Abstract
The potential adverse effects of Bisphenol A (BPA), a synthetic xenoestrogen, have long been debated. Although standard toxicology tests have revealed no harmful effects, recent research highlighted what was missed so far: BPA-induced alterations in the nervous system. Since 2004, our laboratory has been investigating one of the central effects of BPA, which is interference with gonadal steroid-induced synaptogenesis and the resulting loss of spine synapses. We have shown in both rats and nonhuman primates that BPA completely negates the ~70–100% increase in the number of hippocampal and prefrontal spine synapses induced by both estrogens and androgens. Synaptic loss of this magnitude may have significant consequences, potentially causing cognitive decline, depression, and schizophrenia, to mention those that our laboratory has shown to be associated with synaptic loss. Finally, we discuss why children may particularly be vulnerable to BPA, which represents future direction of research in our laboratory.
The potential risk of Bisphenol A
Since the 1950s, the synthetic xenoestrogen, Bisphenol A (BPA), has been employed in the manufacturing of plastics that have a broad range of uses, including dental prostheses and sealants [143], the polycarbonate lining of metal cans used to preserve foods [66], as well as such items as baby bottles [8] and clear plastic cages for housing laboratory animals [57]. BPA is also used as an additive in many products, with a global production rate of >6 billion pounds per year. Polycarbonate is less durable than commonly believed, because the ester-bond linking BPA molecules can be hydrolyzed, and this hydrolysis increases dramatically at high and low pH and as temperature increases. Thus, BPA leaches out from tin cans and polycarbonate plastic containers and gets into food and beverages, even under normal conditions of use like washing and sterilizing [8, 9, 57]. As a result, exposure measurement data from several countries, including the United States, consistently indicate that human beings are widely exposed to low levels of BPA, probably on a continuous basis [162]. There is considerable debate, however, whether this exposure represents an environmental hazard.
Because BPA has been believed to be an estrogen-agonist compound, much of the perceived risks of BPA exposure are derived from the large body of knowledge about the neurotrophic effects of estrogens, evolving from studies of sexual differentiation. As a result, to understand the risks of BPA exposure, we first need to discuss the neurodevelopmental actions of gonadal steroid hormones. In most vertebrates, males and females exhibit striking differences in sexual behavior and neuroendocrine function that are appropriate for the sex of the individual. The development and expression of these sexually differentiated patterns of behavior and neuroendocrine function occur as a result of a temporally ordered sequence of responses, in which gonadal steroid hormones play a critical role. Testosterone secreted by the testis, in concert with estradiol-17β derived either from the circulation or from local androgen metabolism, acts on the entire reproductive axis to bring about the development of normal masculine or feminine phenotype. Although many of them are involved in reproduction, sex differences are by no means confined to reproductive functions. In human beings, for example, there are sex differences in normal cognitive behavior and, quite importantly, in the incidence of neurological disorders and the ability of the brain to recover from accidental or disease-related damage [97, 132, 173, 174]. It has long been hypothesized that certain morphological/cellular mechanisms are related to cognitive performance, especially in the prefrontal cortex and hippocampus, implying sex differences in brain morphology also. Indeed, the effects of gonadal steroid hormones on the structure and function of the rodent hippocampus are evident as early as the first postnatal week of life. Androgens produced by the testis mediate sexual differentiation of the hippocampus. In some strains of mice, males have more granule cells in the dentate gyrus than females [172]. Likewise, male rats have a larger and more asymmetric dentate gyrus when compared with females [121, 122], while sex differences have also been demonstrated in the apical dendritic structure and dendritic branching patterns of CA3 pyramidal neurons. Because the apical dendrites of CA3 pyramidal cells are the targets of afferent mossy fibers from granule cells, the above observations are consistent with the hypothesis that more granule cells in a larger dentate gyrus provide an increased input to CA3 in males when compared with females [114].
Because circulating gonadal steroid hormones regulate the development and expression of sexually dimorphic functions, the potential exists for biologically active environmental substances to mimic or antagonize the effects of endogenous hormones, interfering with normal sexual differentiation [38, 127]. Indeed, there have been numerous studies demonstrating effects of substances derived from either plant foods [76, 115, 160, 169–171], agricultural chemical usage [68, 163], or industrial chemical synthesis [99, 118, 123] on aspects of sexual differentiation in animals. These observations have prompted research and regulatory efforts around the world to develop simple and cost-effective screening methods that can be used for identifying potentially harmful chemical agents. From these efforts came a wide range of bioassay systems, such as the rat uterotrophic assay [4], that can predict, to a considerable extent, the likely hormonal activity of novel substances introduced into the environment. At the same time, however, it became clear that there are certain hormonally active chemical agents that exhibit radically different potencies in different bioassay systems, making it difficult to assess their potential developmental effects [42, 104, 123]. This is also true in the case of our synthetic xenoestrogen, BPA, spurring much of the debate surrounding the usage of this substance. On the one hand, BPA has been reported, for example, not to induce significant reproductive abnormalities in a two-generation trial in rats, at doses up to 200 µg/kg [25], which can be explained by the relatively low affinity of BPA toward the nuclear estrogen receptors (ER), ERα and ERβ, that mediate much of the estrogenic activity along the reproductive axis. On the other hand, a number of reports indicate that doses of BPA in the 2–100 µg/kg range induce changes in the development of the prostate, preputial, and mammary glands in mice [41, 99, 108, 118, 161, 168]. These developmental actions of BPA are particularly worrisome, because relatively high levels of BPA have been found in the human amniotic fluid (8.3 ng/ml) and placenta (12.7 ng/g) [59, 129] versus adult human serum levels of 0.3–4.4 ng/ml [158]. Despite the obvious uncertainty, however, the initial conclusion regarding the safety of BPA has affirmed that human exposure to BPA is insufficient to elicit significant estrogenic responses [16].
More relevant to our topic is that unexpected anomalies have emerged from studies on the effects of BPA in the developing central nervous system (Table 1). While estradiol-17β defeminizes the development of sex behavior and reproductive neuroendocrine function, by substituting for local aromatization of circulating testosterone [97], low doses of BPA do not masculinize the behavior of female rats [26, 27], suggesting weak or no estrogen-agonist activity from BPA. However, there are some unexpected long-term effects of BPA on the development of non-reproductive behaviors. At doses that are below the 50 µg/kg/day ‘reference safe daily limit’ for human exposure recommended by the US Environmental Protection Agency (EPA), BPA interferes with the development of play and maze learning behaviors in both female and male rodents [11, 17, 18, 26, 27]. These observed effects are diametrically opposite to what could be predicted for a substance with estrogen-agonist activity, raising the possibility that in addition to being a weak estrogen-agonist, BPA may act as an estrogen-antagonist as well.
TABLE 1.
Effects of Bisphenol A (BPA) exposure on higher brain functions.
| Reference | Species | BPA dose | Findings |
|---|---|---|---|
| Farabollini et al, 1999 [27] |
rat, m1&f2 | 40 µg/kg, po3 G4(−10)-P521; 400 µg/kg, po, G14-P6 |
Reduced exploration and anxiety in males. Reduced exploration and motor activity in females. |
| Aloisi et al, 2002 [2] |
rat, m&f | 40 µg/kg, po, G1–21 or P1–21 |
Altered behavioral response to pain. |
| Dessi-Fulgheri et al, 2002 [18] |
rat, f | 40 µg/kg, po, P0–21; 400 µg/kg, po, G14-P6 |
Altered social behavior in a play situation. |
| Farabollini et al, 2002 [26] |
rat, m&f | 40 µg/kg, po G1–21 or P1–21 |
Increased defensive behavior and reduced sexual performance in males. Increased sexual behavior in females. |
| Carr et al, 2003 [11] |
rat, m&f | 100 µg/kg or 250 µg/kg, po, P1–21 |
Low dose disrupts normal gender dependent pattern of acquisition in Morris water maze. High dose alters retention of spatial information. |
| Negishi et al, 2004 [109] |
rat, m | 100 µg/kg, po, G3-P20 |
Increased number of escape failures in active avoidance. |
| Della Seta et al, 2006 [17] |
rat, m | 40 µg/kg, po, P23–30 |
Reduced exploratory drive in juveniles Altered socio-sexual behavior in adults. |
| Fujimoto et al, 2006 [30] |
rat, m&f | 15 µg/kg, po, G15–21 |
Increased immobility of males in forced swimming. |
| Tian et al, 2010 [151] |
mouse, m&f | 100 µg/kg or 500 µg/kg, po, P(−7)-36 |
Decreased anxiety in open field and elevated plus maze. Working memory impairment in Y-maze. Recognition memory Impairment in object recognition test. |
Abbreviations: 1male; 2female; 3per os; 4gestational day; 5postnatal day.
Gonadal steroid-induced synaptogenesis: A major target of Bisphenol A?
In the central nervous system, gonadal steroid hormones not only influence neurodevelopment and sexual differentiation, but they also have a so-called ‘activational effect’, an effect involving the modulation of function and activity in the mature brain [97]. While earlier studies have investigated the developmental effects of BPA, as we discussed in the previous section, the potential interference of BPA with the central activational effects of gonadal steroid hormones has remained largely unnoticed. This may become a substantial problem considering that these activational effects are rather important in higher brain functions including cognition. It is well documented that both estrogens and androgens significantly influence cognitive performance in human beings as well as in laboratory animals [20, 24, 63, 73, 106]. Whether BPA has the capability of interfering with the activational effects of estrogens, and thus disrupting cognitive functions, remains currently unknown, except for a preliminary study from our laboratories indicating that acute BPA exposure (300 µg/kg) indeed reduces learning capabilities in rats (VN Luine, unpublished observation).
In order to understand how BPA may disrupt estrogen’s activational effect on cognition, we need to review some of the cellular mechanisms that are thought to underlie these activational effects. Until quite recently, the prevailing view has been that the mature mammalian brain has only limited capacity for repair and regeneration. Neurotrophic mechanisms, in which new neurons and glial cells are added and new synaptic connections are formed, have been assumed to be largely or completely suppressed in adulthood. Effects of gonadal steroid hormones on the brain have been considered within the context of a similar conceptual framework, based on the assumption that effects on neurotrophic processes are confined to early development. Responses to gonadal steroid hormones later in life have been viewed as being primarily ‘activational’, modulating the function of pre-existing organized pathways [97]. Over the last two decades, however, a growing body of experimental evidence has emerged, which challenges these simple generalizations. It is now clear that the mature central nervous system can in fact undergo significant remodeling, including the generation of new neurons and synaptic connections [39, 58, 102, 103]. Nowhere it is more obvious than in the hippocampus (Figure 1) and prefrontal cortex. In adulthood, these areas retain the potential for considerable plasticity in response to changing levels of circulating gonadal steroid hormones. This was first recognized in studies on the cyclical alterations in hippocampal activity that occurs during the female reproductive cycle [175]. Subsequent extensive work has demonstrated that both estrogen and androgen administration reverses the loss of CA1 dendritic spines and spine synapses observed following gonadectomy, in both male and female rodents and nonhuman primates [48, 52, 81, 83, 84, 86, 87, 92, 93, 95, 176, 177]. Similar structural responses to estrogens have been revealed in the prefrontal cortex of monkeys [53, 147]; and our recent study also shows robust synaptogenesis induced by both estrogens and androgens in the rat prefrontal cortex [47].
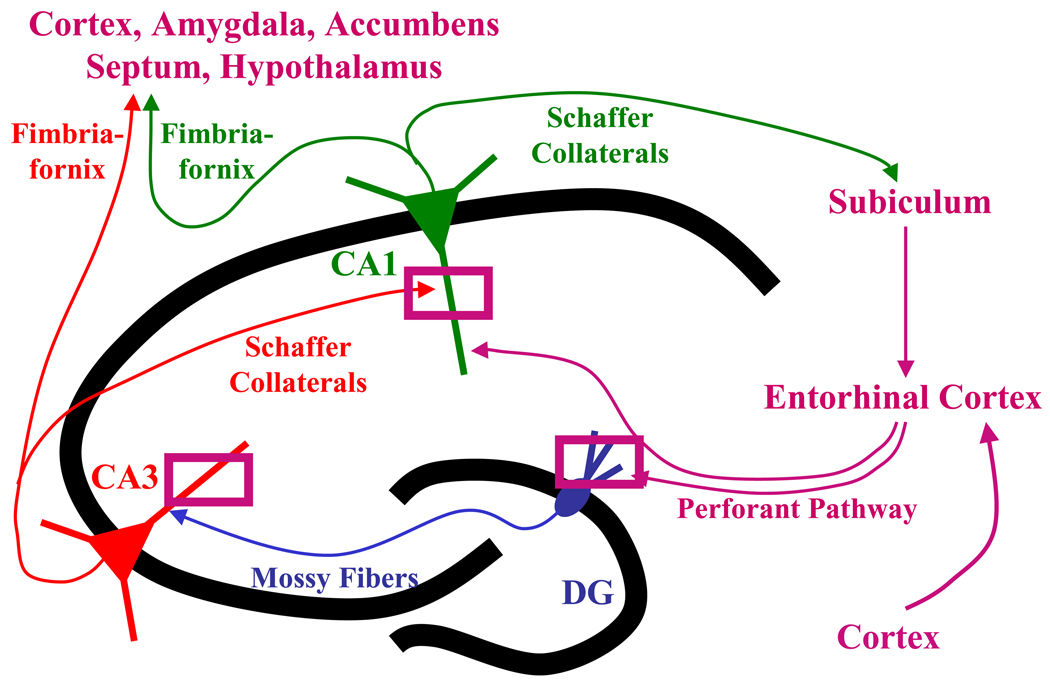
Figure 1.
A schematic drawing depicting the hippocampal circuitry. The three rectangles represent our sampling areas in the CA1 and CA3 stratum radiatum and in the dentate gyrus (DG) stratum moleculare, where we take samples for electron microscopic stereological estimation of spine synapse numbers.
What conceptually links estrogen-induced synaptic remodeling with its activational effect on cognition is the hypothesis that rapid remodeling of dendritic spines and their synapses may represent morphological substrates for learning and memory [64, 67]. We define the term “remodeling of spine synapses” as a neuroplasticity mechanism that includes the loss of existing and generation of new spine synapses. Our laboratories have demonstrated that estradiol-17β is capable of inducing the formation of new synaptic contacts with extraordinary rapidity (within 30 minutes) in the hippocampus of ovariectomized rats, which is clearly associated with enhanced performance in the object placement test of spatial memory [90, 96]. There are many such examples from other laboratories, see for example [89], reinforcing the fact that there is an excellent correlation between spine synapse remodeling and cognitive performance in various tests of cognitive function. These observations suggest that remodeling of dendritic spines and their synapses in the hippocampus and prefrontal cortex are critical mechanisms that appear to contribute to the activational effect of gonadal steroid hormones on cognitive function. If we apply the conceptual framework of this cognition / synaptic plasticity association to the above-discussed interference from BPA with the development of cognitive functions [11, 17, 18, 26, 27, 151], the question arises whether BPA exposure has the ability to impact spine synapses in the prefrontal cortex and hippocampus.
Bisphenol-A blocks the rapid synaptogenic response to estradiol in the female rat hippocampus
Although the suggestion that BPA may influence spine synapses came originally from observations that BPA affects the development of cognitive functions [11, 17, 18, 26, 27, 151], we have initially tested BPA in an experimental paradigm that is based on the activational effects of estradiol. The main reason behind our decision came from an interesting ambiguity in the peripheral vs. central effectivity of estradiol, and from the fact that the developmental influence of BPA appears to be limited. We have reported earlier that estradiol-17α more powerfully induces hippocampal spine synapse growth than its isomer, estradiol-17β. This is contrary to what is seen in the periphery, where unlike estradiol-17β, estradiol-17α possesses minimal uterotrophic activity [96]. These observations suggest that the response of spine synapses to estradiol may depend on mechanisms that are different from those in the periphery. The periphery is rich in nuclear ERs, mainly ERα, for which BPA has low affinity, as indicated by the weak uterotrophic activity of BPA [94]. If BPA followed the signaling pathways and characteristics of estradiol-17α, i.e., weak in the periphery and strong centrally, BPA exposure should result in a substantial remodeling of hippocampal spine synapses. The question was, is this response synapse growth or synapse loss?
As we report in our relevant paper [94], ovariectomized adult female rats were treated subcutaneously with various combinations of estradiol-17α (45 µg/kg), estradiol-17β (60 µg/kg), BPA (40, 120, 300, and 400 µg/kg), and sesame oil vehicle. At the 30-min time point following drug treatment, the volumetric density of spine synapses in the CA1 hippocampal area was estimated using electron microscopic stereology. In line with our earlier findings [96], treatment of ovariectomized rats with either estradiol-17α or estradiol-17β both increased CA1 spine synapse density almost two-fold when compared to oil-treated controls. Estradiol-17α was more potent as it elicited the same response at a much lower dose than estradiol-17β. On the other hand, administration of 300 µg/kg BPA alone did not increase CA1 spine synapse density in ovariectomized rats. Instead, surprisingly, we observed a significant decrease in spine synapse levels, demonstrating for the first time that BPA exposure elicits an acute hippocampal spine synapse loss. This loss is similar to that found in animals that are incapable of showing CA1 spine synapse responses to estrogens, such as males and reproductively senescent females [83]. We hypothesized that the ability of BPA to further reduce CA1 spine synapse density in ovariectomized rats may reflect the antagonism of either residual gonadal estrogen effects or estrogen actions derived from adrenal steroidogenesis or dietary phytoestrogen intake. When testing this hypothesis, at a dose range of 300–400 µg/kg, BPA completely blocked the synaptogenic effects of both estradiol-17α and estradiol-17β in the CA1 area, confirming that BPA acts as an estrogen-antagonist in this context. Importantly, the blockade was dose-dependent, with even the 40 µg/kg BPA dose providing significant reductions in CA1 synapse density. This anti-synaptogenic effect of BPA is in line with our above-mentioned preliminary study indicating that acute BPA exposure reduces learning capabilities in rats (VN Luine, unpublished observation). By contrast, BPA administration did not influence the uterotrophic response to estradiol-17β, even at a high dose (300 µg/kg), which is in line with an earlier study that finds BPA to be safe at up to 200 µg/kg in a two-generation trial of reproductive abnormalities [25].
From these observations, we can conclude that responses to BPA are much stronger centrally than in the periphery, implying different underlying mechanisms.
Bisphenol-A prevents the synaptogenic response to testosterone in the hippocampus and medial prefrontal cortex of male rats
As we mentioned above, BPA has been considered as a xenoestrogen, a compound with estrogenic activity. This thinking may lead to the speculation that BPA probably does not interfere with the activational effects of androgens, suggesting a sex difference in the response to BPA in adults. This potential sex difference could be considered in the context of cognition and spine synapse remodeling, because modulation by androgens is just as critical in cognitive functions in males [63], as estrogens are in females. More importantly, our earlier studies have demonstrated that spine synapse remodeling in adult male rats responds primarily to androgens, while synaptic responses of males to estrogens, especially in the hippocampus, are quite limited [50, 83, 84, 92]. However, recent experiments have unexpectedly revealed that BPA also antagonizes androgen receptor-mediated transcriptional activities [126, 138, 142, 178], questioning the above generalizations. These experiments raise the possibility that BPA, quite unexpectedly for a compound believed to be estrogenic, also interferes with the physiology and morphology of the adult male brain, including prefrontal and hippocampal synaptogenic responses to testosterone.
As we report in our relevant paper [88], castrated or sham-operated adult male rats were treated subcutaneously with different combinations of BPA (300 µg/kg), testosterone propionate (1.5 mg/kg), and sesame oil vehicle for 3 days. Twenty-four hours after the last treatment, brains were processed for electron microscopic stereology, as well as for immunocytochemical detection and light microscopic semi-quantitative analysis of astroglia processes. The number of asymmetric spine synapses, as well as the density of astroglia processes in layer II/III of medial prefrontal cortex and stratum radiatum of the CA1 hippocampal area were estimated. In both regions analyzed, BPA reduced the number of spine synapses in sham-operated, gonadally intact animals, which was accompanied by a compensatory increase in astroglia process density that is likely secondary to the loss of spine synapses. To test the hypothesis that this BPA-induced reduction in synapse levels is mediated by antagonism of synaptogenic responses to testosterone, we coadministered BPA with testosterone supplementation to castrated males. In this paradigm, BPA prevented both prefrontal and hippocampal synaptogenic responses to testosterone, confirming that BPA acts as an anti-androgen in this context.
In summary, our findings demonstrate that BPA exposure results in a severe loss of spine synapses in both hippocampus and prefrontal cortex of adult male rats, confirming that there is no sex difference in the response to BPA in adult rats, at least in the context of spine synapse remodeling. Although we discuss it later, it has to be noted here that these findings in male rats provide important insights into the potential mechanisms underlying the anti-synaptogenic effect of BPA.
Bisphenol-A inhibits gonadal steroid hormone-induced prefrontal and hippocampal synaptogenesis in female and male nonhuman primates
A potential limitation of our BPA studies in rodents, detailed above, is that the predictive validity of these rodent models is supposedly restricted based on considerable differences between the rodent and the human endocrine systems and brains. In spite of the fact that standard toxicology tests adopted to justify current safe limits for BPA are based on rodent models (see http://www.epa.gov/NCEA/iris/subst/0356.htm), nowadays the EPA and the Food and Drug Administration are calling for studies based on primate models that are more relevant to human health (see http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-0038b1_01_02FDA%20BPA%20Draft%20Assessment.pdf). In response to these requests, we have set out to reproduce our rodent findings in monkeys, to demonstrate for the first time the harmful effects of BPA exposure in a nonhuman primate model.
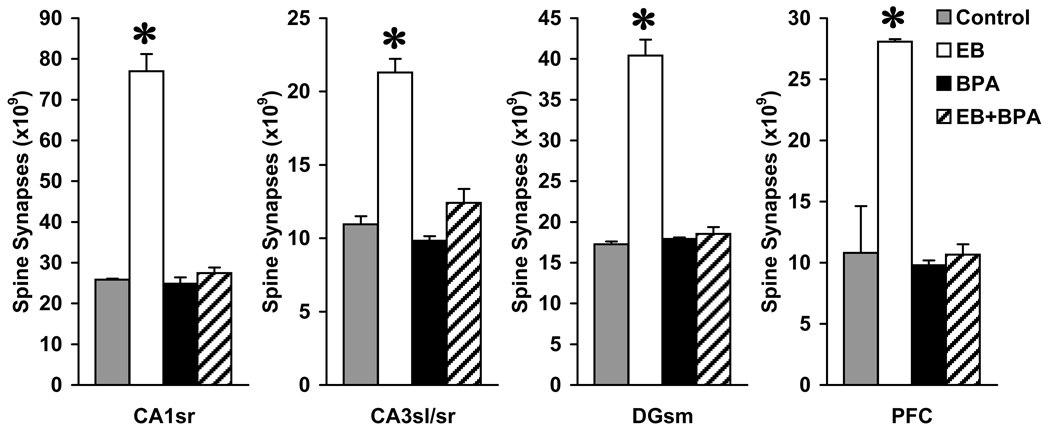
As we report in our relevant paper [82], ovariectomized adult female African green monkeys (Chlorocebus aethiops sabaeus) of reproductive age were treated with different combinations of estradiol-17β benzoate (a long-acting estradiol ester), BPA (50 µg/kg/day - the ‘reference safe daily limit’ of the EPA), and vehicle for 28 days. Estradiol-17β benzoate was delivered with silastic capsules, while BPA was administered with Alzet osmotic minipumps, both implanted subcutaneously at the time of ovariectomy. At the end of the 28-day treatment period, blood samples were collected and the brains processed for electron microscopic stereological calculation of spine synapse numbers in CA1 and CA3 stratum radiatum, dentate gyrus stratum moleculare, and layer II/III of Walker’s area 46 (the region corresponding to the rat medial prefrontal cortex). Serum estradiol-17β levels in monkeys that did not receive estradiol supplementation were <15 pg/ml, which is the sensitivity threshold of the immunoassay used. In animals that received estradiol supplementation, serum estradiol-17β levels were 80–90 pg/ml, which is equivalent of levels seen in the low estrogen days of the monkey menstrual cycle [56]. In all areas analyzed, estradiol-17β supplementation dramatically increased the number of spine synapses compared to vehicle-treated controls. By contrast, spine synapse numbers in BPA-treated monkeys were not significantly different from controls, irrespective of conditions of estradiol exposure (Figure 2).
Figure 2.
The number of spine synapses in CA1 stratum radiatum (CA1sr), CA3 stratum lucidum and radiatum (CA3sl/sr), dentate gyrus stratum moleculare (DGsm), and layer II/III of prefrontal cortex (PFC) of vehicle-treated (Control), estradiol-treated (EB), Bisphenol A-treated (BPA), and estradiol + BPA-treated (EB + BPA) monkeys. The asterisks indicate that the number of spine synapses in all examined areas of estradiol-treated animals is significantly higher than those in any other groups (Tukey test, p<0.001).
In separate studies, we have shown that CA1 spine synapse density is reduced by castration in male nonhuman primates [84], in line with our findings in male rats [83]. In addition, we had a fortunate opportunity that in parallel with the above-detailed female experiment, we could also treat two gonadally intact male monkeys with BPA and analyze their prefrontal and hippocampal spine synapses. For comparison, we pooled some spine synapse data obtained from intact male monkeys in our earlier studies [84]. Although this experimental setup is far from accepted standards and the insufficient number of subjects excludes statistical analysis, it is still informative that spine synapse numbers in BPA-treated animals were considerably decreased, to levels similar to those observed in castrated monkeys [84].
These observations demonstrate that gonadal steroid hormones are responsible for maintaining physiological levels of spine synapses in the prefrontal cortex and the hippocampus of nonhuman primates. BPA appears to strongly interfere with these mechanisms, as continuous exposure to a low, 50 µg/kg daily dose completely abolishes gonadal steroid hormone-induced spine synapse growth. This was the first demonstration of the harmful effects of BPA in a primate model, providing valuable information with significant relevance to human health. In addition, refuting earlier criticisms of rodent models, we show that the extensive spine synapse remodeling in rats observed in response to both gonadal steroid hormones and BPA exposure can fully be reproduced in a nonhuman primate model.
No difference in the synaptogenic effect of oral vs. subcutaneous Bisphenol A
As we detailed above, BPA antagonizes spine synapse growth in the prefrontal cortex and hippocampus of both rats [88, 94] and nonhuman primates [82]. Based on these findings, it is conceivable that low-dose BPA may have widespread influence on the structure and function of the brain, because remodeling of prefrontal and hippocampal spine synapses plays a critical role in higher brain activities such as cognition [64] and mood [43]. However, we have used subcutaneous injections as route for BPA administration, which is less relevant for everyday human BPA exposure. Subcutaneously delivered BPA escapes normal first-pass metabolism in the liver [116, 153, 156], by which BPA is rapidly metabolized and excreted [7, 101]. To address this limitation of our previous studies, we compared the effects of oral versus subcutaneous exposure to BPA on the number of spine synapses in the prefrontal cortex and hippocampus of male rats.
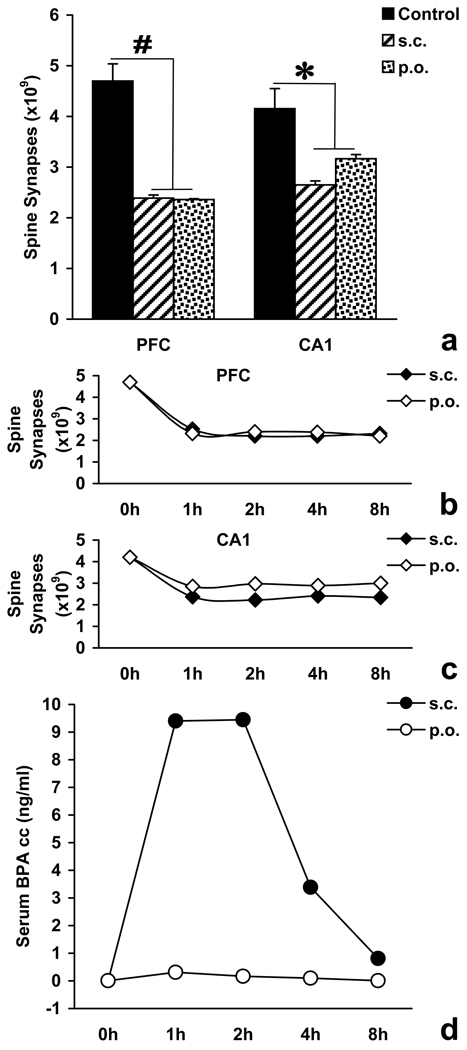
In this recently performed preliminary study, gonadally intact adult male Sprague Dawley rats were treated with either BPA (300 µg/kg) or vehicle for three days. BPA was delivered either subcutaneously as in earlier studies, or directly into the stomach via gastric cannula. Animals were sacrificed 24 h after the last treatment by transcardial perfusion fixation, their brains removed and processed for electron microscopic stereological estimation of prefrontal and hippocampal spine synapses as we described earlier [43, 44]. The number of asymmetric spine synapses in layer II/III of the medial prefrontal cortex and stratum radiatum of the CA1 hippocampal subfield was counted. We analyzed these particular regions because our earlier studies have demonstrated strong synaptogenic responses to androgens in these areas of the adult male brain [47, 83]. Two-way ANOVA revealed significant treatment (F2,12=44.122 p<0.001) and interaction effects (F2,12=4.724 p=0.031), while the sampling area effect was not significant (F1,12=0.919 p=0.357). When compared with vehicle-treated controls, both subcutaneous and oral BPA administrations significantly decreased the number of prefrontal spine synapses by 49.1% and 49.8%, respectively, and by 36.2% and 23.8%, respectively, in the CA1 area. Spine synapse levels after subcutaneous versus oral treatments were not significantly different (Figure 3a).
Figure 3.
Panel a: Effect of subcutaneous (s.c.) or per oral (p.o.) administration of Bisphenol A (300 mg/kg/day, dissolved in sesame oil vehicle, for three days) on the number of spine synapses in layer II/III of medial prefrontal cortex (PFC) and in stratum radiatum of CA1 hippocampal area, compared to vehicle-treated controls (Control). #p<0.001; *p<0.05. Panels b–c: Changes in the number of spine synapses in PFC and CA1 over time following single s.c. and p.o. doses of 300 mg/kg Bisphenol A. Panel d: Clearance curves for single s.c. and p.o. doses of 300 mg/kg Bisphenol A.
We also analyzed the temporal characteristics of serum BPA levels and spine synapse changes by sacrificing a rat at each of the 0, 1, 2, 4, and 8 h time points after both oral and subcutaneous BPA (300 µg/kg) administrations. Blood samples were collected, followed immediately by transcardial perfusion fixation and brain tissue processing for electron microscopic stereological analysis [43, 44]. Serum samples were analyzed for BPA content as described earlier [149]. From individual serum BPA measurements, clearance curves were constructed for both oral and subcutaneous exposures. Maximum serum concentration (Cmax) and time of maximum concentration (Tmax) were obtained from the curves. Area under the curve was calculated by using the linear trapezoidal rule and the assumption that serum BPA levels before administration (at T0) were zero. We observed marked changes in serum BPA concentrations (Figure 3d). Cmax for subcutaneous administration was 9.45 ng/ml with Tmax = 2 h, while in the case of oral treatment, Cmax was only 0.31 ng/ml with Tmax = 1 h. According to expectations, we observed an approximately 40-fold higher BPA exposure level following subcutaneous administration versus oral treatment, as the subcutaneous / oral area under the curve ratio was 35.37 / 0.89 = 39.74. Quite surprisingly, however, both administration methods resulted in similarly severe spine synapse losses in the prefrontal cortex and the CA1 area, despite the extreme differences in serum BPA concentrations, indicating that spine synapse remodeling is very sensitive to BPA (Figure 3b–c). The synaptic loss occurred rapidly, within the first hour of BPA administration, in line with our earlier observations in females [94]. Thereafter, spine synapse levels remained constantly reduced without signs of recovery, although serum BPA concentrations showed a rapid descent after the first two hours, suggesting that BPA-induced synaptic loss is persistent.
In our previous studies [82, 88, 94], we have used the subcutaneous administration route to deliver BPA, which does not fully reproduce human BPA exposure [7, 101]. Human beings mostly ingest BPA with contaminated food and drink, which is then transported through the enterohepatic circulation to the liver. During first-pass metabolism, the liver enzyme, UDP-glucuronosyltransferase conjugates BPA [179], and the conjugate is then excreted into the urine in adult humans [158]. This effective clearance of BPA by first-pass metabolism [146, 153, 156] does not occur in case of subcutaneous exposure. The importance of first-pass metabolism is further emphasized in postnatal animals that normally display limited UDP-glucuronosyltransferase activity, resulting in similar serum BPA concentrations following both oral and subcutaneous administrations [149]. Our serum BPA measurements are in line with these earlier reports, showing much higher BPA concentrations after subcutaneous treatment. Most importantly, however, lower serum BPA concentrations achieved via oral administration were able to induce the same magnitude of spine synapse losses as were induced by subcutaneous treatment.
One could still argue that the 300 µg/kg/day oral BPA dose we applied is much higher than the 50 µg/kg/day EPA reference limit. However, serum BPA concentrations achieved by the particular treatment are considered more important by having more relevance to human health. It has been reported in several studies that circulating BPA levels in adult humans are in the range of 0.3–4.4 ng/ml [158]. It is remarkable that even the maximum serum BPA concentration we achieved with oral treatment (0.31 ng/ml) is at the very low end of this range, further supporting our earlier argument that environmental BPA exposure may have a serious adverse effect on human synaptic remodeling [82, 88, 94]. Our findings indicate that serum BPA levels usually detectable in adult human beings are sufficient to significantly interfere with the remodeling of prefrontal and hippocampal spine synapses, at least in rodents. In addition, first-pass metabolism, which effectively clears BPA from the body, is limited by the reduced rate of hepatic blood flow in primates and humans [15] when compared with that in rats [10, 165]. As a result, it may take longer for human beings to clear BPA from their circulation, leading to prolonged exposure to the compound.
Mediators of Bisphenol A effects
Our knowledge about the mediators of BPA’s effects, especially about those of its anti-synaptogenic effect, is very limited. However, as the potential risks of BPA exposure were derived from BPA’s estrogenic properties, we can speculate about these mediators by applying our knowledge of the signaling mechanisms employed by gonadal steroid hormones. Throughout most of the last three decades, the actions of gonadal steroid hormones on the brain have been believed to be mediated almost entirely through control of gene transcription via nuclear steroid receptor proteins resembling those found in non-neural target tissues [91]. Initially, only a single ER, ERα has been known. Work during the 1990s demonstrated that there is also a second nuclear ER, ERβ, encoded by a different gene with extensive (>90%) sequence homology to ERa in the DNA-binding domain but only partial homology in other regions of the molecule. ERα and ERβ have different estrogen binding specificity, as well as different distributions within the brain, although the two receptors are co-expressed in many regions in adulthood. In some structures, however, there is a preferential expression of one or the other sub-type. In the mediobasal hypothalamus, for example, ERα expression predominates. By contrast, in the olfactory bulb, cerebral cortex, cerebellum and hypothalamic paraventricular nucleus, ERβ is expressed with little or no ERα [135]. Considering the androgen receptor (AR), it is enriched in the hippocampus, particularly in the CA1 subfield, localized both at nuclear [71, 124, 137] and extranuclear sites [21, 145]. Our extensive knowledge about nuclear ERs, however, is less useful here because, as we mentioned above, BPA has relatively low affinity for nuclear ERα and ERβ, explaining the weak uterotrophic activity of BPA [94].
In search for alternative receptor targets of BPA, recent research has concentrated on the membrane ER [181] and on the AR [126, 142, 178], highlighting their importance as possible mediators of BPA action. During the last few years, a number of laboratories have demonstrated the presence of ER and AR proteins in the cell membrane [69, 100, 107, 125, 136, 145, 159], as well as in both the pre- and postsynaptic sites of hippocampal spine synapses [55, 105]. There now seems to be no doubt that these systems play a functional role in mediating responses to gonadal steroid hormones, and their involvement may underlie strong BPA effects seen in the brain. A particularly important feature of membrane ERs and ARs is that by being associated with fast-acting intracellular signaling molecules, they provide a mechanism that potentially underlies rapid cellular responses to gonadal steroid hormones. For example, our earlier findings suggest that the rapid hippocampal synaptogenic response to estradiol-17α is also mediated by these membrane ERs [96]. The observation that BPA blocks this rapid synaptic response [94] implicates the membrane ER as a potential site where BPA interferes with the central effects of estrogens. It remains unclear whether these membrane proteins represent distinct, novel receptor systems or subsets of nuclear receptors diverted to the cell membrane, possibly in association with chaperones. In the membrane environment, however, interactions with other membrane constituents may alter the properties of membrane receptors in such a way that they exhibit response characteristics different from those observed when the same receptors are in the cell nucleus. For example, transfection of ER-negative rat-2 fibroblasts with ERα or ERβ results in cells that respond to estrogens with increased MAP-kinase activity [164]. However, in contrast to the activation of the nuclear receptor system, which is more sensitive to estradiol-17β than estradiol-17α, the MAP-kinase response is equally induced by both estradiol-17α and estradiol-17β [164]. Furthermore, in plasma membrane fractions from the developing brain, estradiol-17α and estradiol-17β both activate extracellular signal-regulated kinase (ERK) phosphorylation; and both compete equally well with [3H]estradiol for binding to high-affinity plasma membrane estradiol binding sites [154]. As a result, these characteristics of the centrally located membrane ERs may explain the considerable differences in the peripheral versus central effectivity of estradiol-17α and estradiol-17β, as well as BPA.
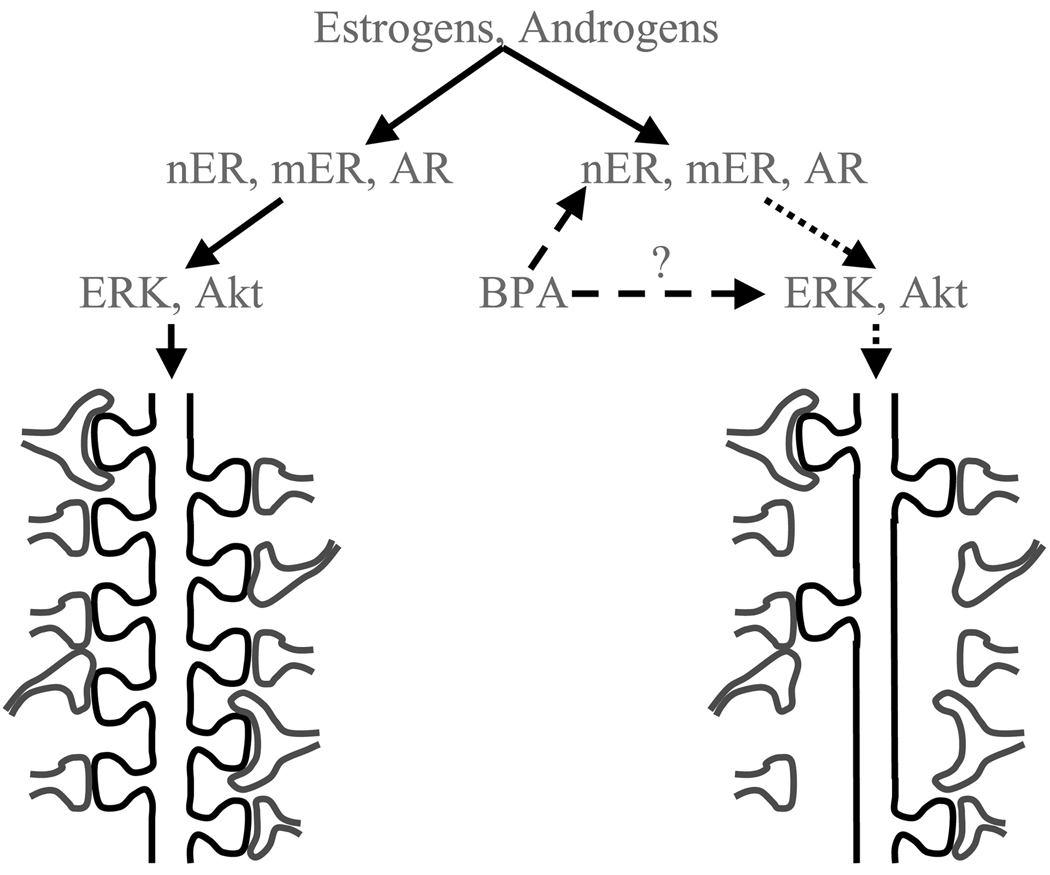
As we mentioned above, our findings in male rats provide further important insights that question the above-detailed involvement of gonadal steroid hormone receptors, at least in the context of the anti-synaptogenic effect of BPA. Actions at the level of gonadal steroid receptors may explain the interference of BPA with prefrontal cortical synaptogenesis in males [88], as androgen-induced spine synapse remodeling in the male medial prefrontal cortex could be mediated by both the ER (via conversion of the androgens to estrogenic compounds) and the AR [46]. However, extensive work from our laboratory indicates that the synaptogenic action of androgens is independent of both the ER and AR in the male hippocampus. First, spine synapses in the male hippocampus do not respond to estrogens, excluding the ERs [83], while androgen-induced spine synapse growth is retained in males with both pharmacologically [93] and genetically [92] impaired ARs, excluding this receptor type also. These findings indicate that BPA does not interfere with gonadal steroid hormone receptor functions, at least in the male hippocampus. A potential resolution could be that BPA directly targets intracellular signaling mechanisms downstream of the receptors, which are involved in the remodeling of hippocampal spine synapses, such as the ERK and Akt pathways (Figure 4). A recent study indeed suggests that the ERK1/2 signaling pathway may be involved in the effects of BPA [181]. Considering available data, the ERK1/2 pathway is known to be activated by both estrogens [6, 155, 181] and androgens [33, 111], and it plays a critical role in both synaptic remodeling [3, 34] and cognitive functions [144, 150, 167]. The phosphatidylinositol 3-kinase / protein kinase B (Akt) pathway is also implicated in downstream signaling of the ER [180]. There is evidence that androgens induce Akt phosphorylation in non-neural tissue [65], and that Akt is involved in spine growth [77]. Unfortunately, very limited data are currently available about the effects of BPA on intracellular signaling mechanisms [181], and this issue is an important avenue for future research.
Figure 4.
Mediators of the anti-synaptogenic effect of Bisphenol A (BPA). The prefrontal and hippocampal synaptogenic effects of estrogens and androgens are mediated by their respective receptors and the extracellular signal-regulated kinase (ERK) and Akt pathways (arrows). BPA may interfere with this action at the level of receptors, such as the nuclear and membrane estrogens receptors (nER and mER) and the androgen receptor (AR). Based on our findings in male rats, we speculate that BPA may also interfere at the level of intracellular signaling mechanisms (dashed arrows), exerting a blockade on the effects of gonadal steroid hormones and resulting in the loss of spine synapses (dotted arrows).
Finally, another potential pathway has been revealed by recent research. Our earlier work with gonadal steroid hormones and available data on BPA actions both suggest that BPA may exert its anti-synaptogenic effect by influencing gonadal steroid hormone-sensitive subcortical structures. Strong evidence indicates that certain subcortical areas play a critical role in the prefrontal and hippocampal synaptogenic effects of estradiol. Transection of the fimbria/fornix, that contains the majority of input fibers coming from these subcortical areas to the hippocampus, completely abolishes the hippocampal synaptogenic effect of systemic estradiol-17β administration in ovariectomized animals [86]. On the other hand, local estradiol-17β administration into estrogen-sensitive subcortical brain areas, including the medial septum diagonal band of Broca, results in a dramatic increase of CA1 spine synapse density in ovariectomized rats [79]. Furthermore, we have shown that ovariectomy reduces the number of dopaminergic neurons in the ventral tegmental area and substantia nigra of non-human primates [85]. Castration also has a selective negative effect on cortical dopaminergic innervation in adult male rats, especially in the prefrontal cortex [72], while ovariectomy results in profound reductions in the density of prefrontal cholinergic, dopaminergic, and serotonergic axons in monkeys [74, 75]. We have also reported that local implantation of estradiol-17β into the median raphe decreases the density of serotonergic fibers in the CA1 area of ovariectomized rats [117]. In light of the critical role of these subcortical areas in mediating the actions of gonadal steroid hormones, it may not be surprising that BPA exposure strongly influences their functions [113]. For example, oral administration of BPA to male rats aged 5 days to 3 weeks results in hyperactivity (a symptom of dopaminergic malfunction) at 4–5 weeks of age, degeneration of mesencephalic dopaminergic neurons at 7 weeks of age, as well as decreased gene expression levels for dopamine transporter in adult animals [60, 61].
Potential clinical consequences of Bisphenol A-induced spine synapse loss
Up until now, we only mentioned cognitive dysfunction as the primary correlate of prefrontal and hippocampal spine synapse loss. The perturbation of play and maze learning behaviors reported in both female and male rodents after developmental BPA exposure [11, 17, 18, 26, 27, 151] may be based, as we mentioned above, on this mechanism. In addition to cognition, however, alterations in patterns of synaptogenesis appear to play critical roles in several other neurologic/neuropsychiatric disorders, such as mental retardation and developmental disabilities [112], Alzheimer’s disease [128], schizophrenia [44, 54], and depression [51]. On this basis, the ability of BPA to interfere with spine synapse formation in the prefrontal cortex and hippocampus may have some clinical implications.
Of the above-mentioned diseases, here we discuss in more detail the possibility that BPA exposure may contribute to the development depressive behavior, because our laboratory took a pioneering role in clarifying the involvement of spine synapse remodeling in depression neurobiology [43, 49, 51]. It has been postulated for many years that stress and depression are associated with the loss of hippocampal dendritic spines and spine synapses, while antidepressant treatment elicits the formation of new synapses [12, 45, 110]. Prior to our work, this hypothesis has been quite controversial. Although considerable evidence [49, 62, 80, 120, 139, 148, 166], as well as clinical observations about the comorbidity of depression with cognitive impairments [28] support the “synaptogenesis hypothesis” of depression [45], there are many reports that contradict this postulation [19, 22, 133]. More importantly, electron microscopic data, which is the only method having sufficient resolution to directly visualize synapses, have been limited in this field. After these earlier confusions, our laboratory has provided the first direct, electron microscopic evidence that antidepressant treatment is associated with formation of new spine synapses in the hippocampus [49]. More recently, we have shown that there is extensive remodeling of hippocampal spine synapses in the rat learned helplessness model of depression and antidepressant response [43]. Specifically, there is a statistically significant negative linear correlation between the number of hippocampal spine synapses and the severity of depressive symptoms, i.e., the less synapses the animals have, the more ‘depressed’ they are [51]. Based on this strong correlation between depressive behavior and hippocampal spine synapse remodeling, it is conceivable that exposure to BPA and the resulting loss of hippocampal spine synapses may elicit depressive behavior. Although there are limited data available, a couple of studies have demonstrated that BPA indeed promotes helpless behavior in the learned helplessness paradigm [109] and increases immobility in the forced swim test [30], signs of depressive behavior in two widely-accepted animal models of depression.
Considering the estrogen- and androgen-antagonist properties of BPA along with the sensitivity of prefrontal and hippocampal spine synapse remodeling to gonadal steroid hormones, estrogens and androgens are expected to influence mood and/or depression neurobiology. Indeed, women are twice as likely to suffer from depression as men [70], supporting the above view that gonadal steroid hormones contribute to the neurobiology of depression. Clinical data also show that distinct events of reproductive physiology, especially those accompanied by the abrupt decline of gonadal steroid hormone levels, are associated with mood disorders, such as premenstrual dysphoric disease, postpartum dysphoria/depression, and peri/postmenopausal depression [134, 141]. Postpartum dysphoria, for example, affects up to ~80% of postpartum women, usually lasting from the 5th to the 12th day following childbirth [140]. Clinical studies have demonstrated that administration of estrogens or antidepressants are both effective in treating postpartum and postmenopausal depressions [1, 40, 119, 141]. In addition, a potential role for androgens in depression neurobiology has also been suggested in men, especially in elderly men [130]. These clinical findings support the view that withdrawal of the neurotrophic effects of gonadal steroid hormones may contribute to mood disturbances, and even to depression.
As BPA appears to induce depressive behavior [30, 109], there are further requirements in order to uphold our hypothesis that BPA directly targets certain intracellular signaling mechanisms. Namely, as these potential targets of BPA are involved in the signaling of estrogens and androgens, in spine synapse remodeling, as well as in cognition, they also need to play a role in depression neurobiology. Our earlier finding in rats demonstrates that the antidepressant, fluoxetine substitutes for estrogens to reverse the loss of hippocampal spine synapses caused by ovariectomy [49], suggesting that estrogens and fluoxetine may exert their synaptogenic effects via the same mechanisms. Antidepressants do not act via the ER or AR, but there is evidence that estrogens and antidepressants both activate common intracellular signaling mechanisms, such as the ERK1/2 pathway [6, 152, 155, 157], Akt [5, 14, 180], and STAT3 [29, 32], the same pathways that are involved in synaptic remodeling [3, 34, 77] and cognition [144, 150, 167]. Acute administration of fluoxetine to mice causes a clear increase in ERK1/2 phosphorylation in the prefrontal cortex and hippocampus [157], while treatment of rats for 2–3 weeks appears to elicit no change or even a reduction in phosphorylation [31, 152] but increases the expression of the ERK1/2 proteins [152]. The apparently common intracellular signaling pathways for the effects of gonadal steroid hormones and antidepressants represent another argument in favor of our hypothesis that BPA actually acts at the level of intracellular signaling mechanisms.
Future direction of research: Perinatal exposure to Bisphenol A
Our earlier research that we describe above has been focused on BPA exposure of adults. Recently, our attention has turned toward the potential consequences of BPA exposure during the perinatal period. We have documented that even circulating serum BPA levels normally found in adult human beings (0.3–4.4 ng/ml) are devastating for spine synapses, at least in a rodent model. Most notable, however, is that according to a recent National Toxicology Program Report (http://www.niehs.nih.gov/health/docs/bpa-factsheet.pdf), the estimated daily BPA exposure of infants and children may be as high as 10-times the exposure level of adults. The unusually high perinatal serum BPA level is probably explained by the low-level expression of UDP-glucuronosyltransferase in fetuses and neonates, the enzyme that conjugates BPA and contributes to the rapid metabolization of the chemical in adults [149]. Perinatal exposure starts in utero, as BPA ingested by the mother is passed through the placenta and gets into fetal blood [59, 129]. Exposure continues postnatally by breastfeeding [78], and also by intake of BPA that leaches out from plastic baby bottles [8]. As a result, Canada and certain stores in the U.S. have already banned baby products that contain BPA, such as plastic baby bottles and toys.
Exposure of juveniles is a sensitive issue because this enhanced level of BPA exposure comes during a particularly sensitive period of neuronal development. Extensive information about neuronal development is available from rhesus monkey histological investigations, and more recently from noninvasive imaging studies [98], and also from behavioral experiments. The peak of neurogenesis in rhesus occurs in the third trimester of pregnancy, somewhat earlier than in humans [35], while the peak in synaptic density is seen during the first half of the first year of life [35]. Cortical layers and connectivity are established by the end of the first year [23, 36, 131], with advances in cognitive function being associated with these early developmental events [37]. Taking into account the differences in lifespan and in time of sexual maturation, the first year of age in rhesus corresponds roughly to four years of age in human beings. Thus, the functional architecture of the brain is laid down during an enhanced level of BPA exposure, raising the possibility that BPA interferes with juvenile brain development, and may cause lasting and irreversible alterations in behavior and brain morphology. There is indeed evidence that BPA at puberty increases the number of cells that express ERα in the hypothalamus of rats [13], which may contribute to alterations in sociosexual behavior [26]. More importantly, we have already described above that BPA seems to affect the development of many other behaviors, such as anxiety [151], play and pain behaviors [2, 18, 27], differently in males and females (Table 1).
We hypothesize that developmental cognitive dysfunctions are associated with disrupted synaptogenesis induced by perinatal BPA exposure, because early derailment of synapse development has been implicated in developmental disabilities [112]. Although there are several findings in rodent models, as mentioned above, we currently don’t know whether BPA may disturb the development of cognitive functions in primates. Considering synapses, in particular, no data exist at all, neither from rodents nor from primates, about potential interference from BPA with synapse development. As a result, our laboratory is currently working with a nonhuman primate model that reproduces human perinatal exposure to BPA, based on the same African green monkey species we have used in our earlier study [82], in order to investigate the effects of perinatal BPA exposure on the development of synapses and cognitive functions.
ACKNOWLEDGMENTS
This work was supported by NIH grants ES014893 (Leranth), MH074021 (Hajszan), and a NARSAD Young Investigator Award (Hajszan). We would like to thank Drs. Frederick S. vom Saal and Julia A. Taylor for measuring Bisphenol A levels for this study, and Klara Szigeti-Buck and Jeremy Bober for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J. Clin. Psychiatry. 2001;62:332–336. doi: 10.4088/jcp.v62n0504. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi AM, Della Seta D, Rendo C, Ceccarelli I, Scaramuzzino A, Farabollini F. Exposure to the estrogenic pollutant bisphenol A affects pain behavior induced by subcutaneous formalin injection in male and female rats. Brain Res. 2002;937:1–7. doi: 10.1016/s0006-8993(02)02446-0. [DOI] [PubMed] [Google Scholar]
- 3.Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn. Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashby J. Increasing the sensitivity of the rodent uterotrophic assay to estrogens, with particular reference to bisphenol A. Environ. Health Perspect. 2001;109:1091–1094. doi: 10.1289/ehp.011091091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basta-Kaim A, Budziszewska B, Jaworska-Feil L, Tetich M, Kubera M, Leskiewicz M, Lason W. Inhibitory effect of imipramine on the human corticotropin-releasing-hormone gene promoter activity operates through a PI3-K/AKT mediated pathway. Neuropharmacology. 2005;49:156–164. doi: 10.1016/j.neuropharm.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bird J. An exploratory study which is not relevant for human risk assessment. Brussels: 2008. www.plasticseurope.org. [Google Scholar]
- 8.Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 2003;20:684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 9.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ. Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- 11.Carr R, Bertasi F, Betancourt A, Bowers S, Gandy BS, Ryan P, Willard S. Effect of neonatal rat bisphenol a exposure on performance in the Morris water maze. J. Toxicol. Environ. Health A. 2003;66:2077–2088. doi: 10.1080/713853983. [DOI] [PubMed] [Google Scholar]
- 12.Castren E. Neurotrophic effects of antidepressant drugs. Curr. Opin. Pharmacol. 2004;4:58–64. doi: 10.1016/j.coph.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Ceccarelli I, Della Seta D, Fiorenzani P, Farabollini F, Aloisi AM. Estrogenic chemicals at puberty change ERalpha in the hypothalamus of male and female rats. Neurotoxicol. Teratol. 2007;29:108–115. doi: 10.1016/j.ntt.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res. Mol. Brain Res. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 16.Degen GH, Janning P, Wittsiepe J, Upmeier A, Bolt HM. Integration of mechanistic data in the toxicological evaluation of endocrine modulators. Toxicol. Lett. 2002;127:225–237. doi: 10.1016/s0378-4274(01)00504-5. [DOI] [PubMed] [Google Scholar]
- 17.Della Seta D, Minder I, Belloni V, Aloisi AM, Dessi-Fulgheri F, Farabollini F. Pubertal exposure to estrogenic chemicals affects behavior in juvenile and adult male rats. Horm. Behav. 2006;50:301–307. doi: 10.1016/j.yhbeh.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Dessi-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ. Health Perspect. 2002;110 Suppl 3:403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- 20.Dohanich GP. Gonadal steroids, learning and memory. In: Rubin RI, editor. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 265–327. [Google Scholar]
- 21.DonCarlos LL, Garcia-Ovejero D, Sarkey S, Garcia-Segura LM, Azcoitia I. Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology. 2003;144:3632–3638. doi: 10.1210/en.2002-0105. [DOI] [PubMed] [Google Scholar]
- 22.Donohue HS, Gabbott PL, Davies HA, Rodriguez JJ, Cordero MI, Sandi C, Medvedev NI, Popov VI, Colyer FM, Peddie CJ, Stewart MG. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 23.Eckenhoff MF, Rakic P. A quantitative analysis of synaptogenesis in the molecular layer of the dentate gyrus in the rhesus monkey. Brain Res. Dev. Brain Res. 1991;64:129–135. doi: 10.1016/0165-3806(91)90216-6. [DOI] [PubMed] [Google Scholar]
- 24.Edinger KL, Frye CA. Androgens' effects to enhance learning may be mediated in part through actions at estrogen receptor-beta in the hippocampus. Neurobiol. Learn. Mem. 2007;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ema M, Fujii S, Furukawa M, Kiguchi M, Ikka T, Harazono A. Rat two-generation reproductive toxicity study of bisphenol A. Reprod. Toxicol. 2001;15:505–523. doi: 10.1016/s0890-6238(01)00160-5. [DOI] [PubMed] [Google Scholar]
- 26.Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessi-Fulgheri F. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ. Health Perspect. 2002;110 Suppl 3:409–414. doi: 10.1289/ehp.02110s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farabollini F, Porrini S, Dessi-Fulgheri F. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol. Biochem. Behav. 1999;64:687–694. doi: 10.1016/s0091-3057(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 28.Fossati P, Coyette F, Ergis AM, Allilaire JF. Influence of age and executive functioning on verbal memory of inpatients with depression. J. Affect. Disord. 2002;68:261–271. doi: 10.1016/s0165-0327(00)00362-1. [DOI] [PubMed] [Google Scholar]
- 29.Fricker AD, Rios C, Devi LA, Gomes I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res. Mol. Brain Res. 2005;138:228–235. doi: 10.1016/j.molbrainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 31.Fumagalli F, Molteni R, Calabrese F, Frasca A, Racagni G, Riva MA. Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J. Neurochem. 2005;93:1551–1560. doi: 10.1111/j.1471-4159.2005.03149.x. [DOI] [PubMed] [Google Scholar]
- 32.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat. Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 33.Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028–2034. doi: 10.1210/en.2005-1395. [DOI] [PubMed] [Google Scholar]
- 34.Goldin M, Segal M. Protein kinase C and ERK involvement in dendritic spine plasticity in cultured rodent hippocampal neurons. Eur. J. Neurosci. 2003;17:2529–2539. doi: 10.1046/j.1460-9568.2003.02694.x. [DOI] [PubMed] [Google Scholar]
- 35.Goldman-Rakic PS. Prenatal formation of cortical input and development of cytoarchitectonic compartments in the neostriatum of the rhesus monkey. J. Neurosci. 1981;1:721–735. doi: 10.1523/JNEUROSCI.01-07-00721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman-Rakic PS. The corticostriatal fiber system in the rhesus monkey: organization and development. Prog. Brain Res. 1983;58:405–418. doi: 10.1016/S0079-6123(08)60043-6. [DOI] [PubMed] [Google Scholar]
- 37.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–622. [PubMed] [Google Scholar]
- 38.Gore AC. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front. Neuroendocrinol. 2008;29:358–374. doi: 10.1016/j.yfrne.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol. Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- 40.Gregoire AJ, Kumar R, Everitt B, Henderson AF, Studd JW. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347:930–933. doi: 10.1016/s0140-6736(96)91414-2. [DOI] [PubMed] [Google Scholar]
- 41.Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc. Soc. Exp. Biol. Med. 2000;224:61–68. doi: 10.1046/j.1525-1373.2000.22402.x. [DOI] [PubMed] [Google Scholar]
- 42.Gutendorf B, Westendorf J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology. 2001;166:79–89. doi: 10.1016/s0300-483x(01)00437-1. [DOI] [PubMed] [Google Scholar]
- 43.Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol. Psychiatry. 2009;65:392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajszan T, Leranth C, Roth RH. Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol. Psychiatry. 2006;60:639–644. doi: 10.1016/j.biopsych.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Hajszan T, MacLusky NJ. Neurologic links between epilepsy and depression in women: is hippocampal neuroplasticity the key? Neurology. 2006;66:S13–S22. doi: 10.1212/wnl.66.66_suppl_3.s13. [DOI] [PubMed] [Google Scholar]
- 46.Hajszan T, Maclusky NJ, Johansen JA, Jordan CL, Leranth C. Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology. 2007;148:1963–1967. doi: 10.1210/en.2006-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C. Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology. 2007;148:1963–1967. doi: 10.1210/en.2006-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajszan T, MacLusky NJ, Leranth C. Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized female rats. Endocrinology. 2004;145:1042–1045. doi: 10.1210/en.2003-1252. [DOI] [PubMed] [Google Scholar]
- 49.Hajszan T, Maclusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur. J. Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 50.Hajszan T, MacLusky NJ, Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm. Behav. 2008;53:638–646. doi: 10.1016/j.yhbeh.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hajszan T, Szigeti-Buck K, Sallam NL, Bober J, Parducz A, Maclusky NJ, Leranth C, Duman RS. Effects of estradiol on learned helplessness and associated remodeling of hippocampal spine synapses in female rats. Biol. Psychiatry. 2010;67:168–174. doi: 10.1016/j.biopsych.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J. Comp. Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- 53.Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J. Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 55.Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res. 2006;1121:46–58. doi: 10.1016/j.brainres.2006.08.084. [DOI] [PubMed] [Google Scholar]
- 56.Hess DL, Hendrickx AG, Stabenfeldt GH. Reproductive and hormonal patterns in the African green monkey (Cercopithecus aethiops) J. Med. Primatol. 1979;8:273–281. doi: 10.1159/000460211. [DOI] [PubMed] [Google Scholar]
- 57.Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, vom Saal FS, Welshons WV. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ. Health Perspect. 2003;111:1180–1187. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hung AJ, Stanbury MG, Shanabrough M, Horvath TL, Garcia-Segura LM, Naftolin F. Estrogen, synaptic plasticity and hypothalamic reproductive aging. Exp. Gerontol. 2003;38:53–59. doi: 10.1016/s0531-5565(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 59.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 60.Ishido M, Masuo Y, Kunimoto M, Oka S, Morita M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J. Neurosci. Res. 2004;76:423–433. doi: 10.1002/jnr.20050. [DOI] [PubMed] [Google Scholar]
- 61.Ishido M, Yonemoto J, Morita M. Mesencephalic neurodegeneration in the orally administered bisphenol A-caused hyperactive rats. Toxicol. Lett. 2007;173:66–72. doi: 10.1016/j.toxlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 62.Iwata M, Shirayama Y, Ishida H, Kawahara R. Hippocampal synapsin I, growthassociated protein-43, and microtubule-associated protein-2 immunoreactivity in learned helplessness rats and antidepressant-treated rats. Neuroscience. 2006;141:1301–1313. doi: 10.1016/j.neuroscience.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 63.Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn. Sci. 2006;10:77–82. doi: 10.1016/j.tics.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 65.Kang HY, Cho CL, Huang KL, Wang JC, Hu YC, Lin HK, Chang C, Huang KE. Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. J. Bone Miner. Res. 2004;19:1181–1190. doi: 10.1359/JBMR.040306. [DOI] [PubMed] [Google Scholar]
- 66.Kang JH, Kito K, Kondo F. Factors influencing the migration of bisphenol A from cans. J. Food Prot. 2003;66:1444–1447. doi: 10.4315/0362-028x-66.8.1444. [DOI] [PubMed] [Google Scholar]
- 67.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 68.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p'-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 69.Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol. Cell. Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 71.Kritzer M. The distribution of immunoreactivity for intracellular androgen receptors in the cerebral cortex of hormonally intact adult male and female rats: localization in pyramidal neurons making corticocortical connections. Cereb. Cortex. 2004;14:268–280. doi: 10.1093/cercor/bhg127. [DOI] [PubMed] [Google Scholar]
- 72.Kritzer MF, Adler A, Marotta J, Smirlis T. Regionally selective effects of gonadectomy on cortical catecholamine innervation in adult male rats are most disruptive to afferents in prefrontal cortex. Cereb. Cortex. 1999;9:507–518. doi: 10.1093/cercor/9.5.507. [DOI] [PubMed] [Google Scholar]
- 73.Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm. Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J. Comp. Neurol. 1998;395:1–17. [PubMed] [Google Scholar]
- 75.Kritzer MF, Kohama SG. Ovarian hormones differentially influence immunoreactivity for dopamine beta- hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J. Comp. Neurol. 1999;409:438–451. doi: 10.1002/(sici)1096-9861(19990705)409:3<438::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 76.Kudwa AE, Boon WC, Simpson ER, Handa RJ, Rissman EF. Dietary phytoestrogens dampen female sexual behavior in mice with a disrupted aromatase enzyme gene. Behav. Neurosci. 2007;121:356–361. doi: 10.1037/0735-7044.121.2.356. [DOI] [PubMed] [Google Scholar]
- 77.Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuruto-Niwa R, Tateoka Y, Usuki Y, Nozawa R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere. 2007;66:1160–1164. doi: 10.1016/j.chemosphere.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 79.Lam TT, Leranth C. Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. Eur. J. Neurosci. 2003;17:1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- 80.Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am. J. Psychiatry. 2004;161:1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- 81.Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J. Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J. Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leranth C, Prange-Kiel J, Frick KM, Horvath TL. Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cereb. Cortex. 2004;14:503–510. doi: 10.1093/cercor/bhh012. [DOI] [PubMed] [Google Scholar]
- 85.Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DEJ. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson's disease and memory. J. Neurosci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leranth C, Shanabrough M, Horvath TL. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience. 2000;101:349–356. doi: 10.1016/s0306-4522(00)00369-9. [DOI] [PubMed] [Google Scholar]
- 87.Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J. Comp. Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- 88.Leranth C, Szigeti-Buck K, Maclusky NJ, Hajszan T. Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology. 2008;149:988–994. doi: 10.1210/en.2007-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 91.MacLusky NJ. Sex steroid receptors. In: Rosenwaks Z, editor. Reproductive Endocrinology, Surgery and Technology. Philadelphia: Lippincott-Raven; 1996. pp. 627–663. [Google Scholar]
- 92.MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006;147:2392–2398. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- 93.MacLusky NJ, Hajszan T, Leranth C. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology. 2004;145:4154–4161. doi: 10.1210/en.2004-0477. [DOI] [PubMed] [Google Scholar]
- 94.MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol A inhibits estradiol-induced hippocampal synaptogenesis. Environ. Health Perspect. 2005;113:675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138:957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 96.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 97.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 98.Malkova L, Heuer E, Saunders RC. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur. J. Neurosci. 2006;24:3204–3212. doi: 10.1111/j.1460-9568.2006.05175.x. [DOI] [PubMed] [Google Scholar]
- 99.Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol. Reprod. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 100.Marquez DC, Pietras RJ. Membrane-associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene. 2001;20:5420–5430. doi: 10.1038/sj.onc.1204729. [DOI] [PubMed] [Google Scholar]
- 101.Mathews RA. Publication incorrectly characterizes dose of bisphenol A. Proc. Natl. Acad. Sci. U. S. A. 2008;105:E97. doi: 10.1073/pnas.0808990105. author reply E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 103.McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48:S8–S15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 104.McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr. Rev. 2001;22:319–341. doi: 10.1210/edrv.22.3.0432. [DOI] [PubMed] [Google Scholar]
- 105.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 106.Mukai H, Tsurugizawa T, Ogiue-Ikeda M, Murakami G, Hojo Y, Ishii H, Kimoto T, Kawato S. Local neurosteroid production in the hippocampus: Influence on synaptic plasticity of memory. Neuroendocrinology In Press. 2006 doi: 10.1159/000097747. PMID17142999. [DOI] [PubMed] [Google Scholar]
- 107.Nadal A, Ropero AB, Fuentes E, Soria B. The plasma membrane estrogen receptor: nuclear or unclear? Trends Pharmacol. Sci. 2001;22:597–599. doi: 10.1016/s0165-6147(00)01846-0. [DOI] [PubMed] [Google Scholar]
- 108.Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ. Health Perspect. 1997;105:70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Negishi T, Kawasaki K, Suzaki S, Maeda H, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ. Health Perspect. 2004;112:1159–1164. doi: 10.1289/ehp.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 111.Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J. Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 112.Okado N, Narita M, Narita N. A biogenic amine-synapse mechanism for mental retardation and developmental disabilities. Brain Dev. 2001;23 Suppl 1:S11–S15. doi: 10.1016/s0387-7604(01)00371-0. [DOI] [PubMed] [Google Scholar]
- 113.Panzica GC, Viglietti-Panzica C, Mura E, Quinn MJJ, Lavoie E, Palanza P, Ottinger MA. Effects of xenoestrogens on the differentiation of behaviorally-relevant neural circuits. Front. Neuroendocrinol. 2007;28:179–200. doi: 10.1016/j.yfrne.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 114.Parducz A, Garcia-Segura LM. Sexual differences in the synaptic connectivity in the rat dentate gyrus. Neurosci. Lett. 1993;161:53–56. doi: 10.1016/0304-3940(93)90138-b. [DOI] [PubMed] [Google Scholar]
- 115.Patisaul HB. Phytoestrogen action in the adult and developing brain. J. Neuroendocrinol. 2005;17:57–64. doi: 10.1111/j.1365-2826.2005.01268.x. [DOI] [PubMed] [Google Scholar]
- 116.Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JMJ. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol. Sci. 2000;54:3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 117.Prange-Kiel J, Rune GM, Leranth C. Median raphe mediates estrogenic effects to the hippocampus in female rats. Eur. J. Neurosci. 2004;19:309–317. doi: 10.1111/j.0953-816x.2003.03124.x. [DOI] [PubMed] [Google Scholar]
- 118.Ramos JG, Varayoud J, Sonnenschein C, Soto AM, Munoz De Toro M, Luque EH. Prenatal exposure to low doses of bisphenol A alters the periductal stroma and glandular cell function in the rat ventral prostate. Biol. Reprod. 2001;65:1271–1277. doi: 10.1095/biolreprod65.4.1271. [DOI] [PubMed] [Google Scholar]
- 119.Rasgon NL, Altshuler LL, Fairbanks LA, Dunkin JJ, Davtyan C, Elman S, Rapkin AJ. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J. Clin. Psychiatry. 2002;63 Suppl 7:45–48. [PubMed] [Google Scholar]
- 120.Reines A, Cereseto M, Ferrero A, Sifonios L, Podesta MF, Wikinski S. Maintenance treatment with fluoxetine is necessary to sustain normal levels of synaptic markers in an experimental model of depression: Correlation with behavioral response. Neuropsychopharmacology. 2008;33:1896–1908. doi: 10.1038/sj.npp.1301596. [DOI] [PubMed] [Google Scholar]
- 121.Roof RL. The dentate gyrus is sexually dimorphic in prepubescent rats: testosterone plays a significant role. Brain Res. 1993;610:148–151. doi: 10.1016/0006-8993(93)91228-k. [DOI] [PubMed] [Google Scholar]
- 122.Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992;572:310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- 123.Safe SH. Endocrine disruptors and human health--is there a problem? An update. Environ. Health Perspect. 2000;108:487–493. doi: 10.1289/ehp.00108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology. 1990;127:3180–3186. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- 125.Sarkey S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D, DonCarlos LL. Classical androgen receptors in non-classical sites in the brain. Horm. Behav. 2008;53:753–764. doi: 10.1016/j.yhbeh.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Satoh K, Ohyama K, Aoki N, Iida M, Nagai F. Study on anti-androgenic effects of bisphenol A diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives using cells stably transfected with human androgen receptor, AR-EcoScreen. Food Chem. Toxicol. 2004;42:983–993. doi: 10.1016/j.fct.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 127.Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environ. Health Perspect. 2001;109:1197–1206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scheff SW, Price DA. Synaptic pathology in Alzheimer's disease: a review of ultrastructural studies. Neurobiol. Aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 129.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seidman SN. Testosterone deficiency and mood in aging men: pathogenic and therapeutic interactions. World J. Biol. Psychiatry. 2003;4:14–20. doi: 10.3109/15622970309167905. [DOI] [PubMed] [Google Scholar]
- 131.Seress L, Ribak CE. Postnatal development of CA3 pyramidal neurons and their afferents in the Ammon's horn of rhesus monkeys. Hippocampus. 1995;5:217–231. doi: 10.1002/hipo.450050308. [DOI] [PubMed] [Google Scholar]
- 132.Sherwin BB. Horomones, mood, and cognitive functioning in postmenopausal women. Obstet. Gynecol. 1996;87:20S–26S. doi: 10.1016/0029-7844(95)00431-9. [DOI] [PubMed] [Google Scholar]
- 133.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J. Affect. Disord. 2003;74:85–96. doi: 10.1016/s0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 136.Shughrue PJ, Merchenthaler I. Estrogen is more than just a "sex hormone": novel sites for estrogen action in the hippocampus and cerebral cortex. Front. Neuroendocrinol. 2000;21:95–101. doi: 10.1006/frne.1999.0190. [DOI] [PubMed] [Google Scholar]
- 137.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 138.Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 139.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 140.Stein G, Marsh A, Morton J. Mental symptoms, weight changes, and electrolyte excretion in the first post partum week. J. Psychosom. Res. 1981;25:395–408. doi: 10.1016/0022-3999(81)90054-4. [DOI] [PubMed] [Google Scholar]
- 141.Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J. Affect. Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 142.Sun H, Xu LC, Chen JF, Song L, Wang XR. Effect of bisphenol A, tetrachlorobisphenol A and pentachlorophenol on the transcriptional activities of androgen receptor-mediated reporter gene. Food Chem. Toxicol. 2006;44:1916–1921. doi: 10.1016/j.fct.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 143.Suzuki K, Ishikawa K, Sugiyama K, Furuta H, Nishimura F. Content and release of bisphenol A from polycarbonate dental products. Dent. Mater. J. 2000;19:389–395. doi: 10.4012/dmj.19.389. [DOI] [PubMed] [Google Scholar]
- 144.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 145.Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 146.Takahashi O, Oishi S. Testicular toxicity of dietarily or parenterally administered bisphenol A in rats and mice. Food Chem. Toxicol. 2003;41:1035–1044. doi: 10.1016/s0278-6915(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 147.Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb. Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- 148.Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J. Comp. Neurol. 2006;498:363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- 149.Taylor JA, Welshons WV, vom Saal FS. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reprod. Toxicol. 2008;25:169–176. doi: 10.1016/j.reprotox.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thiels E, Klann E. Klann, Extracellular signal-regulated kinase, synaptic plasticity, and memory. Rev. Neurosci. 2001;12:327–345. doi: 10.1515/revneuro.2001.12.4.327. [DOI] [PubMed] [Google Scholar]
- 151.Tian YH, Baek JH, Lee SY, Jang CG. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse. 2010;64:432–439. doi: 10.1002/syn.20746. [DOI] [PubMed] [Google Scholar]
- 152.Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, Racagni G, Popoli M. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29:1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- 153.Tominaga T, Negishi T, Hirooka H, Miyachi A, Inoue A, Hayasaka I, Yoshikawa Y. Toxicokinetics of bisphenol A in rats, monkeys and chimpanzees by the LC-MS/MS method. Toxicology. 2006;226:208–217. doi: 10.1016/j.tox.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 154.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ESJ, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Toran-Allerand CD, Singh M, Setalo GJ. Novel mechanisms of estrogen action in the brain: new players in an old story. Front. Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 156.Upmeier A, Degen GH, Diel P, Michna H, Bolt HM. Toxicokinetics of bisphenol A in female DA/Han rats after a single i.v. and oral administration. Arch. Toxicol. 2000;74:431–436. doi: 10.1007/s002040000144. [DOI] [PubMed] [Google Scholar]
- 157.Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur. J. Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- 158.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 159.Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Viglietti-Panzica C, Mura E, Panzica G. Effects of early embryonic exposure to genistein on male copulatory behavior and vasotocin system of Japanese quail. Horm. Behav. 2007;51:355–363. doi: 10.1016/j.yhbeh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 161.vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol. Ind. Health. 1998;14:239–260. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 162.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.vom Saal FS, Nagel SC, Palanza P, Boechler M, Parmigiani S, Welshons WV. Estrogenic pesticides: binding relative to estradiol in MCF-7 cells and effects of exposure during fetal life on subsequent territorial behaviour in male mice. Toxicol. Lett. 1995;77:343–350. doi: 10.1016/0378-4274(95)03316-5. [DOI] [PubMed] [Google Scholar]
- 164.Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 165.Ward KW, Smith BR. A comprehensive quantitative and qualitative evaluation of extrapolation of intravenous pharmacokinetic parameters from rat, dog, and monkey to humans. II. Volume of distribution and mean residence time. Drug Metab. Dispos. 2004;32:612–619. doi: 10.1124/dmd.32.6.612. [DOI] [PubMed] [Google Scholar]
- 166.Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- 167.Weeber EJ, Sweatt JD. Molecular neurobiology of human cognition. Neuron. 2002;33:845–848. doi: 10.1016/s0896-6273(02)00634-7. [DOI] [PubMed] [Google Scholar]
- 168.Welshons WV, Nagel SC, Thayer KA, Judy BM, vom Saal FS. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicol. Ind. Health. 1999;15:12–25. doi: 10.1177/074823379901500103. [DOI] [PubMed] [Google Scholar]
- 169.Whitten PL, Lewis C, Naftolin F. A phytoestrogen diet induces the premature anovulatory syndrome in lactationally exposed female rats. Biol. Reprod. 1993;49:1117–1121. doi: 10.1095/biolreprod49.5.1117. [DOI] [PubMed] [Google Scholar]
- 170.Whitten PL, Lewis C, Russell E, Naftolin F. Potential adverse effects of phytoestrogens. J. Nutr. 1995;125:771S–776S. doi: 10.1093/jn/125.3_Suppl.771S. [DOI] [PubMed] [Google Scholar]
- 171.Whitten PL, Patisaul HB, Young LJ. Neurobehavioral actions of coumestrol and related isoflavonoids in rodents. Neurotoxicol. Teratol. 2002;24:47–54. doi: 10.1016/s0892-0362(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 172.Wimer CC, Wimer RE. On the sources of strain and sex differences in granule cell number in the dentate area of house mice. Brain Res. Dev. Brain Res. 1989;48:167–176. doi: 10.1016/0165-3806(89)90073-4. [DOI] [PubMed] [Google Scholar]
- 173.Witelson SF. Sex and the single hemisphere: specialization of the right hemisphere for spatial processing. Science. 1976;193:425–427. doi: 10.1126/science.935879. [DOI] [PubMed] [Google Scholar]
- 174.Witelson SF. Neural sexual mosaicism: sexual differentiation of the human temporoparietal region for functional asymmetry. Psychoneuroendocrinology. 1991;16:131–153. doi: 10.1016/0306-4530(91)90075-5. [DOI] [PubMed] [Google Scholar]
- 175.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J. Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, Wang XR. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216:197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 179.Yokota H, Iwano H, Endo M, Kobayashi T, Inoue H, Ikushiro S, Yuasa A. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem. J. 1999;340(Pt 2):405–409. [PMC free article] [PubMed] [Google Scholar]
- 180.Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J. Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zsarnovszky A, Le HH, Wang HS, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146:5388–5396. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]