Abstract
The conjunctival microcirculation in 14 pediatric and 8 adult sickle cell anemia (SCA) patients was studied using computer-assisted intravital microscopy. The bulbar conjunctiva in SCA patients in both age groups exhibited a blanched/avascular appearance characterized by decreased vascularity. SCA patients from both age groups had many of the same abnormal morphometric {vessel diameter, vessel distribution, morphometry (shape), tortuosity, arteriole:venule (A:V) ratio, and hemosiderin deposits} and dynamic {vessel sludging/sludged flow, boxcar blood (trickled) flow and abnormal flow velocity} abnormalities. A severity index (SI) was computed to quantify the degree of vasculopathy for comparison between groups. The severity of vasculopathy differed significantly between the pediatric and adult patients (SI: 4.2 ± 1.8 vs 6.6 ± 2.4; p=0.028), indicative of a lesser degree of overall severity in the pediatric patients. Specific abnormalities that were less prominent in the pediatric patients included abnormal vessel morphometry and tortuosity. Sludged flow, abnormal vessel distribution, abnormal A:V ratio, and boxcar flow, appeared in high prevalence in both age groups. The results indicate that SCA microvascular abnormalities develop in childhood and the severity of vasculopathy likely progresses with age. Intervention and effective treatment/management modalities should target pediatric patients to ameliorate, slow down or prevent progressive microvascular deterioration.
Sickle cell anemia (SCA) is a genetic disorder that affects millions of people worldwide, for which there is no cure despite substantial understanding of its underlying pathogenesis [1, 2]. Anemia caused by ineffective erythropoiesis and hemolysis is a contributing factor, but vascular complications and abnormal blood flow dynamics account for much of SCA morbidity and mortality. However, there are few real-time in vivo studies on the microcirculation in SCA patients, except for Lipowsky’s work on intravital microscopy of nailfold capillary hemodynamics in sickle cell anemia [3].
We have previously reported three real-time in vivo studies on the microcirculation of the bulbar conjunctiva in SCA patients using computer-assisted intravital microscopy (CAIM) [4–6]. The microvascular bed of the bulbar conjunctiva offers a readily accessible site for non-invasive measurements from which it is possible to extrapolate the in vivo condition of the microvasculature within soft tissues, and to quantify changes in microvascular condition of critical end-organs over time. Using our imaging studies of the bulbar conjunctiva in SCA patients, we have characterized and quantified the morphometric and dynamic microvascular abnormalities (vasculopathy) of the disease [4], demonstrated that abnormal microvascular blood flow dynamics correlate with intracranial blood flow velocity in the Circle of Willis measured by transcranial Doppler ultrasonography [5], and evaluated the efficacy of the drug Poloxamer 188 (RheothRxR and Flocor ) on vasoocclusion [6]. Thus, microvascular characteristics from image analysis of the bulbar conjunctiva can serve as a reliable surrogate biomarker of the severity of microvascular pathology and the efficacy of interventions designed to treat and ameliorate complications resulting from SCA-associated vasculopathy.
These real-time in vivo studies using CAIM have included both adult [4, 6] and pediatric SCA patients [5, 6]. However, in pediatric patients, these studies have focused primarily on the measurements of vessel diameter and blood flow velocity, and assessments of vasculopathy have not been reported. Moreover, there have been no direct comparisons of microvascular abnormalities and severity of vasculopathy between pediatric and adult SCA patients. Accordingly, the goal of the present study was to characterize and compare real-time measurements on the degree of in vivo vasculopathy in pediatric and adult SCA patients, and to test the hypothesis that the severity of vasculopathy increases with age as a natural course of the disease.
Fourteen pediatric and eight adult SCA patients participated in the study. Mean ages of the two groups were significantly different (13.6 ± 4.4 vs. 36.8 ± 11.9y, p<0.001). Conjunctival microvasculature was compared between the pediatric and adults patients, and contrasted with that of healthy, non-SCA control subjects analyzed in previous studies [4, 11, 12]. Figure 1A shows a typical image of the conjunctival microvasculature in a non-SCA subject frame-captured from a videotape sequence from an unrelated study [4]. There is an orderly presence of anastomosing networks of capillaries, arterioles and venules without the presence of ischemic (avascular) zones (Figure 1A). The normal A:V ratio is typically ~1:2 and the arterioles and venules exhibit an even distribution without the presence of dilations, narrowing, distension, microaneurysm, sacculated (beaded) vessels, broken/damaged vessels, or hemosiderin deposits. Normal conjunctival blood flow, though variable in red-cell velocity, is smooth and non-intermittent. Blood sludging, tortuous vessels and boxcar blood flow (trickled flow) patterns are typically not observed.
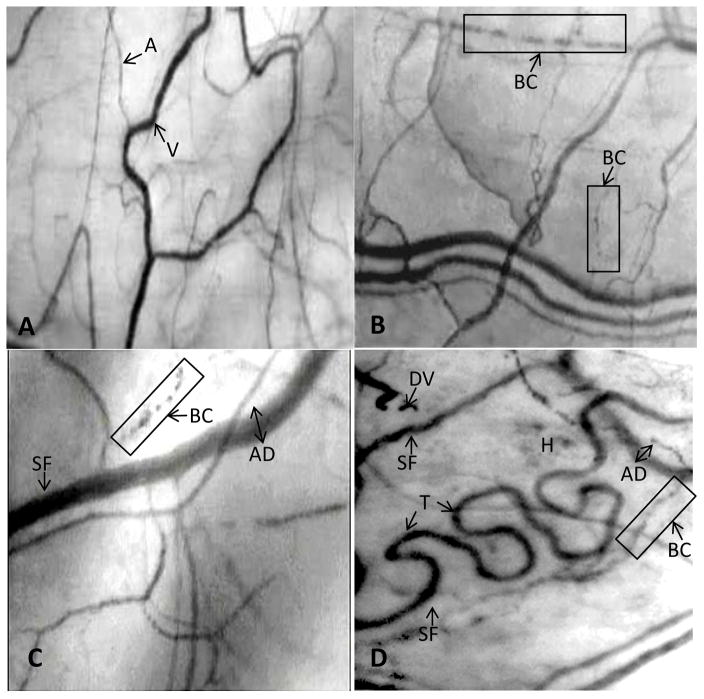
Figure 1.
Figure 1A. A frame-captured image of the conjunctival microcirculation in a healthy non-SCA control subject [10, 11].
Optical magnification 4.5X; onscreen magnification 125X. This image illustrates a typical view of the conjunctival microcirculation in a healthy (non-SCA) control subject who has no history of any vascular disease. Note the even and orderly distribution of normal-sized arterioles, venules and capillaries in a richly vascularized network.
Abbreviations: A = arteriole; V = venule.
Figure 1B. A frame-captured image of the conjunctival microcirculation in a pediatric SCA patient (Patient #P-3; age 8y).
Optical magnification 4.5X; onscreen magnification 125X. The SI of this patient is 3 and the microvascular abnormalities include only sludged blood flow (vessel sludging), boxcar (trickled) blood flow and abnormal A:V ratio. Overall, the vasculopathy observed is mild.
Abbreviations: BC = boxcar (trickled) blood flow.
Figure 1C. A frame-captured image of the conjunctival microcirculation in another pediatric SCA patient (Patient #P-8; age 15y).
Optical magnification 4.5X; onscreen magnification 125X. Patient P-8 is 7 years older than the patient described in Figure 2. The microcirculation shows a greater level of vasculopathy, which includes abnormal vessel diameter, sludged blood flow, boxcar (trickled) blood flow, abnormal vessel distribution, hemosiderin deposit, and abnormal A:V ratio in this captured frame. The overall vasculopathy in this pediatric patient is severe, with an SI of 7 (compared with the SI of 3 in the pediatric patient described in Figure 2).
Abbreviations: SF = sludged blood flow [stop-and-go pattern of blood flow as evidenced by area(s) of darker or uneven coloration within the vessel]; BC = boxcar (trickled) blood flow; AD = abnormal diameter (wide).
Figure 1D. A frame-captured image of the conjunctival microcirculation in an adult SCA patient (Patient #A-7; age 58y).
Optical magnification 4.5X; onscreen magnification 125X. The microvascular abnormalities in this adult patient include abnormal vessel diameter, pronounced vessel tortuosity, abnormal vessel distribution, abnormal A:V ratio, sludged (trickled) blood flow, boxcar flow pattern, damaged vessel, and hemosiderin deposits.
Abbreviations: SF = sludged blood flow [stop-and-go pattern of blood flow as ecidenced by area(s) of darker or uneven coloration within the vessel]; BC = boxcar (trickled) blow flow; DV = damaged vessel; AD = abnormal diameter (wide); H = hemosiderin deposits; T = tortuosity.
The conjunctival microcirculation in the pediatric and adult SCA patients uniquely differs from those found in non-SCA control subjects [See real-time video sequences in the on-line Supplementary Materials section]. There is a lower amount of vascularity (diminished presence of conjunctival vessels) and abnormal vascular distribution in most patients in both age groups, giving the bulbar conjunctiva a “blanched” avascular appearance. The prevalence of specific microvascular abnormalities in both patient groups is summarized in Tables 1 and 2 [See on-line Supplementary Materials section] and some of the abnormalities are shown in Figures 1B–D. SCA patients from both age groups exhibit, to varying degrees, the same morphometric and dynamic abnormalities, including abnormal vessel diameter, abnormal vessel distribution, abnormal vessel morphometry (shape), sludged flow, vessel tortuosity, abnormal A:V ratio, boxcar blood flow, hemosiderin deposits, and abnormal flow (red-cell) velocity. These microvascular abnormalities are rarely found in the bulbar conjunctiva of healthy non-SCA subjects [4, 11, 12]. The severity of vasculopathy, as indicated by the Severity Index (SI), was significantly lower in the pediatric patients than in the adult patients (4.2 ± 1.8 vs. 6.6 ± 2.4, p=0.028). For comparison, the mean SI values for both the pediatric and adult SCA patients were significantly higher than the mean SI value determined for a previous cohort of healthy non-SCA subjects (n=10; SI = 0.31 ± 0.72; p<0.05) [12]. In comparing the prevalence of microvascular abnormalities between pediatric and adult SCA groups, the following significant differences were observed:
Abnormal vessel morphometry was observed in 3 out of 8 adult patients (38%), but was not observed in any of the pediatric patients. The OR (95% CI) for the difference in prevalence was ∞ (1.2, ∞) (p=0.036).
Vessel tortuosity was observed in 7 out of 8 adult patients (88%) compared with only 3 out of 14 pediatric patients (21%). The OR (95% CI) for the difference in prevalence was 25.7 (1.7, 1258) (p=0.006).
In addition, several microvascular abnormalities were highly prevalent in both the pediatric and adult patients. Ten out of 14 pediatric patients (71%) and seven out of eight adult patients (88%) had vessel sludging. Ten out of 14 pediatric patients (71%) and eight out of eight adult patients (100%) had an abnormal A:V ratio. Eight out of 14 pediatric patients (57%) and seven out of eight adult patients (88%) had an abnormal vessel distribution. Eleven out of 14 pediatric patients (79%) and six out of eight adult patients (75%) exhibited boxcar flow patterns.
CAIM is a real-time technology that can be used to non-invasively videotape, analyze and quantify real-time microvascular abnormalities in vascular diseases. The technique has been used successfully in our laboratory to assess microvascular abnormalities in type-1 and type-2 diabetes, Alzheimer’s disease, and SCA [4–12]. The in vivo microvascular bed of the bulbar conjunctiva (conjunctival microcirculation) is particularly amenable to the use of CAIM because it is non-invasively and easily accessible, and yields images of excellent quality and clarity. Results from some of the studies on the identification and quantification of microvascular abnormalities in the conjunctival microcirculation [4, 8] have been used as a basis for subsequent translational research and interventional efficacy studies [6, 7].
The present study was designed to extend our knowledge base on real-time vasculopathy in pediatric and adult SCA patients. Our overall goal is to understand the ontogeny of vasculopathy based on the hypothesis that, as a genetic disorder, SCA microvascular complications and vasculopathy begin to develop after birth and continue to progress into adulthood as part of the natural course of the disease. Results from the present study support this hypothesis: the severity of microvascular abnormalities in the pediatric patients was significantly lower than that observed in the adult patients. Secondary analyses of specific microvascular abnormalities revealed that the observed difference in severity was primarily due to a lower prevalence of abnormal vessel morphometry and vessel tortuosity in the pediatric patients compared with the adults. These findings suggest that these two specific abnormalities develop at a slower rate than other microvascular abnormalities.
The primary limitation of this study is that it is cross-sectional. The observed difference in the severity of vasculopathy between the pediatric and adult patients could be attributable to advances in management of the disease that were not available to the adult patients during their childhood. A longitudinal study in which the microvasculature of SCA patients is evaluated at regular intervals from childhood to adulthood would be required to definitively test the hypothesis that the severity of vasculopathy progresses with age. If confirmed, the results of this study suggest that the pediatric years represent a window of opportunity during which effective treatment and management modalities may slow or ameliorate complications of SCA caused by vasculopathy that arises as a natural progression of the disease from childhood to adulthood. Specific abnormalities, e.g. abnormal vessel morphometry and vessel tortuosity, may serve as landmark biomarkers to evaluate the efficacy of treatment and disease management modalities over time. Moreover, the high prevalence of other abnormalities in both pediatric and adult patients, including vessel sludging, abnormal A:V ratio, abnormal vessel distribution, and boxcar flow patterns -- indicative of rapid development of vasculopathy in childhood -- suggests an urgency to identify better interventions and treatments that ameliorate or slow the progression of microvascular abnormalities and can be used to treat pediatric SCA patients more aggressively.
Conjunctival vessels have unique shapes and forms (Figures 1A–D) and can be easily re-identified for follow-up studies using CAIM -- each individual vessel can serve as its own baseline (reference) control and then re-localized and re-assessed in longitudinal studies [6, 7]. This makes the conjunctival microcirculation an ideal arena and CAIM an excellent non-invasive real-time technology for longitudinal studies of SCA disease progression and evaluations of the efficacy of medications and other treatment or management modalities. At this time, CAIM is not yet widely used as a research tool. However, two identical CAIM systems have been built recently and are functional in other laboratories. A blinded interventional collaborative study to compare independently obtained real-time in vivo vasculopathy data is in progress. These studies will eventually allow for independent confirmation of our results at other institutions and will validate the utility of CAIM as a clinical tool to objectively and non-invasively study vasculopathy in SCA and other vascular diseases.
Methods
Patient groups to be studied
The University of California Davis Institutional Review Board approved the study and written informed consent was obtained from all patients or from their parents or guardians. Pediatric SCA patients (HbSS; ages 6–18y) were recruited from the Pediatric Sickle Cell Clinic at the University of California Davis Medical Center (UCDMC). Adult SCA patients (HbSS; ages 27–58y) were recruited from the Adult Sickle Cell Clinic at UCDMC. Before initiation of the study, all patient records were evaluated to ensure that each patient was not having any sickling complications (i.e. in steady-state condition) and had not suffered a vasoocclusive (painful) crisis for at least a month before the study. SCA patients on chronic transfusion were allowed to participate in this study.
Computer-assisted intravital microscopy (CAIM)
A CAIM system substantially modified and adapted from the earlier prototype originally designed to study the conjunctival microcirculation in adult subjects [4, 7] has been utilized successfully thereafter to study pediatric patients [5, 6, 8]. The CAIM system uses macro-optics in which image acquisition is based on real-time video-documentation of selected regions in the in vivo conjunctival microcirculation. The procedural details of this technique have been described in detail in previous publications [10–12].
Quantification of severity of vasculopathy and prevalence of microvascular abnormalities
Videotape sequences made of the conjunctival microcirculation in each patient were coded for subsequent viewing and analysis to ensure objectivity, with the medical history and identity of each pediatric and adult patient blinded to the investigators prior to and during data analysis. Data analysis, which was described in detail in previous reports [4, 10–12], was conducted in two phases:
Visualization phase -- Identification of morphometric characteristics. Videotape sequences of each patient were viewed in their entirety. Key landmark features (characteristics), including comma signs, vessel sludging (sludged flow), box-car (trickled) blood flow pattern, microaneurysms (micropools), ischemia, vessel morphometry (pattern or shape), vessel distribution, distended vessels, tortuous vessels, sacculated (beaded) vessels, damaged vessels, and hemosiderin deposits were identified and tabulated for their presence in each experimental subject [See Tables 1 and 2 in Supplementary Materials section]. The same coded videotape sequences were analyzed by at least two observers. Differences in the identification of the morphometric features, though infrequent, were discussed and reconciled through a third adjudicator.
Quantification phase -- Computer-assisted image analysis. Four to five short coded videotape sequences of ~30 seconds each from each experimental subject were selected and frame-captured for data quantification, including vessel diameter, total lengths of arterioles and venules per area for arteriole-venule (A:V) ratio computation and measurement of red-cell flow velocity [4, 10–12].
Based on previous studies on microvascular abnormalities in various vascular diseases, 15 possible aberrations can be found in the conjunctival microvasculature [7, 8, 10–12]. A Severity Index (SI) is computed to quantify the degree (severity) of vasculopathy in each patient, based on the arithmetic summation of the presence of any of the 15 microvascular abnormalities listed above on a binary (yes = 1; no = 0) basis. The SI ranges from a score of 0 (no abnormalities present) to 15 (all 15 abnormalities present). This SI computation methodology has been validated in previous studies [7, 8, 10–12] and has an inter-investigator variation coefficient of <5%.
Statistical analysis
Results were reported as means ± standard deviation and medians with ranges. The two-sided Wilcoxon rank-sum test was used to compare SI, which is a numerical variable, between the two groups. The two-sided Fisher’s exact test was used to compare the prevalence of each of the 15 microvascular abnormalities between the two groups, which can be constructed as a 2 × 2 contingency table. An odds ratio (OR) for each abnormality in the adult patients with 95% exact confidence intervals (95% CI) was reported with the pediatric patients serving as the reference group. This OR is the ratio of the odds of an abnormality appearing in the adult patient group to the odds of it appearing in the pediatric patient group. An OR with 95% CI represents a statistically significant difference in the appearance of a specific microvascular abnormality between the two patient groups. All statistical analyses in this study were performed using the SAS v9.2 software (SAS Institute Inc., Cary, NC, USA). A p-value ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This study was supported in part by a National Institutes of Health (NIH) grant (R01 HL83276) and a National Center for Research Support (NCRS) grant (UL1-RR024146). We would like to thank the University of California Davis Medical Center Sickle Cell Center nurses, social workers, SCA patients, caregivers, and parents for their support and generous participation.
Footnotes
Conflict of Interest Disclosure: The authors report no conflict(s) of interest.
Contribution: ATWC developed the intravital microscope and CAIM methodology, designed and conducted the study, analyzed and critically interpreted the data, and wrote the manuscript. JWM contributed to study design and interpretation of the data, and critically reviewed and edited the manuscript. SMC, PLT and XL performed and interpreted the CAIM analysis and reviewed the manuscript. SLS coordinated the study, recruited patients and reviewed the manuscript. PCYC co-designed the intravital microscope and CAIM methodology with ATWC and independently verified the CAIM results off-site. TZ and TW provided access to and assisted in patient recruitment, contributed to study design and interpretation of data and critically reviewed the manuscript. CSL served as biostatistician for the study and critically reviewed the manuscript. RG, who is principal investigator of NIH grant R01 HL83276 which funded this project, contributed to study design and interpretation of data and critically reviewed and edited the manuscript.
References
- 1.U.S. Department of health & Human Services, National Institutes of Health; 2009. National Heart Lung and Blood Institute–Diseases and Conditions Index on Sickle Cell Anemia (all sections) pp. 1–17. [ http://www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_All.html] [Google Scholar]
- 2.Embury SH, Hebbel RP, Mohandas N, Steinberg MH, editors. Sickle Cell Disease: Basic Principles and Clinical Practice. New York, NY: Raven Press; 1994. [Google Scholar]
- 3.Lipowsky HH, Sheikh NU, Katz DM. Intravital microscopy of capillary hemodynamics in sickle cell anemia. J Clin Invest. 1987;80:117–127. doi: 10.1172/JCI113036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung ATW, Chen PCY, Larkin EC, et al. Microvascular abnormalities in sickle cell disease: a computer-assisted intravital microscopy study. Blood. 2002;99:3999–4005. doi: 10.1182/blood.v99.11.3999. [DOI] [PubMed] [Google Scholar]
- 5.Cheung ATW, Harmatz P, Wun T, et al. Correlation of abnormal intracranial vessel velocity (measured by transcranial Doppler ultrasonography) with abnormal conjunctival vessel velocity (measured by computer-assisted intravital microscopy) in sickle cell disease. Blood. 2002;97:3401–3404. doi: 10.1182/blood.v97.11.3401. [DOI] [PubMed] [Google Scholar]
- 6.Cheung ATW, Chan MS, Ramanujam S, et al. Effects of Poloxamer 188 treatment on sickle cell vaso-occlusive crisis: computer-assisted intravital microscopy study. J Invest Med. 2004;52:402–406. doi: 10.1136/jim-52-06-35. [DOI] [PubMed] [Google Scholar]
- 7.Cheung ATW, Perez RV, Chen PCY. Improvements in diabetic microangiopathy after successful simultaneous pancreas-kidney transplantation: a computer-assisted intravital microscopy study on the conjunctival microcirculation. Transplantation. 1999;68:927–932. doi: 10.1097/00007890-199910150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Cheung ATW, Price AR, Duong PL, et al. Microvascular abnormalities in pediatric diabetic patients. Microvasc Res. 2002;63:252–258. doi: 10.1006/mvre.2001.2386. [DOI] [PubMed] [Google Scholar]
- 9.Cheung ATW, Ramanujam S, Greer DA, et al. Microvascular abnormalities in the bulbar conjunctiva of patients with type 2 diabetes mellitus. Endocr Prac. 2001;7:358–363. doi: 10.4158/EP.7.5.358. [DOI] [PubMed] [Google Scholar]
- 10.Devaraj S, Cheung ATW, Jialal I, et al. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes. 2007;56:2790–2796. doi: 10.2337/db07-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung ATW, Tomic (Smith) MM, Chen PCY, et al. Correlation of microvascular abnormalities and endothelial dysfunction in type-1 diabetes mellitus (T1DM): a real-time intravital microscopy study. Clin Hemorheo Microc. 2009;42:285–295. doi: 10.3233/CH-2009-1199. [DOI] [PubMed] [Google Scholar]
- 12.Smith MM, Chen PCY, Li CS, et al. Whole blood viscosity and microvascular abnormalities in Alzheimer’s disease. Clin Hemorheo Microcirc. 2008;41:229–239. doi: 10.3233/CH-2009-1174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.