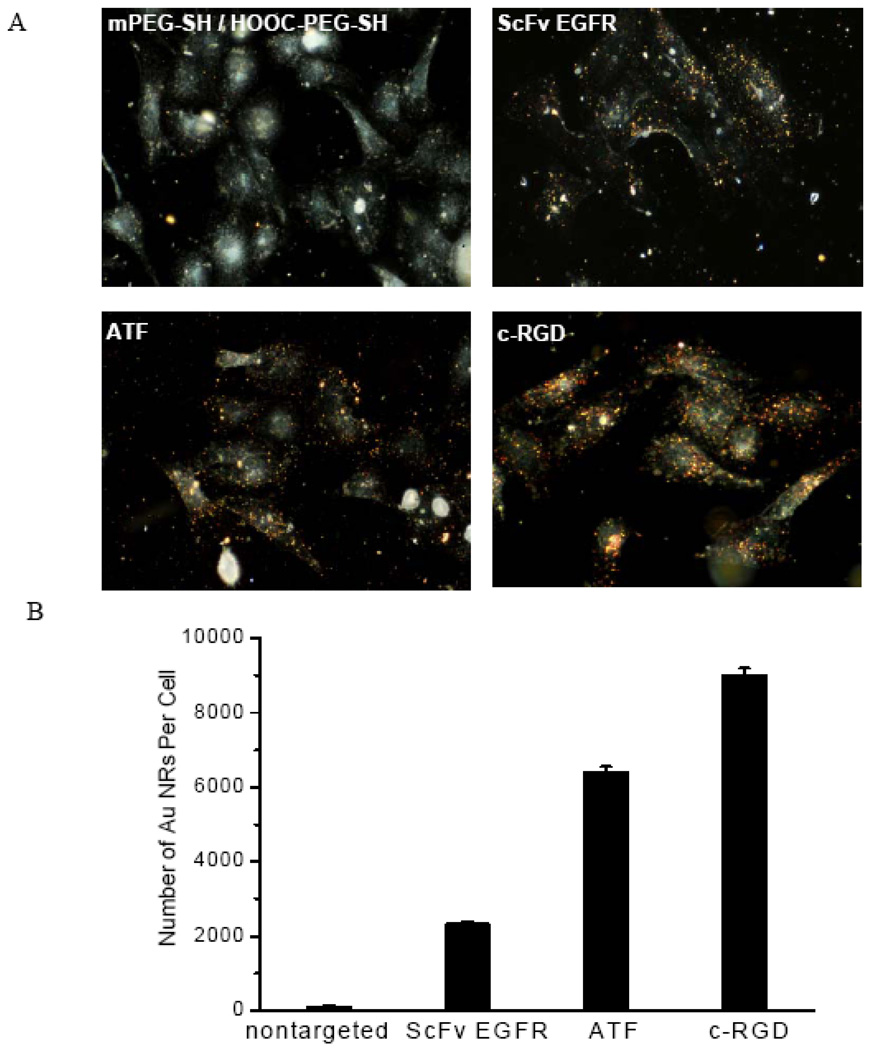
As mentioned earlier, the A549 tumor is used as an animal model for aggressive and metastatic non-small-cell-lung carcinoma. Histological staining studies have shown that the xenograt tumors have high expressions of EGFR, uPAR, and avβ3 receptors.50–54 By using cultured cancer cells, we have obtained in-vitro cellular binding and uptake data for both targeted and nontargeted Au NRs. As shown in Figure 3, both dark-field imaging and quantitative ICP-MS data demonstrate specific cellular binding of the peptide-conjugated particles that is 20–100 times stronger than the control particles under the same experimental conditions. The amount of bound nanoparticles is believed to depend on the receptor expression level and the ligand-receptor binding affinity, but other factors such as steric hindrance (accessibility) and the rate of receptor-mediated endocytosis are likely important as well. Taken together, these cellular binding data indicate that (i) the covalent conjugation chemistry is successful; (ii) the conjugated ligands are still able to recognize their target receptors; and (iii) the A549 cancer cells indeed have high expressions of the three extracellular receptors.
Figure 3. (A) Dark-field imaging and (B) quantitative Au ICP-MS studies of NR binding to cultured A549 cancer cells.
The data show specific binding of peptide-conjugated Au NRs to cultured A549 lung cancer cells, and negligible binding of nontargeted particles to the same tumor cells. Cells were incubated with 1 nM Au NRs for 2 h at 37 °C in the culture medium and were washed with 1 × PBS buffer. After trypsin treatment, approximately one million cells were counted and analyzed to minimize statistical errors.
Pharmacokinetics
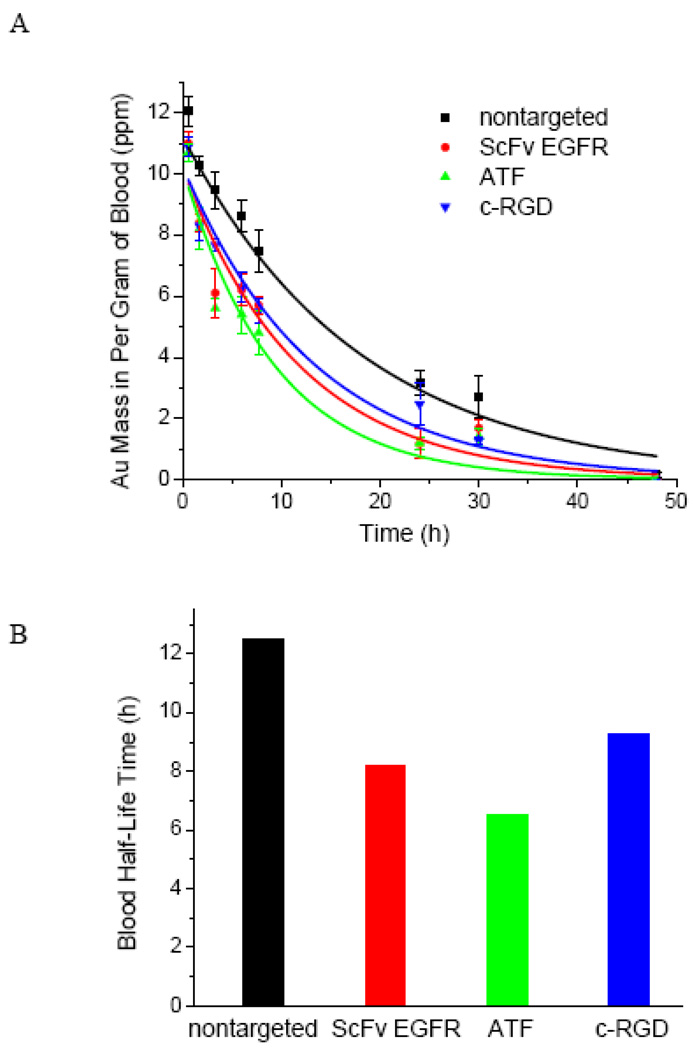
To examine the pharmacokinetic properties of the Au NRs, we have used ICP-MS to measure the Au concentration in blood samples at various time points after tail vein injection (between 0 and 48 hours) (see Figure 4). The experimental data can be fitted to a mono-exponential decay model, resulting in a half decay time (t1/2) of 12.5 h for the nontargeted nanoparticle (which are coated with a mixture of mPEG and HOOC-PEG), a half time of 8.3 h for the ScFv-conjugated nanoparticle, a half time of 6.5 h for the ATF-conjugated nanoparticle, and a half time of 9.3 h for the cRGD-conjugated nanoparticle. These quantitative data indicate that the addition of tumor targeting ligands can indeed accelerate nanoparticle opsonization and blood clearance. Previously, Montet et al. reported that the attachment of just 20 c-RGD molecules to each amino-dextran coated iron oxide nanoparticle reduced the blood circulation halftime by 5 h.28 Also, McNeeley et al. showed that adding just 0.15% folate to PEGylated liposomes reduced the t1/2 value from 18 h to 6.7 h.29 Clearly, the density of targeting ligands needs to be optimized so that the targeted nanoparticles have sufficiently long blood circulation times for active receptor binding and tumor accumulation.61
Figure 4. Blood circulation and pharmacokinetic data obtained for targeted and nontargeted Au NRs in healthy mice models.
Au NRs were injected via tail veins, and their blood concentrations were measured by ICP-MS analysis of blood samples at various time intervals (0 to 48 hours). The measured blood halftime (t1/2) is 12.5 hours for the control particles, 8.5 hours for the ScFv EGFR particles, 6.5 hours for the ATF particles, and 9.3 hours for the c-RGD particles.
Tumor and Organ Uptake
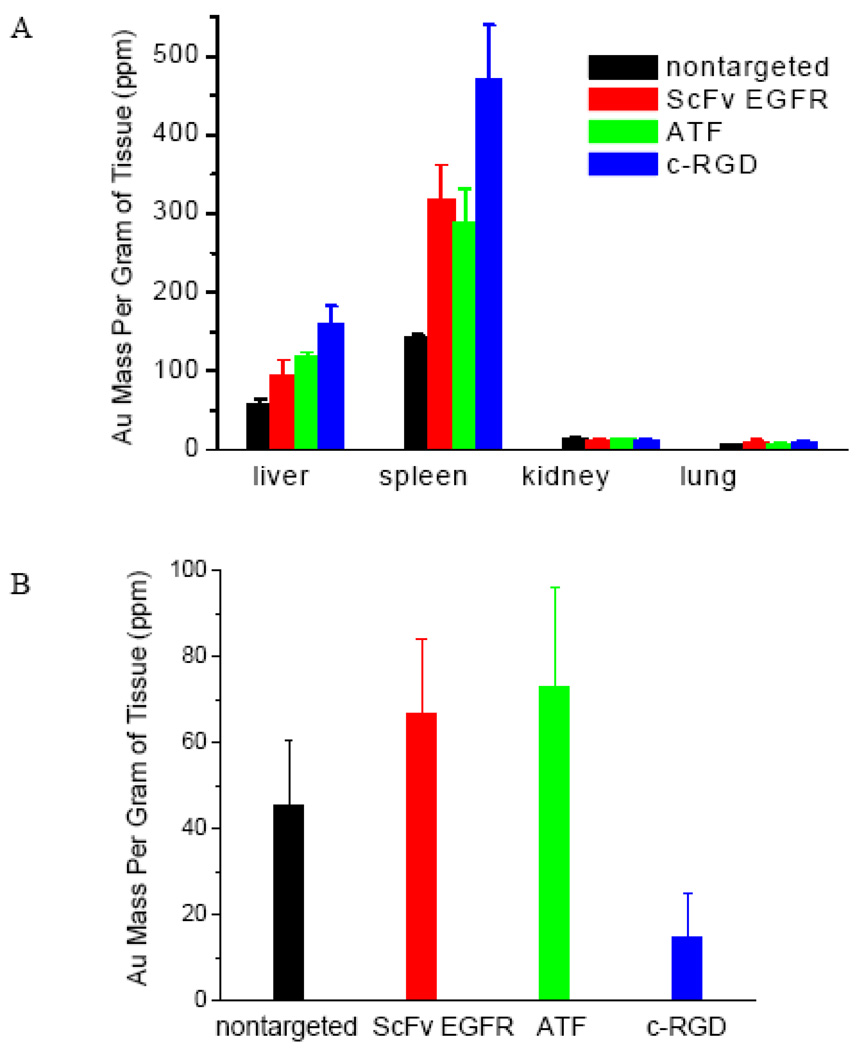
Next, we investigated the organ distribution and tumor uptake for both the targeted and nontargeted nanoparticles at 24 h post-injection, again by using ICP-MS to measure the accumulated Au in dried tissues (see Figure 5). As expected, all the Au particles are efficiently taken up by the RES organs (e.g., the liver and spleen), with little accumulation in the kidney or lung. Interestingly, the three peptide-conjugated nanoparticles are taken up more efficiently by the RES organs than the control particle (which has the lowest Au concentration in the liver and spleen). This is perhaps not surprising because the nanoparticles with exposed peptide ligands are less “stealthy” and can be recognized by the immune system for clearance. Also, the ScFv- and ATF-conjugated particles are more efficient in tumor accumulation than the control particle, but the cRGD-conjugated particles shows 2–3 fold lower tumor accumulation than the control particles. Considering their similar blood circulation times, we believe that the low tumor accumulation of the RGD particle is related to its high uptake in the liver and spleen. Kinetic studies have shown that targeted nanoparticles are taken up by tumors faster than nontargeted particles in the short term (within 6 hours), but after 20 hours, the nontargeted particles often attain a higher accumulation level than the targeted particles.27 To further explore the effect of ligand density on tumor uptake, we have obtained quantitative organ and tumor biodistribution data for non-targeted (control) and cRGD-conjugated Au nanorods at different ligand densities (0, 50, 500, or 5000 cRGD molecules per nanorod) (see Supporting Figure S1). The results indicate that the liver and spleen uptake is increased at higher ligand densities, but the gold accumulation in tumors is significantly reduced for all the ligand densities studied in this work. More data points are still needed to examine the pharmacokinetic and tumor uptake behaviors of targeted gold nanorods at ultralow ligand densities in the range of 1–10 ligand molecules per particle. Also, when the organ and tumor uptake data are analyzed as percentages of injection dose, it becomes clear that the liver and spleen take up 40% of the control nanorods and 60–90% of the targeted nanorods (see Supporting Figure S2). In comparison, the tumors take up only 1–2% of the injected gold nanorods (see Supporting Figure S3). These results indicate that the use of active targeting ligands does not greatly improve the total tumor uptake of nanoparticles, but as discussed below, the nature of targeting ligands can dramatically change the nanoparticle distribution in the tumor cells and in the tumor microenvironments.
Figure 5. Quantitative organ (A) and tumor (B) uptake data obtained from non-targeted and targeted Au NR conjugates measured by ICP-MS at 24 h post-injection in A549 xenografted mice models.
The nontargeted Au NRs showed similar tumor uptake to EGFR- and uPAR-targeted, but 3 times higher than αvβ3 integrin-targeted Au NRs.
Intratumoral Nanoparticle Distribution
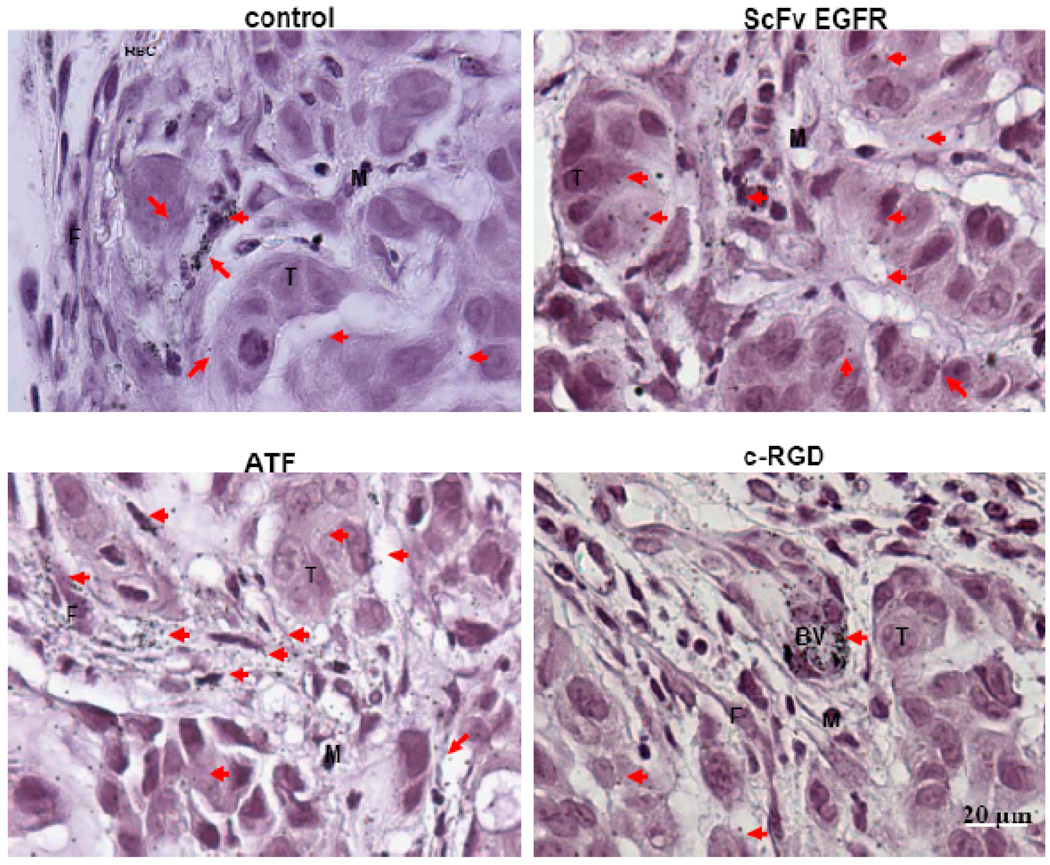
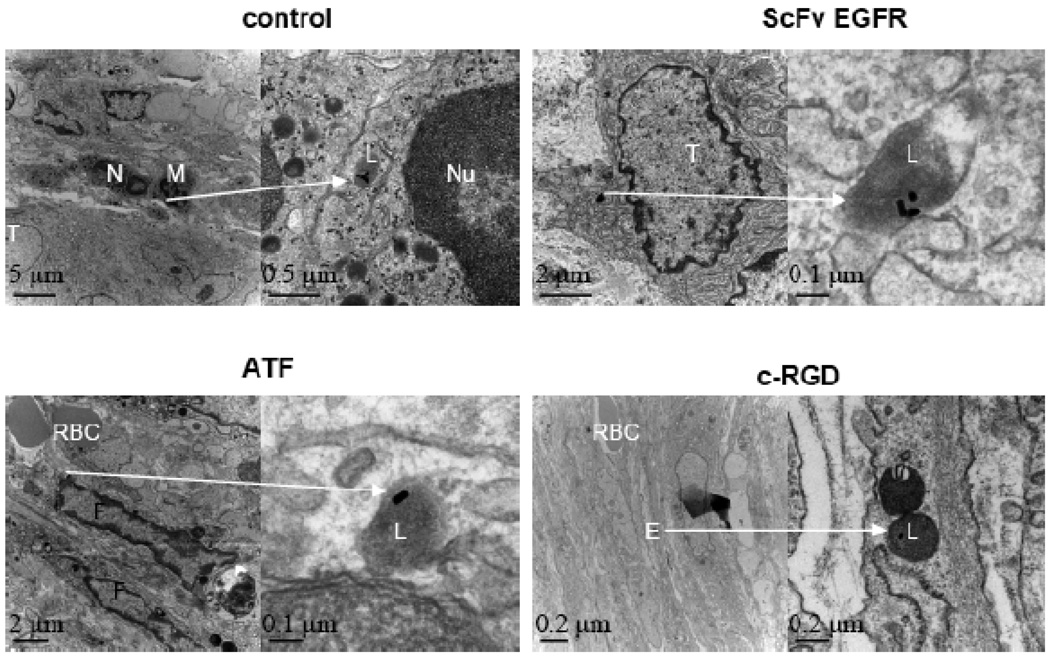
We further examined the intratumoral distributions of targeted and nontargeted Au NRs by using both optical microscopy (via silver enhancement) and transmission electron microscopy (TEM). Silver enhancement renders Au NRs in tissue sections directly visible for observation under bright-field light microscopy. As shown Figure 6, silver enhancement and haematoxylin staining reveal that the nontargeted Au NRs are mainly localized around blood vessels, in the tumor stromal matrix, and inside some macrophage cells. In contrast, the EGFR-targeted NRs are mainly found inside tumor cells; the uPAR-targeted NRs are predominantly localized in tumor stromal cells (but not inside tumor cells); and the integrin-targeted nanorods are accumulated around tumor blood vessels (perivascular regions). These findings are further supported by TEM results (see Figure 7), which show that nanoparticle internalization into tumor cells, fibroblast cells, and endothelial cells are enhanced by using targeting ligands. In particular, the ATF ligand favorably binds to stromal cells, and the c-RGD ligand to blood vessels.
Figure 6. Light microscopic images of silver-enhanced and hematoxylin-stained tumor tissue sections showing intratumoral distribution of targeted and nontargeted Au NRs.
Nontargeted Au NRs were mainly randomly distributed in the extracellular matrix but not inside the tumor cells. The ScFv EGFR/Au NRs were mainly located in the cytoplasm of tumor cells, few particles could be found in the stroma. The ATF/Au NRs were mainly distributed in the stroma, and few particles were internalized by the tumour cells. Most of the RGD/Au NRs are located in the endothelial cells of the blood vessel but not inside the tumor cells. Silver-enhanced Au NRs are marked by arrows, tumor cells marked by T, macrophage cells by M, fibroblast cells by F, red blood cells by RBC, and blood vessels by BV.
Figure 7. TEM images of tumor tissue sections showing intracellular localization of Au NRs.
For each ligand, Au NRs in selected areas (indicated by arrows) are shown in zoomed-in images. The non-targeted NRs were found in the lysosomes of macrophages. ScFv EGFR/Au NRs were located in the endososomes of tumor cells. ATF/Au NRs were found in the endososomes of fibroblasts, and the RGD/Au NRs were mainly distributed in the endososomes of blood vessel endothelial cells. Tumor cells are marked by T; neutrophil cells by N; macrophage cells by M; red blood cells by RBC; vessel endothelial cell by E; cell nuclei by N; and lysosomes by L.
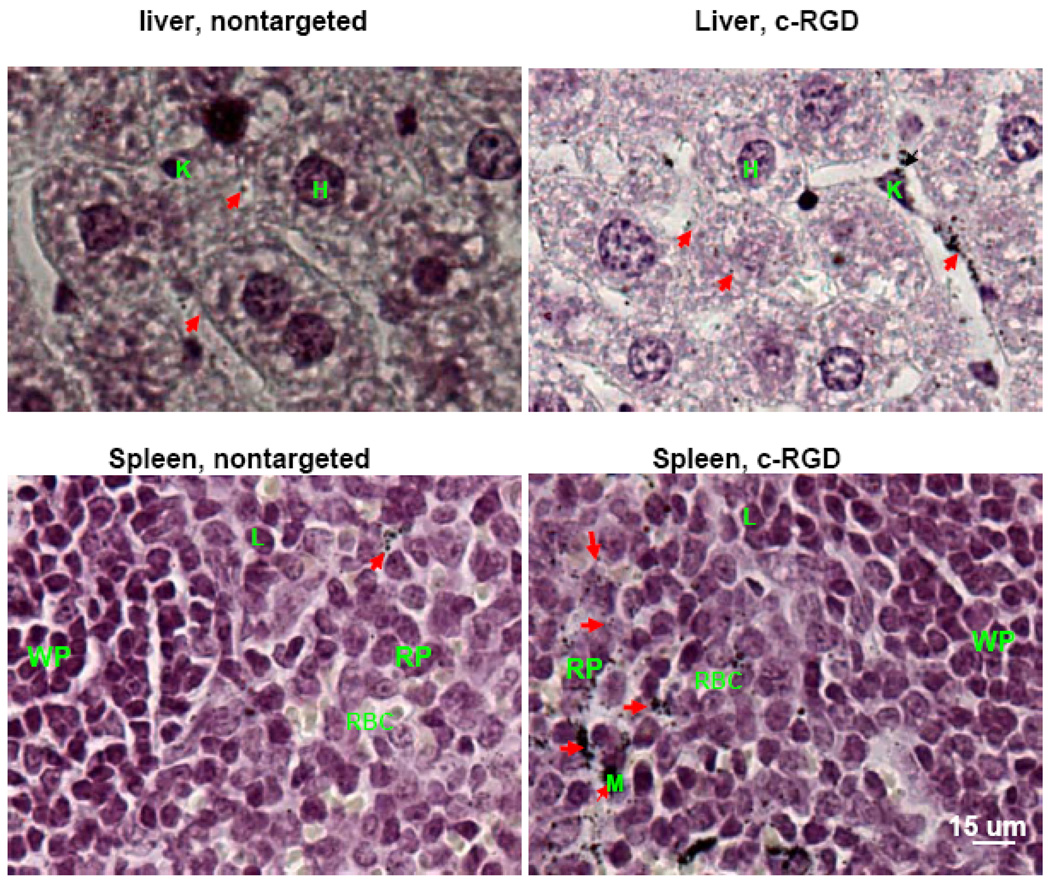
Since Au NRs were mainly accumulated in the RES, we further examined their distribution in the liver and spleen for nontargeted and targeted Au NRs with c-RGD peptide, which is chosen because of their higher uptake than other ligands (see Figure 8). In the liver, targeted Au NRs were found in Kupffer cells or in the Disse space around hepatocytes. Note that the Disse space is located in the liver between hepatocytes and hepatic sinus, and it largely exists in pore-structures in the endothelial cells that do not have basement membrane. Thus, the circulating NRs could enter the Disse space and vice versa, while the non-targeted ones in the Disse space may diffuse back to the hepatic sinus due to lack of cells uptake. A much lower number of nontargeted particles were found in the Disse region. In the spleen, the targeted NRs were mainly found in red pulp and along sinuses, while the nontargeted particles were much less found along the sinuses.
Figure 8. Light microscopic images of silver-enhanced and hematoxylin-stained liver and spleen tissue sections showing intratumoral distribution of nontargeted and αvβ3 integrin targeted Au NRs.
In comparison with nontargeted Au NRs, the αvβ3 integrin targeted Au NRs were mainly found in macrophages in the liver and in red pulp region of the spleen with significant higher affinities. Silver-enhanced Au NRs are marked by arrows, Hepatocyte cell by H; Lymphocyte cell by L; Kupffer cell by K; Microphage cell by M; Spleen red pulp by RP; and Spleen white pulp by WP.
In summary, we have reported quantitative tumor uptake studies for elongated gold nanocrystals that are covalently conjugated to tumor-targeting peptides. Quantitative pharmacokinetic and biodistribution data from xenograft animal models show that the use of active targeting ligands only marginally improves the total tumor uptake in comparison with control particles. However, the nature of targeting ligands can greatly affect the nanoparticle distribution in the tumor cells and the tumor microenvironments. A surprise finding is that active molecular targeting of the tumor microenvironments (including fibroblasts, macrophages, and vasculatures) does not significantly improve the total tumor accumulation of nanoparticles, at least for long-circulating and rigid gold nanoparticles. These results suggest that mass transport across the tumor vasculature is a rate-limiting step for nanoparticle delivery, and the kinetics of this step is largely unaffected by receptor binding. Thus, for photothermal cancer therapy, the preferred route of gold nanorods is intra-tumoral administration rather than intravenous injection.
Materials and Methods
Synthesis and characterization of Au NRs
Au NRs were synthesized according to the well-established seed-mediated growth method.57 In a typical procedure, 600 uL ice-cold 10 mM sodium borohydride (Sigma-Aldrich, St. Louis, MO, USA) was quickly added to 10 mL 0.5 mM auric acid (HAuCl4) (Sigma-Aldrich) dissolved in 0.2 M CTAB (Sigma-Aldrich) surfactant solution. The mixture was stirred for 4 min, producing a brown solution of small seed gold particles (2–5 nm). Gold nanoparticles were formed as indicated by the brown color. In a separate flask, 2 mL, 4 mM silver nitrate (Sigma-Aldrich) was added to 50 mL Au growth solution (0.2 M CTAB and 1 mM HAuCl4). Then, 0.7 mL, 8 mM L-ascorbic acid (Sigma-Aldrich) solution was added, reducing HAuCl4 to colorless HAuCl2. Subsequent injection of 0.12 mL seed solution initiated NR growth. The NR growth process was completed within 2 h. NRs were purified by centrifugation twice at 14,000 rpm for 20 min and were redispersed in DI water. The physical dimension of the NRs was characterized by Hitachi H-7500 transmission electron microscope (TEM) (Hitachi Ltd, Tokyo, Japan), and their hydrodynamic size and surface zeta potential were measured by dynamic light scattering (ZetaSizer NanoZS90, Malvern Instruments, Worcestershire, UK).
Preparation of ScFv EGFR and ATF
The ScFv B10 clone was isolated from the YUAN-FCCC human naive phage display library using solid phase biopanning methods. Large quantities were obtained from lysates of transformed TG1 Escherichia coli competent cells (Biochain Institute Inc, Hayward, CA, USA) after Ni2+ NTA-agarose column (Qiagen, Valencia, CA, USA) separation. The protein purity was greater than 95%, as determined by using sodium dodecyl sulfate (SDS)-PAGE. For ATF peptide production, a complementary DNA fragment encoding amino acids 1 to 135 of mouse uPA was isolated by polymerase chain reaction amplification, and then cloned into the pET101/D-TOPO expression vector (Invitrogen, Carlsbad, CA, USA). Recombinant ATF peptides were expressed in Escherichia coli BL21 (Invitrogen) and purified from bacterial extracts under native conditions using Ni2+ NTA-agarose columns.
Au NR PEGylation and ligand conjugation
Peptide ligands (ScFv EGFR, ATF and c-RGD) (c-RGD, i.e. c(RGDfK) was purchased from Peptide Interactional, Louisville, KY, USA) were conjugated to Au NRs in a three-step procedure: (i1) 50 uL 1 mM mPEG-SH (MW = 5 K. Laysan Bio, Inc, Arab, AL, USA) was added to 10 mL 1nM Au NRs, and the mixture solution was stirred for 10 min. PEGylated NRs were centrifuged at 14,000 rpm for 10 min and were redispersed in 10 mL DI water. (ii) 300 uL 1 mM HOOC-PEG-SH (MW = 5K, Rapp Polymers, Tubingen, German) was added and the solution was stirred for 1h, resulting in a mixed layer of mPEG-SH and HOOC-PEG-SH on the NR surface. The solution was centrifuged twice at 14,000 rpm for 10 min, and was redispersed in 100 uL MES pH 5.5 buffer. (iii) 0.2 mg EDC and 0.5 mg sulfo-NHS (Thermo Scientific, Rockford, IL, USA) were incubated with the functionalized Au NRs for 15 min to activate the carboxyl groups on the Au NRs. Excess EDC/sulfo-NHS was removed by centrifugation at 14,000 rpm for 10 min, and the activated NRs were mixed with the peptide ligands (ScFv EGFR, ATF or c-RGD) in PBS buffer (pH 7.4) in a NR:ligand molar ratio of 1:1000. The solution was gently shaken for 2 h at RT, and then stored at 4 °C. Prior to use, the NRs conjugates were centrifuged to separate free reactants. At each step, hydrodynamic size and seta potential were measured by the ZetaSizer Nano ZS90.
Cell culture and Au NRs cellular binding
Human small lung cancer cell carcinoma A549 cells were cultured in RPMI medium (Mediatech, Inc, Manassas, VA, USA) plus 10% fetal bovine serum (FBS) (Gemini Bio-Products, West Sacramento, CA, USA) at 37 °C under 5% CO2. For dark field imaging, the cells were grown on 8-well Lab-Tek chamber slides (Fisher Scientific, Pittsburgh, PA, USA) for 2 days. Then the Au NR conjugates were incubated with cultured cells for 2 h at 37 °C or 1 h at 4 °C. The cells were washed with 1× PBS and fixed with paramaldehyde before dark field imaging using an Olympus IX70 microscope.
Tumor inoculation and Au NRs injection
Female nu/nu mice, 7–8 weeks of age, were obtained from Taconic (Hudson, NY, USA). Mice were injected subcutaneously in the flank with 1×106 A549 cells suspended in 10 mM PBS. The tumors are allowed to grow for 1–2 weeks to reach a volume of ~100 mm3. Tumor bearing mice were administered with Au NR conjugates via tail vein injection.
ICP – MS
Organs and tumors were collected, washed in PBS buffer and dried at 60°C for 3 days. The dried tissues were dissolved in concentrated nitric acid (70%, 5 mL for liver and 1 mL for all others, gently shaken for 7 days). Tissue debris was removed by centrifugation at 3000 rpm for 10 min. After dilution in DI water, the gold content was analyzed by ICP-MS (HP 4500, Agilent Technologies, Santa Clara, CA, USA).
Histology and Silver Enhancement
Organs and tumors were collected and fixed in 10% buffered formalin (Sigma-Aldrich). Samples were dehydrated, embedded and sectioned to 4 um-thick slices. The tissue sections were de-waxed with xylene and washed consecutively with 100%, 90%, 70% and 30% ethanol, and were immersed in a silver enhancement solution which is composed of equal amount of solution A and B from the Silver Enhancer Kit (Sigma-Aldrich). After rinsing, the tissue sections were fixed with 2.5% sodium thiosulfate (Sigma-Aldrich) for 3 min, immersed in Haematoxylin (Sigma-Aldrich) for 40 sec, washed with DI water, and were then mounted for bright field light imaging.
Transmission Electron Microscopy
Organs and tumors were first fixed in 2.5% glutaraldehyde (in 0.1 M sodium cacodylate, pH 7.4), and then stained by OsO4 and K4Fe(CN)6 according to standard procedures. Tissue blocks in Epon resins were sectioned to around 100 nm in thickness for TEM imaging.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by grants from the NCI Centers of Cancer Nanotechnology Excellence (CCNE) Program (U54CA119338) and the Specialized Program of Research Excellence (SPORE) in Head and Neck Cancer (P50CA128613). We also thank Gregory Adams at Fox Chase Cancer Center for providing the ScFv B10 plasmid construct, and the Emory Robert P. Apkarian Integrated Electron Microscopy Core for TEM imaging of tissue sections. D.M.S. and S.M.N. are Distinguished Cancer Scholars of the Georgia Cancer Coalition (GCC).
Footnotes
Competing Interests Statement. The authors declare that they have no competing financial interests.
References
- 1.Ferrari M. Cancer Nanotechnology: Opportunities and Challenges. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 2.Farokhzad OC, Langer R. Nanomedicine: Developing Smarter Therapeutic and Diagnostic Modalities. Adv. Drug Del. Rev. 2006;58:1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Nie S, Xing Y, Kim GJ, Simons JM. Nanotechnology Applications in Cancer. Annu. Rev. Biomed. Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 4.Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven Challenges for Nanomedicine. Nat. Nanotechol. 2008;3:242–244. doi: 10.1038/nnano.2008.114. [DOI] [PubMed] [Google Scholar]
- 5.Heath JR, Davis ME. Nanotechnology and Cancer. Annu. Rev. Med. 2008;59:251–265. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis ME, Chen Z(G), Shin DM. Nanoparticles Therapeutics: An Emerging New Treatment Modality for Cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 7.Cho KJ, Wang X, Nie S, Chen Z(G), Shin DM. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 8.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine-Challenge and Perspectives. Angew. Chem. Intl. Ed. 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philips MA, Gran ML, Peppas NA. Targeted Nanodelivery of Drugs and Diagnostics. Nano Today. 2010;5:143–159. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie SM. Understanding and Overcoming Major Barriers in Cancer Nanomedicine. Nanomed. 2010;5:523–528. doi: 10.2217/nnm.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain RK. Transport of Molecules, Particles, and Cells in Solid Tumors. Annu. Rev. Biomed. Eng. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 12.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor Vascular Permeability and The EPR Effect in Macromolecular Therapeutics: A Review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura Y, Maeda H. A New Concept For Macromolecular Therapeutics in Cancer-Chemotherapy---Mechanism of Tumoritropic Accumulation of Proteins and The Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 14.Pirollo KF, Chang EH. Does a Targeting Ligand Influence Nanoparticle Tumor Localization or Uptake? Trends in Biotechnol. 2008;26:552–555. doi: 10.1016/j.tibtech.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody Targeting of Long-Circulating Lipidic Nanoparticles Does Not Increase Tumor Localization But Does Increase Internalization In Animal Models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett DW, Su H, Hildebrand IJ, Weber WA, Davis ME. Impact of Tumor-Specific Targeting on The Biodistribution and Efficacy of Sirna Nanoparticles Measured By Multimodality in Vivo Imaging. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi CHJ, Alabi CA, Webster P, Davis ME. Mechanism of Active Targeting in Solid Tumors with Transferrin-Containing Gold Nanoparticles. Proc. Nat. Amer. Soc. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt MM, Wittrup KD. A Modeling Analysis of The Effects Of Molecular Size and Binding Affinity on Tumor Targeting. Mol. Cancer Ther. 2009;8(10):2861–2871. doi: 10.1158/1535-7163.MCT-09-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garanger E, Boturyn D, Dumy P. Tumor Targeting With RGD Peptide Ligands-Design of New Molecular Conjugates for Imaging and Therapy of Cancers. Anticancer Agents Med. Chem. 2007;7:552–558. doi: 10.2174/187152007781668706. [DOI] [PubMed] [Google Scholar]
- 20.de Bruin K, Ruthardt N, Von Gersdorff K, Bausinger R, Wagner E, Ogris M, Bräuchls C. Cellular Dynamics of EGF Receptor-Targeted Synthetic Viruses. Mol. Ther. 2007;15:1297–1305. doi: 10.1038/sj.mt.6300176. [DOI] [PubMed] [Google Scholar]
- 21.Hilgenbrink AR, Low PS. Folate Receptor-Mediated Drug Targeting: From Therapeutics to Diagnostics. J. Pharm. Sci. 2005;94:2135–2146. doi: 10.1002/jps.20457. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Pirollo KF, Tang WH, Rait A, Chang EH. Transferrin-Liposome-Mediated Systemic P53 Gene Therapy in Combination With Radiation Results In Regression of Human Head And Neck Cancer Xenografts. Hum. Gene Ther. 1999;10:2941–2952. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- 23.Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The Transferrin Receptor Part I: Biology and Targeting With Cytotoxic Antibodies for The Treatment of Cancer. Clin. Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Dass CR, Choong PF. Selective Gene Delivery for Cancer Therapy Using Cationic Liposomes: in Vivo Proof of Applicability. J. Control Release. 2006;113:155–163. doi: 10.1016/j.jconrel.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Koyama Y, Hama Y, Urano Y, Nguyen DM, Choyke PL, Kobayashi H. Spectral Fluorescence Molecular Imaging of Lung Metastases Targeting HER2/Neu. Clin. Cancer Res. 2007;13(10):2936–2945. doi: 10.1158/1078-0432.CCR-06-2240. [DOI] [PubMed] [Google Scholar]
- 26.Fidaroval EF, El-Emir E, Boxer GM, Qureshi U, Dearling JLL, Robson MP, Begent RHJ, Trott KlausR, Pedlley RB. Microdistribution of Targeted, Fluorescently Labeled Anti Carcinoembryonic Antigen Antibody In Metastatic Colorectal Cancer: Implications For Radioimmunotherapy. Clin. Cancer Res. 2008;14(9):2639–2646. doi: 10.1158/1078-0432.CCR-07-2031. [DOI] [PubMed] [Google Scholar]
- 27.Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In Vivo Fate of Folate-Targeted Polyethylene-Glycol Liposomes in Tumor-Bearing Mice. Clin. Cancer Res. 2003;9:6551–6559. [PubMed] [Google Scholar]
- 28.Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. Multivalent Effects of RGD Peptides Obtained By Nanoparticle Display. J. Med. Chem. 2006;49:6087–6093. doi: 10.1021/jm060515m. [DOI] [PubMed] [Google Scholar]
- 29.McNeeley KM, Annapragada A, Bellamkonda RV. Decreased Circulation Time Offsets Increased Efficacy of Pegylated Nanocarriers Targeting Folate Receptors of Glioma. Nanotechnol. 2007;18 385101 (11pp) [Google Scholar]
- 30.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape Effects of Filaments Versus Spherical Particles in Flow and Drug Delivery. Nat. Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JH, von Maltzahn G, Zhang L, Derfus AM, Simberg D, Harris TJ, Ruoslahti E, Bhatia SN, Sailor MJ. Systematic Surface Engineering of Magnetic Nanoworms for in Vivo Tumor Targeting. Small. 2009;5(60):694–700. doi: 10.1002/smll.200801789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen AL, Hieftje GM. Inductively Coupled Plasma Mass Spectrometry and Electrospray Mass Spectrometry For Speciation Analysis: Applications and Instrumentation. Spectrochim. Acta, Part B. 2004;59(2):135–146. [Google Scholar]
- 33.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer Cell Imaging and Photothermal Therapy in The Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 34.Ding H, Yong KT, Roy I, Pudavar HE, Law WC, Bergey EJ, Prasad PN. Gold Nanorods Coated with Multilayer Polyelectrolyte as Contrast Agents for Multimodal Imaging. J. Phys. Chem. C. 2007;111:12552–12557. [Google Scholar]
- 35.Oldenburg AL, Hansen MN, Ralston TS, Wei A, Boppart SA. Imaging Gold Nanorods in Excised Human Breast Carcinoma By Spectroscopic Optical Coherence Tomography. J. Mater. Chem. 2009;19:6407–6411. doi: 10.1039/b823389f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal A, Huang SW, ODonnell M, Day KC, Day M, Kotov N, Ashkenazi S. Targeted Gold Nanorod Contrast Agent For Prostate Cancer Detection by Photoacoustic Imaging. J. Appl. Phys. 2007;102 064701 (4pp) [Google Scholar]
- 37.Li PC, Wang CRC, Shieh DB, Wei CW, Liao CK, Poe C, Jhan S, Ding AA, Wu YN. In Vivo Photoacoustic Molecular Imaging With Simultaneous Multiple Selective Targeting Using Antibody-Conjugated Gold Nanorods. Opt. Exp. 2008;16(23):18605–18615. doi: 10.1364/oe.16.018605. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Huff TB, Zweifel DA, He W, Low PS, Wei A, Cheng JX. In Vitro and In Vivo Two-Photon Luminescence Imaging of Single Gold Nanorods. Proc. Natl. Acad. Sci. U.S.A. 2005;102(44):15752–15756. doi: 10.1073/pnas.0504892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durr NJ, Larson T, Smith DK, Korgel BA, Sokolov K, Ben-Yakar A. Two-Photon Luminescence Imaging of Cancer Cells Using Molecularly Targeted Gold Nanorods. Nano lett. 2007;7(4):941–945. doi: 10.1021/nl062962v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu C, Irudayaraj J. Quantitative Evaluation of Sensitivity and Selectivity of Multiplex Nanospr Biosensor Arrays. Biophy. J. 2007;93:3684–3692. doi: 10.1529/biophysj.107.110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C, Chen Y, Wang T, Ma Z, Su Z. Biorecognition-Driven Self-Assembly of Gold Nanorods: A Rapid and Sensitive Approach Toward Antibody Sensing. Chem. Mater. 2007;19:5809–5811. [Google Scholar]
- 42.Mayer KM, Lee S, Liao H, Rostro BC, Fuentes A, Scully PT, Nehl CL, Hafner JH. A Label-Free Immunoassay Based Upon Localized Surface Plasmon Resonance of Gold Nanorods. ACS Nano. 2008;2(4):687–692. doi: 10.1021/nn7003734. [DOI] [PubMed] [Google Scholar]
- 43.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer Cells Assemble and Align Gold Nanorods Conjugated to Antibodies to Produce Highly Enhanced, Sharp and Polarized Surface Raman Spectra: A Potential Cancer Diagnostic Marker. Nano Lett. 2007;7(6):1591–1597. doi: 10.1021/nl070472c. [DOI] [PubMed] [Google Scholar]
- 44.von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally Guided Photothermal Tumor Therapy Using Long-Circulating Gold Nanorod Antennas. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi H, Niidome T, Nariai A, Niidome Y, Yamada S. Gold Nanorod-Sensitized Cell Death: Microscopic Observation of Single Living Cells Irradiated by Pulsed Near-Infrared Laser Light in The Presence of Gold Nanorods. Chem. Lett. 2006;35(5):500–501. [Google Scholar]
- 46.Tong L, Zhao Y, Huff TB, Hansen MN, Wei A, Cheng JX. Gold Nanorods Mediate Tumor Cell Death by Compromising Membrane Integrity. Adv. Mater. 2007;19:3136–3141. doi: 10.1002/adma.200701974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Gold Nanorod Assisted Near-Infrared Plasmonic Photothermal Therapy (PPTT) of Squamous Cell Carcinoma In Mice. Cancer Lett. 2008;269(1):57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li JL, Day D, Gu M. Ultra-Low Energy Threshold for Cancer Photothermal Therapy Using Transferrin-Conjugated Gold Nanorods. Adv. Mater. 2008;20:3866–3871. [Google Scholar]
- 49.Niidome T, Akiyama Y, Yamagata M, Kawano T, Mori T, Niidome Y, Katayama Y. Poly(ethylene glycol)-Modified Gold Nanorods as a Photothermal Nanodevice for Hyperthermia. J. Biomater. Sci. 2009;20:1203–1215. doi: 10.1163/156856209X452953. [DOI] [PubMed] [Google Scholar]
- 50.Jiang T, Zhang C, Zheng X, Xu X, Xie X, Liu H, Liu S. Noninvasively Characterizing The Different Αvβ3 Expression Patterns in Lung Cancers With RGD-USPIO Using A Clinical 3.0T MR Scanner. Int. J. Nanomed. 2009;4:241–149. doi: 10.2147/ijn.s7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C, Lv F, Zhou L, Li X, Wu XX, Hoffman RM. Effect of Verapamil on The Expression of EGFR and NM23 in A549 Human Lung Cancer Cells. Anticancer Res. 2009;29:27–32. [PubMed] [Google Scholar]
- 52.Kim IY, Kang YS, Lee DS, Park HJ, Choi EK, Oh YK, Son HJ, Kim JS. Antitumor Activity Of EGFR Targeted Ph-Sensitive Immunoliposomes Encapsulating Gemcitabine In A549 Xenograft Nude Mice. J. Control. Release. 2009;140:55–60. doi: 10.1016/j.jconrel.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto M, Ikeda K, Ohshima K, Tsugu H, Kimura H, Tomonaga M. Increased Expression of Low Density Lipoprotein Receptor-related Protein/a2- Macroglobulin Receptor in Human Malignant Astrocytomas. Cancer Res. 1997;57:2799–2805. [PubMed] [Google Scholar]
- 54.Hattori N, Sisson TH, Xu Y, Desai TJ, Simon RH. Participation Of Urokinase-Type Plasminogen Activator Receptor In The Clearance Of Fibrin From The Lung. Am. J. Physiol. Lung Cell Mol. Physiol. 1999;277:573–579. doi: 10.1152/ajplung.1999.277.3.L573. [DOI] [PubMed] [Google Scholar]
- 55.Hood JD, Cheresh DA. Role of integrin in Cell Invasion and Migration. Nat. Rev. Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 56.Jin H, Varner J. Integrins: Roles in Cancer Development and as Treatment Targets. Br. J. Cancer. 2004;90:561–565. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blasi F, Carmeliet P. uPAR: a Versatile Signaling Orchestrator. Nat. Rev. Mol. Biol. 2002;3:933–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 58.Nikoobakht B, El-Sayed MA. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003;15(10):1957–1961. [Google Scholar]
- 59.Josephson L, Tung CH, Moore A, Weissleder R. High-Efficiency Intracellular Magnetic Labeling with Novel Superparamagnetic-Tat Peptide Conjugates. Bioconjugate Chem. 1999;10:186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 60.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In Vivo Cancer Targeting And Imaging With Semiconductor Quantum Dots. Nat. Biotechnol. 2004;22(8):969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 61.Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC. Precise Engineering of Targeted Nanoparticles by Using Self-Assembled Biointegrated Block Copolymers. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.