Abstract
Introduction
Reliable outcome measures that reflect the underlying disease process and correlate with motor function in children with SMA are needed for clinical trials.
Methods
Maximum ulnar compound muscle action potential (CMAP) data were collected at 2 visits over a 4–6 week period in children with SMA types II and III, ages 2–17 years old, at 4 academic centers. Primary functional outcome measures included the Modified Hammersmith Functional Motor Scale (MHFMS) and MHFMS-Extend.
Results
CMAP negative peak amplitude and area showed excellent discrimination between the ambulatory and non-ambulatory SMA cohorts (ROC=0.88). CMAP had excellent test-retest reliability (ICC=0.96–0.97, n=64) and moderate to strong correlation with the MHFMS and MHFMS-Extend (r=0.61–0.73, n=68, p<0.001).
Discussion
Maximum ulnar CMAP amplitude and area is a feasible, valid and reliable outcome measure for use in pediatric multicenter clinical trials in SMA. CMAP correlates well with motor function and has potential value as a relevant surrogate for disease status.
Keywords: spinal muscular atrophy, pediatrics, compound muscle action potential, motor function, outcome measures
INTRODUCTION
Spinal muscular atrophy (SMA) is the most common inherited motor neuron disease. It is a progressive neuromuscular disorder that occurs with an incidence of 1 in 10,000 live births.1, 2 Most patients with SMA have markedly reduced muscle strength and motor function across a breadth of severity ranging from profound generalized weakness to modest proximal muscle weakness. The spectrum of SMA is divided into groups based upon maximum achieved gross motor function: SMA type I defines those children who are unable to sit; type II defines those who achieve the ability to sit independently; and type III defines those who at least for a time achieve the ability to walk.3–7 Recent advances in our understanding of mechanisms of disease pathogenesis in SMA research are driving the development of targeted therapeutic strategies. This in turn is increasing the need to develop and validate meaningful outcome measures and their surrogates for use in clinical trials.
Identification of reliable, objective outcome measures that closely reflect the underlying disease process, correlate with motor function and are feasible for use in clinical trials in both children and adults with SMA are important in assessing the potential benefit of a therapy and in understanding its mechanism of action. Muscle weakness in SMA is clearly related to degree of innervation. However, compensatory mechanisms that result in changes in motor unit size, muscle fiber size and recruitment may differ between individual patients and over the range of SMA phenotype severity.
We have previously demonstrated that disease severity in SMA is correlated to the degree of innervation as determined by maximum ulnar compound muscle action potential (CMAP) amplitude and motor unit number estimation.8 Although SMA is traditionally considered a proximal muscle disease, all 89 subjects in our initial study, regardless of degree of weakness or type, had clearly evident denervation in a distal muscle, the abductor digiti minimi. Even in those with CMAP amplitude values that overlapped with normal controls, changes in motor unit size and morphology suggested collateral reinnervation in this nerve-muscle group.
Maximum ulnar CMAP amplitude demonstrated excellent test-retest reliability in this single-center SMA natural history study, and it correlated with general functional status using a basic ordinal scale to depict overall gross motor function at the time of CMAP testing independently from SMA type.8 In this paper, we provide additional data in support of maximum ulnar CMAP amplitude and area as a candidate surrogate of disease status and motor function in SMA. Furthermore, we document the performance characteristics, feasibility and rationale for use of this outcome measure in children with SMA Types II and III in the pediatric multicenter clinical trials setting.
MATERIALS AND METHODS
Participants
All data were obtained as a result of participation in the multicenter SMA CARNI-VAL trial (Clinicaltrials.gov ID NCT00227266). The SMA CARNI-VAL trial was a phase II multicenter trial involving two subject cohorts that evaluated the safety, tolerability, and efficacy of combined regimen of oral valproic acid and carnitine in patients with SMA types II and III.9 All participants had documented homozygous deletion of survival motor neuron (SMN) 1 to verify diagnosis of SMA. The Non-Ambulatory cohort participants were SMA “sitters” 2 to 8 years of age and included children with SMA types II and III after loss of ambulatory function. The Ambulatory cohort participants were SMA “standers and walkers” 3 to 17 years of age and included children with SMA type III who could still walk. Participation was excluded for any subject taking any pharmacological agent known to modify the disease or modulate expression of SMN2 for at least 3 months prior to enrollment.
A total of 94 participants were enrolled in the SMA CARNI-VAL trial: 61 in the Non-Ambulatory cohort (ages 1.8 to 8.7 years, mean 4.3 ± 2 years) and 33 in the Ambulatory cohort (ages 2.8 to 16.3 years, mean 7.3 ± 3.7 years). Data on the first screening visit (S1) was initially obtained from patients at all 6 CARNI-VAL sites. Upon review of the submitted waveforms and before any analysis, two of these sites' studies were found to have frequent technical problems that were outside the boundaries defined by the protocol. Individuals at these sites were not certified in electrophysiology, and had more limited pre-trial experience using the protocol in children. However, board-certified electrophysiologists with more experience performed studies at the remaining 4 sites. Analysis was done on patient data obtained from only the latter four sites. Therefore, for the current report, we performed a cross sectional analysis of data obtained at two screening visits for children with SMA types II and III who were enrolled at four of six clinical trial sites in the SMA CARNI-VAL trial to assess the feasibility, reliability, and validity of CMAP amplitude and area as a surrogate of SMA disease burden, and to explore associations between CMAP and motor function.
Ethics Statement
Written informed parental consent (for participants < 18 years) and assent (for participants ≥ 7 years) were obtained from all participants. The study was approved by the Institutional Review Board at each clinical trial site participating in the SMA CARNI-VAL trial and contributing data to this paper (University of Utah, Wayne State University, Ohio State Biomedical, and Johns Hopkins University).
Methods
All assessments took place during the CARNI-VAL trial screening visits. Participants were assessed twice during a 4–6 week baseline screening period by the study PI (CMAP) and clinical evaluator (motor function) at the study site where they were enrolled. Content, administration and scoring directions for functional tests (MHFMS and MHFMS-Extend) and CMAP are available in detail at http://smaoutcomes.org.
CMAP
Maximum ulnar CMAP amplitude and area were obtained by recording from the abductor digiti minimi muscle following ulnar nerve stimulation at the wrist. All electrophysiologic testing was performed by electromyographers who were experienced in the assessment of pediatric patients and who were blinded to previous test results. Maximum values for both negative peak (NP) amplitude and NP area were obtained from a total of 5 G1 electrode placements, using a disposable surface electrode with a 7 × 4 mm recording area (Alpine biomed adhesive surface electrode, part 9013L0203). Oral midazolam was used for anxiolysis at one center at the discretion of the site PI and approved by local institutional protocol, for children in whom anxiety or discomfort was considered likely to interfere with reliable testing and to facilitate repeated evaluations. Dosing was 0.2 to 0.5 mg/kg, with a maximum of 5 mg per dose. At the other three centers, no medications were used. Children were instead distracted using standard techniques that were regularly in use at the sites for pediatric electromyography studies.
All original CMAP waveforms were printed and faxed to the data-coordinating center with the completed clinical research form for independent review. CMAP amplitude and area data from all sites were screened by a single reviewer to ensure strict adherence to protocol and ascertain accuracy of placement of markers for amplitude and area measurements. CMAP data was excluded for one or more of the following reasons: 1) less than 3 technically adequate waveforms associated with unique G1 electrode placement, 2) electrical artifact precluding accurate amplitude and/or area measurements, and/or 3) initial positive deflection exceeding 1/3 of the negative peak amplitude.
Motor function
Motor performance was assessed using the Modified Hammersmith Functional Motor Scale (MHFMS) in the Non-Ambulatory cohort, and the MHFMS-Extend in the Ambulatory cohort. The MHFMS is reliable and stable over at least a six month duration in non-ambulatory children with SMA types II and III.10 The MHFMS-Extend is comprised of the 20 original MHFMS gross motor items, plus an additional 8 higher-level gross motor items. (ClinicalTrials.gov). Evaluators were trained to ensure they met specific criteria for standardization of performance and reliability for both the MHFMS and MHFMS-Extend. The possible MHFMS score range was 0–40, and the MHFMS-Extend possible score range was 0–56. Specific test administration and scoring criteria for functional tests and CMAP amplitude and area are described in detail at www.smaoutcomes.org.
Statistical analysis
Feasibility of CMAP amplitude and area was assessed by the percentage of participants who successfully completed the procedure at the four sites that provided CMAP data.
Validity was assessed by the ability of CMAP amplitude and area to discriminate between the Non-Ambulatory and the Ambulatory cohorts using area under the receiver operator characteristics curve (ROC). ROC provides a validity assessment, since it is a direct function of both sensitivity and specificity, where sensitivity is equivalent to assessing “convergent validity” and specificity is equivalent to assessing “divergent validity”.11
Test-retest reliability of CMAP amplitude and area measurements from the first (S1) to the second (S2) screening visit was measured with an intraclass correlation coefficient (ICC).
To assess correlation between CMAP values (NP amplitude and area) and motor function scores (MHFMS and MFMS-Extend), we used Pearson's correlation (r). Scores from the same visit were used to determine the relationship of CMAP amplitude and area to motor function; when available, data from the S2 visit was used in preference to that from S1.
RESULTS
Of the 69 participants at the 4 centers with CMAP amplitude and area data from the SMA CARNI-VAL trial, 68 participants had good quality CMAP data for at least one screening visit, 44 from the Non-Ambulatory cohort and 24 from the Ambulatory cohort (Table 1). Sixty-four participants had CMAP data obtained at both screening visits. 2 patients had usable CMAP data from only one screening visit, and 3 patients had only one screening visit with CMAP data. CMAP NP amplitude mean +/− SD (range) was 2.3 +/− 1.7 (0.5–7.6) mV for the Non-Ambulatory cohort and 5.5 +/− 2.6 (1.2–10.4) mV for the Ambulatory cohort. CMAP NP area mean +/− SD (range) was 5.5 +/− 4.6 (0.7–19.7) mVms for the Non-Ambulatory cohort and 14.9 +/− 7.7 (2.6–38.3) mV for the Ambulatory cohort. For functional motor testing, all 68 participants with CMAP amplitude and area data completed either the MHFMS [44 participants in the Non-Ambulatory cohort, MHFMS score mean +/− SD (range) was 18.8 +/− 9.6 (3–38)], or the MHFMS-Extend [24 participants in the Ambulatory cohort, MHFMS-Extend score mean +/− SD was 47.3 +/− 5.5 (36–56)].
Table 1.
Demographics of study participants with CMAP negative peak amplitude and area data
| Characteristic | Non-Ambulatory Cohort (N = 44) | Ambulatory Cohort (N = 24) |
|---|---|---|
| Age Mean (years) | 4.6 | 7.0 |
| Age SD (years) | 2.2 | 3.3 |
| Age Median (years) | 4.0 | 7.2 |
| Age Range (years) | 1.8 – 8.6 | 2.8 – 15.3 |
| Female (N / %) | 24 (55%) | 14 (58%) |
| Non-Hispanic (N / %) | 39 (88%) | 21 (87%) |
SD, standard deviation.
Feasibility
CMAP data were successfully obtained from 99% (68/69) of participants during at least one screening visit during the CARNI-VAL trial at the 4 clinical trial sites that rigorously followed the CMAP amplitude and area study protocol. Additionally, adequate CMAP amplitude and area values were obtained for 93% (64/69) of participants during both screening visits S1 and S2.
Validity
Validity was established with an ROC analysis between the CMAP and cohort membership (Ambulatory versus Non-Ambulatory). For CMAP NP amplitude, the ROC was 0.88, and for CMAP NP area the ROC was also 0.88. Using the Hosmer and Lemeshow12 rule of thumb for interpreting an ROC, a value between 0.8–0.9 is considered excellent discrimination; therefore, validity of CMAP NP amplitude and area as a measurement of innervation in SMA was clearly established. The discrimination, or separation, between the two cohorts is clearly illustrated in Figures 1 and 2.
Figure 1.
Smoothly drawn histograms for the two cohorts, also called kernel density plots, illustrating the separation of the two cohorts on the CMAP negative peak amplitude.
Figure 2.
Smoothly drawn histograms for the two cohorts, also called kernel density plots, illustrating the separation of the two cohorts on the CMAP negative peak area.
Test-Retest Reliability
Test-retest reliability between the first and second screening visit CMAP NP amplitude was very high when all patients were analyzed as one group (ICC=0.96, CI=0.93–0.97), as well as within the Non-Ambulatory cohort alone (ICC=0.94, CI=0.89–0.97) and the Ambulatory cohort alone (ICC=0.93, CI=0.85–0.97). Similarly, test-retest reliability between the first and second screening visit CMAP NP area was very high when all patients were analyzed together (ICC=0.97, CI=0.95–0.98), as well as within the Non-Ambulatory cohort alone (ICC=0.96, CI=0.93–0.98) and the Ambulatory cohort alone (ICC=0.94, CI=0.88–0.98). The percent difference between first and second screen measurements had an absolute mean +/− standard deviation (SD) of 0.5 +/− 0.6 mV for CMAP NP amplitude and 1.1 +/− 1.4 mVms for CMAP NP area. These absolute differences correspond to percent differences of 16.0 +/− 17.4% and 15.8 +/− 19.5%, respectively. Within the Non-Ambulatory cohort, the difference between first and second screen measurements for CMAP NP amplitude was 0.4 +/− 0.4 mV (19.2 +/− 19.0%), and for CMAP NP area it was 0.9 +/− 0.9 mVms (19.2 +/− 19.0%). Within the Ambulatory cohort, the difference between first and second screen measurements for CMAP NP amplitude was 0.6 +/− 0.7 mV (10.2 +/− 12.3%), and for CMAP NP area it was 1.4 +/− 1.9 mVms (8.5 +/−9.1%).
Correlation with motor function
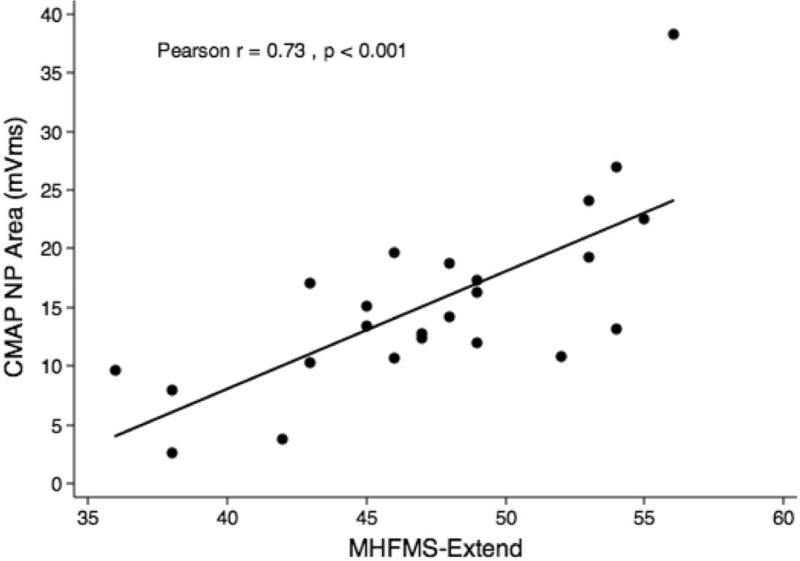
CMAP NP amplitude and area values demonstrated a positive correlation with motor function in both cohorts (r=0.61–0.73, p<0.001). When cohorts were evaluated separately, CMAP NP amplitude and area both correlated with the MHFMS motor function scores from the 44 participants in the Non-Ambulatory cohort (Figs. 3 and 4) and with the MHFMS-Extend scores from the 24 participants in the Ambulatory cohort (Figs. 5 and 6).
Figure 3.
Scatterplot demonstrating correlation between maximum CMAP negative peak amplitude and MHFMS scores for the Non-Ambulatory cohort.
Figure 4.
Scatterplot demonstrating correlation between maximum CMAP negative peak area and MHFMS scores for the Non-Ambulatory cohort.
Figure 5.
Scatterplot demonstrating correlation between maximum CMAP negative peak amplitude and MHFMS-Extend scores for the Ambulatory cohort.
Figure 6.
Scatterplot demonstrating correlation between maximum CMAP negative peak area and MHFMS-Extend scores for the Ambulatory cohort.
DISCUSSION
Data from this multicenter trial in children with SMA types II and III show that maximum ulnar CMAP NP amplitude and area (1) is a feasible, valid, and reliable physiological outcome measure, and that it (2) correlates with motor function. Maximum ulnar CMAP NP amplitude and area can thus be offered as valid and well-tolerated surrogate measures of innervation and hence disease burden in pediatric multi-center trial settings within the range of severity and age of subjects included in this trial.
Although maximum CMAP typically refers to the maximum CMAP obtained from a single G1 electrode placement, actual values can vary as much as 30% in this setting with electrode removal and replacement, reducing the reliability of the CMAP as an outcome measure. However, by specifying a minimum of at least 3 distinct G1 electrode placements, variability was further reduced by about half, to an average percent difference of 16%, such that its feasibility for use as an outcome measure to assess the combined nerve-muscle function was substantially improved. The required 3 distinct G1 placements required by our protocol may in part contribute to the high levels of test-retest reliability of CMAP NP amplitude and area observed in this study.
We initially chose the ulnar nerve-hypothenar muscle group for its ease of applicability across the entire range of disease severity and age from newborns to adult subjects with SMA.8 The current findings support the use of ulnar maximum CMAP NP amplitude and area in SMA. CMAP is noninvasive, easier to perform, requires minimal time and cooperation, is better tolerated by younger patients and is less vulnerable to variation in operator expertise as compared to motor unit number estimation. Therefore, from a practical standpoint, it is preferable to motor unit number estimation in the pediatric clinical trials setting.
We had previously demonstrated a correlation with gross motor functional status using a simple ordinal scale in a single site study.8 This study extends that observed correlation and shows that CMAP NP amplitude and area have a moderate to strong correlation with the SMA disease-specific graduated functional motor scales, the MHFMS and MHFMS-Extend. Higher CMAP amplitude and area values are associated with higher motor function even when each cohort was analyzed individually, providing further evidence for CMAP NP amplitude and area as a valid measure of disease severity within study cohorts and for its power to discriminate more discrete levels of function given the smaller sample sizes and the constrained limits of function of each cohort.
Additional work remains to be done in order to fully assess the clinical utility of CMAP NP amplitude and area markers of disease over time and as predictors or measures of change. It may also be of value to patients younger or older, or weaker or stronger, than the groups evaluated in this trial. The value of CMAP NP amplitude and area as an outcome measure for clinical trials depends upon the hypothesized mechanism of therapeutic action. Therapies that would alter the number of functioning motor units, their capacity for collateral reinnervation, fidelity of junctional or sprout transmission, or muscle fiber bulk could potentially alter CMAP NP amplitude, while other potential targets of therapy may not.13
At present, with no established specific treatment for SMA it is not possible to evaluate to what extent CMAP NP amplitude and area is responsive to the change associated with an effective therapy. Nonetheless, CMAP assessment has potential value for clinical trial design in several ways. A change in CMAP NP amplitude or area might provide an early “read out” or “biomarker” of efficacy that could prove a robust surrogate for disease status in younger children who are not able to cooperate reliably with pulmonary function testing, myometry or certain tests of motor function, such as the six minute walk test. CMAP NP amplitude and area also might prove useful as a means to stratify patient groups, or define potentially responsive patients within a window of values that restrict patients with expected ceiling or floor constraints for the primary outcome measure chosen for a given trial. Given the feasibility, validity, reliability, and correlation with motor function, CMAP NP amplitude and area is a strong candidate as a relevant surrogate for disease status and motor function in future clinical trials.
Acknowledgement
This work was funded entirely by Families of Spinal Muscular Atro- AQ3 phy (FSMA). This investigation was also supported by Grant R01-HD054599 (to K.J.S., University of Utah), Public Health Services, Grants UL1RR025764 (University of Utah, Center for Clinical and Translational Sciences), UL1RR025005 (Johns Hopkins University, General Clinical Research Center), M01-RR00034 (Ohio State University, General Clinical Research Center), and the Clinical Research Center, Children's Hospital of Michigan, from the National Center for Research Resources, National Institutes of Health. The funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- (CMAP)
Compound muscle action potential
- (CI)
Confidence interval
- (ICC)
Intraclass correlation coefficient
- (MHFMS)
Modified Hammersmith Functional Motor Scale
- (NP)
Negative peak
- (r)
Pearson's correlation
- (ROC)
Receiver operator characteristics curve
- (SMA)
Spinal muscular atrophy
- (SMN)
Survival motor neuron
- (SD)
Standard deviation
References
- 1.Emery AE. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 2.Hendrickson BC, Donohoe C, Akmaev VR, et al. Differences in SMN1 allele frequencies among ethnic groups within North America. J Med Genet. 2009;46:641–4. doi: 10.1136/jmg.2009.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerres K, Rudnik-Schoneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52:518–23. doi: 10.1001/archneur.1995.00540290108025. [DOI] [PubMed] [Google Scholar]
- 4.Zerres K, Rudnik-Schoneborn S, Forrest E, Lusakowska A, Borkowska J, Hausmanowa-Petrusewicz I. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci. 1997;146:67–72. doi: 10.1016/s0022-510x(96)00284-5. [DOI] [PubMed] [Google Scholar]
- 5.Chung BH, Wong VC, Ip P. Spinal muscular atrophy: survival pattern and functional status. Pediatrics. 2004;114:e548–53. doi: 10.1542/peds.2004-0668. [DOI] [PubMed] [Google Scholar]
- 6.McCambridge TM, Stricker PR. Strength training by children and adolescents. Pediatrics. 2008;121:835–40. doi: 10.1542/peds.2007-3790. [DOI] [PubMed] [Google Scholar]
- 7.Russman BS, Buncher CR, White M, Samaha FJ, Iannaccone ST. Function changes in spinal muscular atrophy II and III. The DCN/SMA Group. Neurology. 1996;47:973–6. doi: 10.1212/wnl.47.4.973. [DOI] [PubMed] [Google Scholar]
- 8.Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57:704–12. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swoboda KJ, Scott CB, Reyna SP, et al. Phase II open label study of valproic acid in spinal muscular atrophy. PLoS ONE. 2009;4:e5268. doi: 10.1371/journal.pone.0005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krosschell KJ, Maczulski JA, Crawford TO, Scott C, Swoboda KJ. A modified Hammersmith functional motor scale for use in multi-center research on spinal muscular atrophy. Neuromuscul Disord. 2006;16:417–26. doi: 10.1016/j.nmd.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDowell I, Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires. 2nd ed. Oxford University Press; New York: 1996. [Google Scholar]
- 12.Hosmer D, Lemeshow S. Applied logistic regression. 2nd ed. Wiley-Interscience Publication; New York: 2000. [Google Scholar]
- 13.Crawford TO. Concerns about the design of clinical trials for spinal muscular atrophy. Neuromuscul Disord. 2004;14:456–60. doi: 10.1016/j.nmd.2004.04.004. [DOI] [PubMed] [Google Scholar]