Abstract
Voltage-gated Na+ channel (VGSC) β subunits are not “auxiliary.” These multifunctional molecules not only modulate Na+ current (INa), but also function as cell adhesion molecules (CAMs) – playing roles in aggregation, migration, invasion, neurite outgrowth, and axonal fasciculation. β subunits are integral members of VGSC signaling complexes at nodes of Ranvier, axon initial segments, and cardiac intercalated disks, regulating action potential propagation through critical intermolecular and cell-cell communication events. At least in vitro, many β subunit cell adhesive functions occur both in the presence and absence of pore-forming VGSC α subunits, and in vivo β subunits are expressed in excitable as well as non-excitable cells, thus β subunits may play important functional roles on their own, in the absence of α subunits. VGSC β1 subunits are essential for life and appear to be especially important during brain development. Mutations in β subunit genes result in a variety of human neurological and cardiovascular diseases. Moreover, some cancer cells exhibit alterations in β subunit expression during metastasis. In short, these proteins, originally thought of as merely accessory to α subunits, are critical players in their own right in human health and disease. Here we discuss the role of VGSC β subunits in the nervous system.
β subunits are multifunctional CAMs
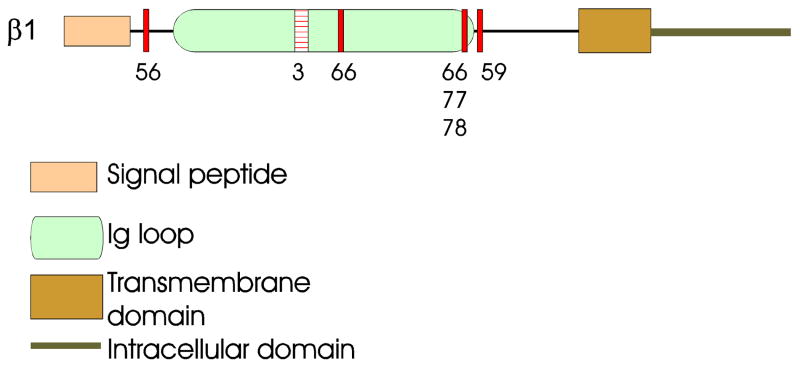
VGSCs in brain are heterotrimers, containing a single α subunit associated with one non-covalently (β1 or β3) and one covalently (β2 or β4) linked β subunit [9, 85]. In mammals, SCN1B - SCN4B encode β1 - β4, respectively. β1, β2, β3, and β4 are type I transmembrane proteins, containing an extracellular N-terminal signal peptide and Ig domain, one transmembrane domain, and an intracellular C-terminal domain (Fig. 1). SCN1B gives rise to two splice variants, β1 and β1B (also called β1A) [32, 61]. β1B is formed through retention of intron 3, containing a stop codon and thus excluding the transmembrane domain in exon 4. The predicted amino acid sequence of the retained intronic region exhibits very low homology between species [61], however, hydrophobicity analysis of these sequences reveals no transmembrane domains in any species, predicting that β1B is a secreted protein that may function as a ligand for cell adhesion [58]. All of the β subunits, including β1B, belong to the Ig superfamily of CAMs [26, 86]. Two SCN1B splice variants, including transmembrane and secreted forms, is consistent with other Ig superfamily CAMs [28, 64]
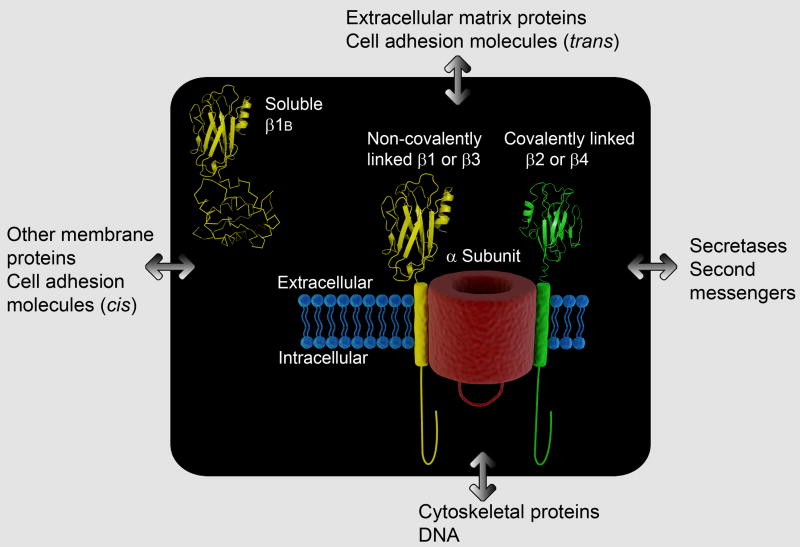
Fig. 1. Subunit structure of VGSCs.
VGSCs in the CNS are multiprotein complexes composed of a single pore-forming α subunit, one non-covalently-linked β subunit (β1 or β3), and one covalently-linked β subunit (β2 or β4). β1B is a secreted, soluble subunit. α and β subunits interact with multiple cell adhesion, ECM, cytoskeletal, and intracellular signal transduction proteins. Models of the β1 and β2 Ig loops and the C-terminus of β1B were obtained by analyzing amino acid sequences on the I-TASSER server [82, 87, 88]. We thank Mauricio Patino for his help in the design of this figure.
VGSC β subunits are widely expressed in excitable and non-excitable cells in the nervous system. Table 1 provides a detailed description of nervous system expression of β1–β4 in mammals and zebrafish. In some tissues there is evidence that β subunits may be expressed in the absence of α, suggesting that they may play cell adhesive roles in the absence of ion conduction in vivo. β subunit expression in the CNS is developmentally regulated. Of the four genes, β1B and β3 mRNA predominate in fetal brain, with levels decreasing during late gestation and after birth. In contrast, levels of β1 and β2 mRNA increase progressively and become dominant after birth [26, 32, 65, 69].
Table 1. Tissue specific and subcellular domain specific expression of VGSC β subunits in the nervous system.
| Tissue – subcellular domain | β1 | β1B | β2 | β3 | β4 |
|---|---|---|---|---|---|
| Central Nervous System | |||||
| Hippocampal neurons | + [12] | + [86] | + (RNA) [52, 69] | + [86] | |
| Cortical neurons | + [32, 61] | + [86] | + (RNA) [52, 69] | + [86] | |
| Basal Ganglia | - (RNA) [52] | + [86] | + (RNA) [52, 69] | + [86] | |
| Retinal ganglion cells | + [18, 29] | + [29] | |||
| Optic nerve nodes of Ranvier | + [12, 18] | + [29] | |||
| Optic nerve myelin | + [19], [29] | ||||
| Astrocytes | + (RNA) [2, 55] | + [54] | |||
| Dorsal root ganglia neurons | + [61] | + [60, 86] | + [8] | + [86] | |
| Ventral horn neurons | + [32] | + [86] | + [86] | ||
| Radial glia | + [19] | ||||
| Cerebellar Purkinje cells soma | + [32, 61] | + [86] | + (RNA, transitory) [69] | + [86] | |
| Cerebellar granule neurons soma | + [4] | - (RNA) [52, 69] | |||
| Cerebellar granule neurons AIS and growth cone | + [4] | ||||
| Deep cerebellar nuclei | + [32] | + [86] | + [86] | ||
| Bergmann glia | + [14] | - [32] | - [14] | - [14] | + [14] |
| Peripheral Nervous System | |||||
| Peripheral nerves | + [61] | + [60] | + [8] | ||
| Sciatic nerve nodes of Ranvier | + [12] | ||||
| Schwann cells (zebrafish) | + [19] |
β subunits associate with multiple VGSC α subunits [16, 41, 48, 70]. In addition, β subunits interact both in cis and in trans with multiple CAMs, with components of the extracellular matrix (ECM), and with intracellular cytoskeletal and signaling molecules. A summary of interactions is presented in Table 2, with the majority of these studies focusing on β1. Similar studies have not been carried out for β1B, however, because β1 and β1B share the extracellular Ig domain, it is safe to assume that these two CAMs share many, if not all, extracellular binding partners.
Table 2.
VGSC β subunit protein-protein interactions
| β SUBUNITS | EXTRACELLUL AR MATRIX PROTEINS | CYTOSKELETAL PROTEINS | ION CHANNELS | ENZYMES | CELL ADHESION MOLECULES | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β1 | β2 | β3 | TN-C | TN-R | AnkyrinG | AnkyrinB | Kv4.2 / Kv4.3 | RPTPβ | Contactin | NF-155 | NF-186 | N-Cadherin | Nr CAM | |
| β1 | trans [39] | trans [43] | (-) [43] | (+) [83] | Non-phosphorylated β1 [39, 40, 44] | Non-phosphorylated β1 [41] | (+) [15] | (+) [62] |
cis [30, 44] trans [43] |
trans [43] |
cis [63] trans [43] |
(+) [41] | cis, trans [43] | |
| β2 | trans [43] | trans [39] | (+) [71, 83] | (+) [71, 83] | (+) [39] | (-) [62] | (-) [30] | (-) [63] | ||||||
| β3 | (-) [43] | (-) [42] | (-) [42] |
cis [63] trans [43] |
||||||||||
| β4 | Cis [1] | |||||||||||||
Abbreviations: NF: neurofascin, RPTPβ: receptor tyrosine phosphatase β, TN: tenascin.
β1 associates with multiple CAMs, including itself, contactin, neurofascin-186 and -155, NrCAM, N-cadherin, and VGSC β2 [30, 41, 43, 63]. β2 does not associate with contactin, but does associate with β1 and the ECM proteins, tenascin-C and tenascin-R [43, 71]. Fibroblasts expressing β1 or β2 are repelled by tenascin-R substrates, suggesting initial binding of this ECM molecule [83]. Trans homophilic β1 or β2 (but not β3) association results in recruitment of ankyrin to points of cell-cell contact [39, 42]. Phosphorylation of a critical tyrosine residue (β1Y181) abolishes β1 association with ankyrinB or ankyrinG and this is postulated to be a mechanism regulating β1 subcellular localization [40, 44]. Indirect evidence suggests that β1 may associate with the lipid raft tyrosine kinase fyn in response to extracellular trans β1-β1 adhesion [5]. Association of the β1 intracellular domain with receptor phosphotyrosine phosphatase β may provide a yin-yang mechanism of phosphorylation and dephosphorylation [62]. β1 and β2 also participate in heterophilic extracellular interactions and some of these interactions require the intracellular domain of at least one of the partners. For example, the intracellular domains of NrCAM and β2, respectively, are necessary for their extracellular association with β1, suggesting inside-out signaling mechanisms [43].
β subunits are substrates for sequential cleavage by the β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) and γ-secretase [81]. β2 is also cleaved by the α-secretase ADAM10 [34]. Cleavage of β subunits by BACE1 or α-secretase at sites in the extracellular juxtamembrane region results in ectodomain shedding, leaving membrane-bound C-terminal fragments (CTFs) [34, 81]. The shed ectodomain of β1 may function as a soluble ligand for cell adhesion to promote neurite outgrowth [14, 39]. The CTFs are processed by γ-secretase, resulting in freed intracellular domains (ICDs) [34, 81]. Inhibition of β2 cleavage by γ-secretase reduces cell-cell adhesion and migration [34] and β4 processing by BACE1 increases neurite outgrowth [49], predicting that proteolytic processing events are critical to the in vivo functioning of these subunits. The β2 ICD translocates to the nucleus and increases Scn1a expression, suggesting that this fragment may function as a transcriptional regulator of VGSC α subunits [33]. Although all four β subunits are BACE substrates in vitro, in vivo processing has only been confirmed for β2 and β4 [81], suggesting that this role may be specific to subunits that can be covalently linked to α.
What do β subunits do?
1. β subunits modulate INa
A large body of literature shows that β subunits modulate INa in heterologous systems. While this approach has yielded valuable structure-function information, these data may have little relevance to the in vivo situation. Given α-β subunit combinations can yield different electrophysiological results in different cell backgrounds, e.g. [27]. β1-mediated effects on Nav1.5 have been particularly difficult to resolve using heterologous expression (see [46] for review). This may be due to the presence of varying levels of endogenous β subunits in cell lines [50, 51] and/or the differential expression of other endogenous VGSC interacting or modifying proteins. Native cells express multiple VGSC α subunits in specific complexes of signaling, cytoskeletal, and adhesion molecules in particular subcellular domains, a situation that cannot be mimicked using a heterologous system. Importantly, while co-expression of β subunits, especially β1, produces significant changes in INa in heterologous systems, mouse models tell us otherwise. While Scn1b null mice have a severe neurological phenotype, only subtle, cell type specific changes in INa are reported [1, 4, 12, 59, 75]. Thus, heterologous systems are not reliable predictors of the effects of β subunits, especially β1, on INa in vivo.
β subunit modulation of INa in vivo is cell type specific and subtle, yet may translate into significant changes in electrical excitability. Scn1b null cerebellar granule neurons (CGNs) have normal transient INa but reduced resurgent INa, likely contributing to the ataxic phenotype of these mice [4]. Minor changes in hippocampal excitability in Scn1b null mice may contribute to severe seizures [59]. A population of Scn2b null hippocampal neurons show negative shifts in the voltage-dependence of inactivation [11, 75], while in small-fast DRG neurons there is slowing of INa activation and inactivation with no change in voltage-dependence [37]. Persistent INa is increased in hippocampal neurons transfected with β4. β4-expressing neurons from Scn1b and Scn1b/Scn2b null mice show slowed entry into inactivated states [1]. β1 and β4 play antagonistic roles in hippocampus in vivo, with the former favoring inactivation, and the latter favoring activation. Because increased VGSC availability may facilitate action potential firing, this may suggest a mechanism for seizure susceptibility of both mice and humans with SCN1B mutations [1].
The interaction of β subunits with other CAM, cytoskeletal, or signaling molecules may influence INa. β1 association with contactin or NF-186 results in increased VGSC cell surface expression [30, 43]. β1 and β2 are ankyrin-binding proteins. AnkyrinB null mice exhibit reduced INa density and abnormal INa kinetics [10], suggesting that β subunits play important roles in the VGSC-ankyrin complex. These interactions may be particularly critical at nodes of Ranvier of myelinated axons. Scn1b null mice have reduced numbers of nodes, dysmyelination, and disruption of axo-glial cell-cell contacts [12]. While nodal proteins are localized normally in these mice, association between VGSCs and contactin is disrupted. Loss of critical β1 subunit-dependent protein-protein interactions may lead to instability of the node, resulting in disrupted saltatory conduction [12, 31]. Finally, β1 and Nav1.6 reciprocally modulate each other in terms of neurite outgrowth, subcellular localization, and regulation of resurgent INa in CGNs [4]. Taken together, these data implicate β1 in brain development.
2. β subunits modulate channel cell surface expression
β subunits, especially β2, increase INa density in heterologous systems by enhancing α subunit cell surface expression [26]. α subunit association with β2 and concomitant plasma membrane insertion are the final steps in VGSC biosynthesis, suggesting that β2 is critical for establishment and maintenance of channel cell surface expression and excitability [67, 68]. Experiments in Scn2b null neurons support this conclusion. Acutely dissociated Scn2b null hippocampal neurons have INa density that is ∼50% of wildtype. Scn2b null neuronal cultures exhibit a ∼50% reduction in cell surface 3H-saxitoxin binding compared to wildtype [11]. Similar to the effects of β1, however, the ability of β2 to increase INa density appears to be cell-type specific. For example, neurons isolated from Scn2b null dentate gyrus have similar INa densities as wildtype [75]. Further, the effects of β2 may be specific to tetrodotoxin-sensitive channels. Scn2b null small-fast DRG neurons have significantly decreased protein expression of Nav1.1 and Nav1.7 and subsequent reduction in tetrodotoxin-sensitive INa density compared to wildtype, but unchanged tetrodotoxin-resistant INa [37].
β1 may be required for the expression or subcellular localization of certain VGSC α subunits in specific cell types. Scn1b null hippocampal neurons in the CA3 region express decreased levels of Nav1.1 and increased levels of Nav1.3 compared to wildtype [12]. Subpopulations of Scn1b null CGNs exhibit reduced expression of Nav1.6 channels, but increased levels of Nav1.1 channels, at axon initial segments compared to wildtype [4].
3. β subunits modulate cellular migration, neurite extension, and axonal fasciculation
As is true for other neuronal and glial CAMs, β subunits are critical for cellular migration, neurite outgrowth, and axonal fasciculation. Interestingly, β subunits are postulated to modulate migration of cancer cells through similar mechanisms [7]. β1 and β2 mediate migration of fibroblasts away from a tenascin-R substrate [83]. β1 and β4 promote neurite extension in a cell type-dependent manner [14, 49]. β1 promotes and β2 inhibits CGN neurite outgrowth, while β4 has no detectable effect [14]. β3 was not tested, but recent results predict that β3 would not affect neurite outgrowth through homophilic interactions [42]. β1-mediated neurite outgrowth in CGNs requires β1-β1 trans interactions: β1 expressed at the CGN cell surface must interact with another β1 that is located either on the cell surface of an adjacent neuron or glial cell [14]. In addition, β1-mediated neurite outgrowth in CGNs requires the presence of Nav1.6 at the axon initial segment, tetrodotoxin-sensitive INa, the CAM contactin, and fyn kinase [4, 5]. Scn1b null mice exhibit significant defasciculation of the corticospinal tract, abnormal migration of CGNs, and defasciculation of cerebellar parallel fibers [5]. Cerebellar defects may contribute to the ataxic phenotype of these mice [12]. Zebrafish scn1bb morphants exhibit defasciculation of the olfactory nerve [19]. Taken together, these data suggest that VGSC β subunits are critical modulators of brain development.
The roles of β subunits in cell migration, adhesion, and neurite extension depend not only on extracellular CAM interactions, but also on intracellular signal transduction events. β1-mediated neurite outgrowth in CGNs requires fyn kinase [5], suggesting that extracellular β1-β1 cell adhesion results in intracellular activation of fyn and initiation of a tyrosine phosphorylation signal transduction cascade. Blockade of processing of β2 by γ-secretase inhibits cell migration [34], suggesting that β2-ankyrin interactions are disrupted by γ-secretase cleavage to allow cytoskeletal remodeling.
What is the role of β subunits in disease?
1. Evidence from mouse models
Scn2b and Scn3b null mice have relatively normal life spans and behaviors [11, 23]. Scn2b null mice have a ∼50% loss of cell surface tetrodotoxin-sensitive VGSCs in neurons, resulting in reduction in compound action potential amplitude and increased action potential threshold. Scn2b null mice show increased susceptibility to seizures and sensitivity to thermal stimuli, and decreased sensitivity in some models of neuropathic pain [11, 37]. The apparent lack of a neurological phenotype in Scn3b mice suggests that Scn1b may compensate for Scn3b in brain [22, 23]. On the other hand, Scn1b null mice have a severe and complex phenotype that includes retarded growth, ataxia, spontaneous seizures, and lethality by postnatal day 21 [12], suggesting that the remaining β subunit genes cannot compensate for the loss of Scn1b. Scn1b null mice may represent a novel model for Dravet Syndrome [59]. In contrast, Scn1b+/- mice have a normal phenotype [12]. Surprisingly, in spite of an extensive literature describing significant electrophysiological effects of β1 co-expression with α subunits in heterologous systems, only subtle changes in INa have been reported in Scn1b null neurons [12, 59, 75]. Thus, the cell adhesive functions of β1 may be more important than INa modulation in vivo.
2. Human inherited epilepsy
β subunits are involved in a number of human neurological diseases (Table 3). While β subunit mutations result in cardiac as well as neurological disease, we will focus our discussion on neuropathology.
Table 3. Neurological diseases associated with β subunits.
| β Subunit | Disease | Relationship | Model | Reference |
|---|---|---|---|---|
| β1 | Febrile seizures | Causal | Human | [3, 56, 66, 77, 78] |
| Dravet Syndrome | Causal | Human, Mouse | [12, 59] | |
| Temporal lobe epilepsy | Causal | Human | [66] | |
| Traumatic nerve injury | Downstream target | Human | [13] | |
| β2 | Multiple sclerosis | Modifying factor | Mouse | [53] |
| Post-traumatic neuropathic pain | Downstream target | Mouse | [60] | |
| Inflammatory pain | Modifying factor | Mouse | [37] | |
| Traumatic nerve injury | Downstream target | Human | [13] | |
| β3 | Temporal lobe epilepsy without hippocampal sclerosis | Causal vs. Downstream target (?) | Human | [76] |
| Traumatic nerve injury | Downstream target | Human | [8] | |
| β4 | Huntington's disease | Downstream target | Human, Mouse | [57] |
Of the four VGSC β subunits genes, to date only mutations in SCN1B are reported to cause epilepsy (Fig. 2). At least two different studies have purposefully screened SCN2B for mutations in epileptic patients with no positive results, suggesting that this gene may not be a target for epilepsy, even though it is a target for inherited cardiac arrhythmia [25, 77, 80]. All epileptic syndromes associated with SCN1B thus far include febrile seizures, assigning them to the disease spectrum of generalized epilepsy with febrile seizures plus (GEFS+) that includes Dravet Syndrome, a catastrophic pediatric epileptic encephalopathy that includes mental retardation [3, 56, 59, 66, 77, 78]. Functional characterization of these mutants has largely been performed in heterologous systems, but these results may not accurately reflect the in vivo situation (results summarized in [59]). Importantly, the majority of SCN1B epilepsy mutations are loss-of-function [1, 47, 59, 73, 84]. Three of these mutants are trafficking-deficient [59, 84] and it is interesting to speculate that this may be a common underlying mechanism for of SCN1B-linked epilepsies. In all cases but one [59], patients carry a single mutant SCN1B allele, making the data difficult to resolve with the apparently normal phenotype of Scn1b+/- mice [3, 66, 77, 78], and raising the possibility that SCN1B mutant alleles may have dominant negative functions that have not been detected using heterologous expression systems. It is likely that genetic background as well as epigenetic factors play important roles in the phenotypes of these mutant alleles in human patients. When thinking about the role of β subunits in disease, it is important to consider that β subunits modulate the response of VGSC α subunits to therapeutic agents that are used to treat both epilepsy and cardiac arrhythmia, [24, 35, 38, 75]. Thus, β subunits must be included in analyzing both the cause and treatment of channelopathies.
Fig. 2. Localization of human GEFS+ spectrum mutations in SCN1B.
Solid bars, missense mutations; Striped bars, deletions. Numbers correspond to cited references.
3. Is there a role for β subunits in neuroprotection or neurodegeneration?
Rodent models of nerve injury suggest a protective role for β2 reduction in neuropathology. β2 expression is increased in peripheral axons and cell bodies in both the spared nerve injury and spinal nerve ligation models of neuropathic pain [60]. Consistent with this, the behavioral response to these models is significantly attenuated in Scn2b null mice [60] and Scn2b null mice manifest a reduced response to the formalin model of inflammatory pain [37]. Scn2b null mice exhibit significantly attenuated symptoms, mortality, and axonal loss compared to wildtype in the Experimental Allergic Encephalomyelitis (EAE) mouse model of Multiple Sclerosis [53]. Brain levels of Scn4b mRNA are reduced in mouse models Huntington's disease [57]. This change is reflected in β4 protein levels in the basal ganglia, and is confirmed in human patients [57]. β4 reduction in mouse models precedes the appearance of motor symptoms. It has been proposed that, in light of its neurite outgrowth promoting ability, a reduction in β4 may cause neurodegeneration as well as alter INa [57].
4. Putative Role of β subunits in Non-epileptic Neurological Disorders
Patients with SCN1B mutations have GEFS+ spectrum epilepsy disorders [59]. Importantly, there is a significant comorbidity of neuropsychiatric disease and seizures [17, 21] and anti-epileptic therapies targeting VGSCs are effective in mood disorders [74], suggesting a shared pathophysiology between these diseases [21]. Bipolar disorder is linked genetically to ANK3, encoding ankyrinG, a protein that is critical for VGSC targeting and localization in neurons [20]. β1 and β2 are ankyrinG-binding proteins [40]. SCN8A, encoding Nav1.6 that associates with β1 and β2 and is also an ankyrinG-binding protein, is another susceptibility gene for bipolar disorder [79]. Scn1b null mice have neuronal migration, pathfinding, and fasciculation defects in the cerebellum [4, 5]. Cerebellar output targets multiple non-motor areas in the prefrontal cortex and posterior parietal cortex associated with attention, memory, learning, and emotion [72]. Thus, a possible novel direction for β subunit research is the idea that mutations in the genes encoding these subunits may be linked to psychiatric disorders as well as neurological disease. Because SCN1B plays important roles in neuronal pathfinding and VGSC expression, disruptions in its expression during development may lead to a spectrum of childhood and adolescent neurological and neuropsychiatric diseases. In vivo data suggest that the primary role of SCN1B in brain is cell adhesion, further, that disruptions in SCN1B-mediated cell adhesive interactions result in pathology. β subunit function may be modulated in the future by interfering with or enhancing cell adhesive interactions, such that patients with diseases-causing β subunit gene mutations might be treated with small molecules that mimic or alter these interactions.
Conclusions and future directions
Previous work has centered on the functional roles of β subunits in INa modulation. However, while β subunits clearly modulate INa in heterologous systems, in vivo models indicate that this may not be their most important role. It is critical that we now challenge our previously held concepts and take a fresh look at the biology of these multifunctional subunits. As discussed above, an important direction in ion channel research focuses on the finding that some voltage-gated ion channels are multi-functional. In addition to regulating electrical excitability through ion conduction, some voltage-gated ion channels contribute to processes as diverse as intracellular signaling, transcriptional regulation, scaffolding, and cell adhesion without requiring changes in ion flux [6, 7, 36]. Results reviewed here demonstrate roles for VGSC β subunits in the regulation of VGSC expression, localization, INa modulation, action potential conduction, and cell adhesion. While many drugs targeting VGSC α subunits are in common use [45], the potential of VGSC β subunits as therapeutic targets has not been considered. Moreover, therapeutic targeting of the non-conducting functions of VGSCs may be critical. In conclusion, important advances in the therapy of diseases that are associated with VGSC β subunit loss- or modulation-of-function may be achieved by targeting β subunits directly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aman TK, Grieco-Calub TM, Chen C, Rusconi R, Slat EA, Isom LL, Raman IM. Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. J Neurosci. 2009;29:2027–2042. doi: 10.1523/JNEUROSCI.4531-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronica E, Troost D, Rozemuller AJ, Yankaya B, Jansen GH, Isom LL, Gorter JA. Expression and regulation of voltage-gated sodium channel beta1 subunit protein in human gliosis-associated pathologies. Acta Neuropathol. 2003;105:515–523. doi: 10.1007/s00401-003-0677-2. [DOI] [PubMed] [Google Scholar]
- 3.Audenaert D, Claes L, Ceulemans B, Lofgren A, Van Broeckhoven C, De Jonghe P. A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology. 2003;61:854–856. doi: 10.1212/01.wnl.0000080362.55784.1c. [DOI] [PubMed] [Google Scholar]
- 4.Brackenbury WJ, Calhoun JD, Chen C, Miyazaki H, Nukina N, Oyama F, Ranscht B, Isom LL. Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proceedings of the National Academy of Sciences of the United States of America. 107:2283–2288. doi: 10.1073/pnas.0909434107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brackenbury WJ, Davis TH, Chen C, Slat EA, Detrow MJ, Dickendesher TL, Ranscht B, Isom LL. Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. J Neurosci. 2008;28:3246–3256. doi: 10.1523/JNEUROSCI.5446-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brackenbury WJ, Djamgoz MB, Isom LL. An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist. 2008;14:571–583. doi: 10.1177/1073858408320293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brackenbury WJ, Isom LL. Voltage-gated Na+ channels: potential for beta subunits as therapeutic targets. Expert Opin Ther Targets. 2008;12:1191–1203. doi: 10.1517/14728222.12.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casula MA, Facer P, Powell AJ, Kinghorn IJ, Plumpton C, Tate SN, Bountra C, Birch R, Anand P. Expression of the sodium channel beta3 subunit in injured human sensory neurons. Neuroreport. 2004;15:1629–1632. doi: 10.1097/01.wnr.0000134927.02776.ae. [DOI] [PubMed] [Google Scholar]
- 9.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacological reviews. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan VS, Tuvia S, Buhusi M, Bennett V, Grant AO. Abnormal cardiac Na(+) channel properties and QT heart rate adaptation in neonatal ankyrin(B) knockout mice. Circulation research. 2000;86:441–447. doi: 10.1161/01.res.86.4.441. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ, 3rd, Kazen-Gillespie KA, Kazarinova-Noyes K, Shrager P, Saunders TL, Macdonald RL, Ransom BR, Scheuer T, Catterall WA, Isom LL. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:17072–17077. doi: 10.1073/pnas.212638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O'Malley HA, Bharucha V, Meadows LS, Knudsen GA, Vilaythong A, Noebels JL, Saunders TL, Scheuer T, Shrager P, Catterall WA, Isom LL. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci. 2004;24:4030–4042. doi: 10.1523/JNEUROSCI.4139-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coward K, Jowett A, Plumpton C, Powell A, Birch R, Tate S, Bountra C, Anand P. Sodium channel beta1 and beta2 subunits parallel SNS/PN3 alpha-subunit changes in injured human sensory neurons. Neuroreport. 2001;12:483–488. doi: 10.1097/00001756-200103050-00012. [DOI] [PubMed] [Google Scholar]
- 14.Davis TH, Chen C, Isom LL. Sodium channel beta1 subunits promote neurite outgrowth in cerebellar granule neurons. The Journal of biological chemistry. 2004;279:51424–51432. doi: 10.1074/jbc.M410830200. [DOI] [PubMed] [Google Scholar]
- 15.Deschenes I, Armoundas AA, Jones SP, Tomaselli GF. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. J Mol Cell Cardiol. 2008;45:336–346. doi: 10.1016/j.yjmcc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhar Malhotra J, Chen C, Rivolta I, Abriel H, Malhotra R, Mattei LN, Brosius FC, Kass RS, Isom LL. Characterization of sodium channel alpha- and beta-subunits in rat and mouse cardiac myocytes. Circulation. 2001;103:1303–1310. doi: 10.1161/01.cir.103.9.1303. [DOI] [PubMed] [Google Scholar]
- 17.Dixon-Salazar TJ, Keeler LC, Trauner DA, Gleeson JG. Autism in several members of a family with generalized epilepsy with febrile seizures plus. Journal of child neurology. 2004;19:597–603. doi: 10.1177/088307380401900806. [DOI] [PubMed] [Google Scholar]
- 18.Fein AJ, Meadows LS, Chen C, Slat EA, Isom LL. Cloning and expression of a zebrafish SCN1B ortholog and identification of a species-specific splice variant. BMC genomics. 2007;8:226. doi: 10.1186/1471-2164-8-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fein AJ, Wright MA, Slat EA, Ribera AB, Isom LL. scn1bb, a zebrafish ortholog of SCN1B expressed in excitable and nonexcitable cells, affects motor neuron axon morphology and touch sensitivity. J Neurosci. 2008;28:12510–12522. doi: 10.1523/JNEUROSCI.4329-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St CD, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60:177–185. doi: 10.1016/j.biopsych.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Hakim P, Brice N, Thresher R, Lawrence J, Zhang Y, Jackson AP, Grace AA, Huang CL. Scn3b knockout mice exhibit abnormal sino-atrial and cardiac conduction properties. Acta Physiol (Oxf) 198:47–59. doi: 10.1111/j.1748-1716.2009.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakim P, Gurung IS, Pedersen TH, Thresher R, Brice N, Lawrence J, Grace AA, Huang CL. Scn3b knockout mice exhibit abnormal ventricular electrophysiological properties. Prog Biophys Mol Biol. 2008;98:251–266. doi: 10.1016/j.pbiomolbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakim P, Thresher R, Grace AA, Huang CL. Effects of flecainide and quinidine on action potential and ventricular arrhythmogenic properties in Scn3b knockout mice. Clin Exp Pharmacol Physiol. doi: 10.1111/j.1440-1681.2010.05369.x. [DOI] [PubMed] [Google Scholar]
- 25.Haug K, Sander T, Hallmann K, Rau B, Dullinger JS, Elger CE, Propping P, Heils A. The voltage-gated sodium channel beta2-subunit gene and idiopathic generalized epilepsy. Neuroreport. 2000;11:2687–2689. doi: 10.1097/00001756-200008210-00016. [DOI] [PubMed] [Google Scholar]
- 26.Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, Catterall WA. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 27.Isom LL, Scheuer T, Brownstein AB, Ragsdale DS, Murphy BJ, Catterall WA. Functional co-expression of the beta 1 and type IIA alpha subunits of sodium channels in a mammalian cell line. The Journal of biological chemistry. 1995;270:3306–3312. doi: 10.1074/jbc.270.7.3306. [DOI] [PubMed] [Google Scholar]
- 28.Jones DC, Roghanian A, Brown DP, Chang C, Allen RL, Trowsdale J, Young NT. Alternative mRNA splicing creates transcripts encoding soluble proteins from most LILR genes. Eur J Immunol. 2009;39:3195–3206. doi: 10.1002/eji.200839080. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan MR, Cho MH, Ullian EM, Isom LL, Levinson SR, Barres BA. Differential control of clustering of the sodium channels Na(v)1.2 and Na(v)1.6 at developing CNS nodes of Ranvier. Neuron. 2001;30:105–119. doi: 10.1016/s0896-6273(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 30.Kazarinova-Noyes K, Malhotra JD, McEwen DP, Mattei LN, Berglund EO, Ranscht B, Levinson SR, Schachner M, Shrager P, Isom LL, Xiao ZC. Contactin associates with Na+ channels and increases their functional expression. J Neurosci. 2001;21:7517–7525. doi: 10.1523/JNEUROSCI.21-19-07517.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazarinova-Noyes K, Shrager P. Molecular constituents of the node of Ranvier. Mol Neurobiol. 2002;26:167–182. doi: 10.1385/MN:26:2-3:167. [DOI] [PubMed] [Google Scholar]
- 32.Kazen-Gillespie KA, Ragsdale DS, D'Andrea MR, Mattei LN, Rogers KE, Isom LL. Cloning, localization, and functional expression of sodium channel beta1A subunits. The Journal of biological chemistry. 2000;275:1079–1088. doi: 10.1074/jbc.275.2.1079. [DOI] [PubMed] [Google Scholar]
- 33.Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM, Woolf CJ, Kovacs DM. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9:755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim DY, Ingano LA, Carey BW, Pettingell WH, Kovacs DM. Presenilin/gamma-secretase-mediated cleavage of the voltage-gated sodium channel beta2-subunit regulates cell adhesion and migration. The Journal of biological chemistry. 2005;280:23251–23261. doi: 10.1074/jbc.M412938200. [DOI] [PubMed] [Google Scholar]
- 35.Lenkowski PW, Shah BS, Dinn AE, Lee K, Patel MK. Lidocaine block of neonatal Nav1.3 is differentially modulated by co-expression of beta1 and beta3 subunits. Eur J Pharmacol. 2003;467:23–30. doi: 10.1016/s0014-2999(03)01595-4. [DOI] [PubMed] [Google Scholar]
- 36.Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nature neuroscience. 2006;9:305–310. doi: 10.1038/nn1647. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Santiago LF, Pertin M, Morisod X, Chen C, Hong S, Wiley J, Decosterd I, Isom LL. Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J Neurosci. 2006;26:7984–7994. doi: 10.1523/JNEUROSCI.2211-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas PT, Meadows LS, Nicholls J, Ragsdale DS. An epilepsy mutation in the beta1 subunit of the voltage-gated sodium channel results in reduced channel sensitivity to phenytoin. Epilepsy research. 2005;64:77–84. doi: 10.1016/j.eplepsyres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. The Journal of biological chemistry. 2000;275:11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra JD, Koopmann MC, Kazen-Gillespie KA, Fettman N, Hortsch M, Isom LL. Structural requirements for interaction of sodium channel beta 1 subunits with ankyrin. The Journal of biological chemistry. 2002;277:26681–26688. doi: 10.1074/jbc.M202354200. [DOI] [PubMed] [Google Scholar]
- 41.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. The Journal of biological chemistry. 2004;279:40748–40754. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 42.McEwen DP, Chen C, Meadows LS, Lopez-Santiago L, Isom LL. The voltage-gated Na+ channel beta3 subunit does not mediate trans homophilic cell adhesion or associate with the cell adhesion molecule contactin. Neuroscience letters. 2009;462:272–275. doi: 10.1016/j.neulet.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen DP, Isom LL. Heterophilic interactions of sodium channel beta1 subunits with axonal and glial cell adhesion molecules. The Journal of biological chemistry. 2004;279:52744–52752. doi: 10.1074/jbc.M405990200. [DOI] [PubMed] [Google Scholar]
- 44.McEwen DP, Meadows LS, Chen C, Thyagarajan V, Isom LL. Sodium channel beta1 subunit-mediated modulation of Nav1.2 currents and cell surface density is dependent on interactions with contactin and ankyrin. The Journal of biological chemistry. 2004;279:16044–16049. doi: 10.1074/jbc.M400856200. [DOI] [PubMed] [Google Scholar]
- 45.McNamara JO. Pharmacotherapy of the epilepsies. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2006. pp. 501–526. [Google Scholar]
- 46.Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovascular research. 2005;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Meadows LS, Malhotra J, Loukas A, Thyagarajan V, Kazen-Gillespie KA, Koopman MC, Kriegler S, Isom LL, Ragsdale DS. Functional and biochemical analysis of a sodium channel beta1 subunit mutation responsible for generalized epilepsy with febrile seizures plus type 1. J Neurosci. 2002;22:10699–10709. doi: 10.1523/JNEUROSCI.22-24-10699.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Messner DJ, Catterall WA. The sodium channel from rat brain. Separation and characterization of subunits. The Journal of biological chemistry. 1985;260:10597–10604. [PubMed] [Google Scholar]
- 49.Miyazaki H, Oyama F, Wong HK, Kaneko K, Sakurai T, Tamaoka A, Nukina N. BACE1 modulates filopodia-like protrusions induced by sodium channel beta4 subunit. Biochemical and biophysical research communications. 2007;361:43–48. doi: 10.1016/j.bbrc.2007.06.170. [DOI] [PubMed] [Google Scholar]
- 50.Moran O, Conti F, Tammaro P. Sodium channel heterologous expression in mammalian cells and the role of the endogenous beta1-subunits. Neuroscience letters. 2003;336:175–179. doi: 10.1016/s0304-3940(02)01284-3. [DOI] [PubMed] [Google Scholar]
- 51.Moran O, Nizzari M, Conti F. Endogenous expression of the beta1A sodium channel subunit in HEK-293 cells. FEBS letters. 2000;473:132–134. doi: 10.1016/s0014-5793(00)01518-0. [DOI] [PubMed] [Google Scholar]
- 52.Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, Jackson AP. beta 3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2308–2313. doi: 10.1073/pnas.030362197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Malley HA, Shreiner AB, Chen GH, Huffnagle GB, Isom LL. Loss of Na+ channel beta2 subunits is neuroprotective in a mouse model of multiple sclerosis. Mol Cell Neurosci. 2009;40:143–155. doi: 10.1016/j.mcn.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh Y, Lee YJ, Waxman SG. Regulation of Na+ channel beta 1 and beta 2 subunit mRNA levels in cultured rat astrocytes. Neuroscience letters. 1997;234:107–110. doi: 10.1016/s0304-3940(97)00694-0. [DOI] [PubMed] [Google Scholar]
- 55.Oh Y, Waxman SG. Differential Na+ channel beta 1 subunit mRNA expression in stellate and flat astrocytes cultured from rat cortex and cerebellum: a combined in situ hybridization and immunocytochemistry study. Glia. 1995;13:166–173. doi: 10.1002/glia.440130303. [DOI] [PubMed] [Google Scholar]
- 56.Orrico A, Galli L, Grosso S, Buoni S, Pianigiani R, Balestri P, Sorrentino V. Mutational analysis of the SCN1A, SCN1B and GABRG2 genes in 150 Italian patients with idiopathic childhood epilepsies. Clin Genet. 2009;75:579–581. doi: 10.1111/j.1399-0004.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- 57.Oyama F, Miyazaki H, Sakamoto N, Becquet C, Machida Y, Kaneko K, Uchikawa C, Suzuki T, Kurosawa M, Ikeda T, Tamaoka A, Sakurai T, Nukina N. Sodium channel beta4 subunit: down-regulation and possible involvement in neuritic degeneration in Huntington's disease transgenic mice. Journal of neurochemistry. 2006;98:518–529. doi: 10.1111/j.1471-4159.2006.03893.x. [DOI] [PubMed] [Google Scholar]
- 58.Patino G, Brackenbury WJ, Isom LL. Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. The β1B splice variant of the voltage-gated sodium channel SCN1B is a soluble protein with a possible role in axon pathfinding Program No 517.12. 2009. Online. [Google Scholar]
- 59.Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, Chen C, O'Malley HA, Gray CB, Miyazaki H, Nukina N, Oyama F, De Jonghe P, Isom LL. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci. 2009;29:10764–10778. doi: 10.1523/JNEUROSCI.2475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pertin M, Ji RR, Berta T, Powell AJ, Karchewski L, Tate SN, Isom LL, Woolf CJ, Gilliard N, Spahn DR, Decosterd I. Upregulation of the voltage-gated sodium channel beta2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons. J Neurosci. 2005;25:10970–10980. doi: 10.1523/JNEUROSCI.3066-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qin N, D'Andrea MR, Lubin ML, Shafaee N, Codd EE, Correa AM. Molecular cloning and functional expression of the human sodium channel beta1B subunit, a novel splicing variant of the beta1 subunit. European journal of biochemistry. 2003;FEBS 270:4762–4770. doi: 10.1046/j.1432-1033.2003.03878.x. [DOI] [PubMed] [Google Scholar]
- 62.Ratcliffe CF, Qu Y, McCormick KA, Tibbs VC, Dixon JE, Scheuer T, Catterall WA. A sodium channel signaling complex: modulation by associated receptor protein tyrosine phosphatase beta. Nature neuroscience. 2000;3:437–444. doi: 10.1038/74805. [DOI] [PubMed] [Google Scholar]
- 63.Ratcliffe CF, Westenbroek RE, Curtis R, Catterall WA. Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J Cell Biol. 2001;154:427–434. doi: 10.1083/jcb.200102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rougon G, Hobert O. New insights into the diversity and function of neuronal immunoglobulin superfamily molecules. Annu Rev Neurosci. 2003;26:207–238. doi: 10.1146/annurev.neuro.26.041002.131014. [DOI] [PubMed] [Google Scholar]
- 65.Sashihara S, Greer CA, Oh Y, Waxman SG. Cell-specific differential expression of Na(+)-channel beta 1-subunit mRNA in the olfactory system during postnatal development and after denervation. J Neurosci. 1996;16:702–713. doi: 10.1523/JNEUROSCI.16-02-00702.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheffer IE, Harkin LA, Grinton BE, Dibbens LM, Turner SJ, Zielinski MA, Xu R, Jackson G, Adams J, Connellan M, Petrou S, Wellard RM, Briellmann RS, Wallace RH, Mulley JC, Berkovic SF. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain. 2007;130:100–109. doi: 10.1093/brain/awl272. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt J, Rossie S, Catterall WA. A large intracellular pool of inactive Na channel alpha subunits in developing rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:4847–4851. doi: 10.1073/pnas.82.14.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt JW, Catterall WA. Biosynthesis and processing of the alpha subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell. 1986;46:437–444. doi: 10.1016/0092-8674(86)90664-1. [DOI] [PubMed] [Google Scholar]
- 69.Shah BS, Stevens EB, Pinnock RD, Dixon AK, Lee K. Developmental expression of the novel voltage-gated sodium channel auxiliary subunit beta3, in rat CNS. The Journal of physiology. 2001;534:763–776. doi: 10.1111/j.1469-7793.2001.t01-1-00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spampanato J, Kearney JA, de Haan G, McEwen DP, Escayg A, Aradi I, MacDonald BT, Levin SI, Soltesz I, Benna P, Montalenti E, Isom LL, Goldin AL, Meisler MH. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J Neurosci. 2004;24:10022–10034. doi: 10.1523/JNEUROSCI.2034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srinivasan J, Schachner M, Catterall WA. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15753–15757. doi: 10.1073/pnas.95.26.15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 73.Tammaro P, Conti F, Moran O. Modulation of sodium current in mammalian cells by an epilepsy-correlated beta 1-subunit mutation. Biochemical and biophysical research communications. 2002;291:1095–1101. doi: 10.1006/bbrc.2002.6570. [DOI] [PubMed] [Google Scholar]
- 74.Thase ME, Denko T. Pharmacotherapy of mood disorders. Annu Rev Clin Psychol. 2008;4:53–91. doi: 10.1146/annurev.clinpsy.2.022305.095301. [DOI] [PubMed] [Google Scholar]
- 75.Uebachs M, Opitz T, Royeck M, Dickhof G, Hostmann MT, Isom LL, Beck H. Efficacy loss of the anticonvulsant carbamazepine in mice lacking sodium channel β subunits via paradoxical effects on persistent sodium currents. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.1534-10.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Gassen KL, de Wit M, van Kempen M, van der Hel WS, van Rijen PC, Jackson AP, Lindhout D, de Graan PN. Hippocampal Nabeta3 expression in patients with temporal lobe epilepsy. Epilepsia. 2009;50:957–962. doi: 10.1111/j.1528-1167.2008.02015.x. [DOI] [PubMed] [Google Scholar]
- 77.Wallace RH, Scheffer IE, Parasivam G, Barnett S, Wallace GB, Sutherland GR, Berkovic SF, Mulley JC. Generalized epilepsy with febrile seizures plus: mutation of the sodium channel subunit SCN1B. Neurology. 2002;58:1426–1429. doi: 10.1212/wnl.58.9.1426. [DOI] [PubMed] [Google Scholar]
- 78.Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 79.Wang X, Koulov AV, Kellner WA, Riordan JR, Balch WE. Chemical and Biological Folding Contribute to Temperature-Sensitive DeltaF508 CFTR Trafficking. Traffic (Copenhagen, Denmark) 2008 doi: 10.1111/j.1600-0854.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watanabe H, Darbar D, Kaiser DW, Jiramongkolchai K, Chopra S, Donahue BS, Kannankeril PJ, Roden DM. Mutations in sodium channel beta1-and beta2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:268–275. doi: 10.1161/CIRCEP.108.779181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. The Journal of biological chemistry. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 82.Wu S, Skolnick J, Zhang Y. Ab initio modeling of small proteins by iterative TASSER simulations. BMC Biol. 2007;5:17. doi: 10.1186/1741-7007-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao ZC, Ragsdale DS, Malhotra JD, Mattei LN, Braun PE, Schachner M, Isom LL. Tenascin-R is a functional modulator of sodium channel beta subunits. The Journal of biological chemistry. 1999;274:26511–26517. doi: 10.1074/jbc.274.37.26511. [DOI] [PubMed] [Google Scholar]
- 84.Xu R, Thomas EA, Gazina EV, Richards KL, Quick M, Wallace RH, Harkin LA, Heron SE, Berkovic SF, Scheffer IE, Mulley JC, Petrou S. Generalized epilepsy with febrile seizures plus-associated sodium channel beta1 subunit mutations severely reduce beta subunit-mediated modulation of sodium channel function. Neuroscience. 2007;148:164–174. doi: 10.1016/j.neuroscience.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 85.Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci. 2003;23:7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y. Template-based modeling and free modeling by I-TASSER in CASP7. Proteins. 2007;69 8:108–117. doi: 10.1002/prot.21702. [DOI] [PubMed] [Google Scholar]