Abstract
Objective
To identify correlates of kidney stone disease in white and African American men and women in a population-based longitudinal study starting in four US communities, and to assess differences in correlates across racial groups.
Methods
12,161 middle-aged participants of the ARIC Study provided information on history of kidney stone disease between 1993–1995. Information on incident kidney stone-related hospitalizations was obtained from ICD-codes on hospital discharge records.
Results
Kidney stone disease was reported by 12.0% of men and 4.8% of women. After multivariable adjustment, prevalent kidney stone disease was significantly (p<0.05) associated with male gender (PR=2.50), increased serum triglycerides (PR=1.07 per SD increase), diabetes (PR=1.27), gallstone disease (PR=1.54), white race (PR= 1.67), and region of residence. Male gender (HR=1.70), diabetes (HR=1.98) and hypertension (HR=1.69) were significantly associated (p<0.05) with incident kidney stone-related hospitalizations (n=94). Race-stratified analyses showed stronger associations of prevalent kidney stone disease with increased triglycerides, older age, and gallstone disease in African Americans compared to whites, whereas male gender showed stronger association in whites (all p-interaction<0.05).
Conclusion
We identified novel correlates of kidney stone disease (triglycerides, gallstone disease) and risk factor interactions by race (age, male gender, triglycerides, gallstone disease).
Keywords: Kidney stones, risk factors, epidemiology
Introduction
In the US, lifetime risk for kidney stone formation exceeds 12% in males and 6% in females. (Johnson, et al, 1979, Stamatelou, et al, 2003, Gillen, et al, 2005b) Increasing prevalence of kidney stones over time has partly been attributed to the increasing prevalence of obesity and type 2 diabetes. (Stamatelou, et al, 2003, Taylor, et al, 2005a, Taylor, et al, 2005b) (Stamatelou, et al, 2003, Taylor, et al, 2005a, Taylor, et al, 2005b) The high prevalence of kidney stones and recurrence rates of 30–40% (Coe, et al, 1977) resulted in annual costs of 2 billion dollars in the US in 2000.
Known risk factors for kidney stones include diabetes (Taylor, et al, 2005a, Taylor, et al, 2005a, Daudon, et al, 2006) and other cardiovascular disease risk factors such as hypertension, hypercholesterolemia and excess weight. (Taylor, et al, 2005b, Ramey, et al, 2004) Individual components of the metabolic syndrome may be independent risk factors for kidney stone disease, and recent evidence suggests that it may be a systemic disorder. (Ramey, et al, 2004, West, et al, 2008, Sakhaee, 2008) Dietary factors are also known to influence risk of stone formation. (Curhan, et al, 1993, Siener, 2006) Known hereditary syndromes and recent population-based studies additionally support a genetic component to kidney stone disease. (Thorleifsson, et al, 2009) Men and individuals of white race seem to be at a higher risk for kidney stone disease than women and African Americans, respectively, (Stamatelou, et al, 2003, Gillen, et al, 2005b, Daudon, et al, 2006, Ramey, et al, 2004, Borghi, et al, 1999, Curhan, et al, 2005, Gillen, et al, 2005a, Hiatt, et al, 1982, Siener, et al, 2004, Soucie, et al, 1996) but many previous studies have focused on only one ethnic group or gender.
We aimed to identify correlates of a history of kidney stone disease in a cohort study among 9,541 white and 2,620 African-American middle-aged participants of the Atherosclerosis Risk in Communities (ARIC) Study, and to assess the importance of these correlates across races and both cross-sectionally and prospectively.
Materials and Methods
Study population
The ARIC Study is an ongoing prospective population-based cohort study of 15,792 adults aged 45–64 years at enrolment. Between 1987 and 1989 (Visit 1), approximately 4,000 individuals were recruited from each of four participating US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; Washington County, Maryland). In-home interviews, laboratory measurements and clinical examinations were conducted after informed consent was obtained. Three additional study visits were conducted (Visit 2: 1990–92; Visit 3: 1993–95; Visit 4: 1996–98). Participants are contacted annually by telephone to provide health status updates. Hospital discharge records and death certificates are collected on a continuous basis. ARIC study design and objectives have been fully described elsewhere. (ARIC investigators, 1989)
Of the 12,887 participants attending ARIC visit 3, when history of kidney stone disease was reported, 190 were missing this information and excluded from further analyses. In addition, participants reporting race other than white or black (n=37) were excluded, as were African American participants at the ARIC centers in Minnesota (n=15) and Washington County (n=26) because of small numbers. Covariates were missing for 458 participants. The final study sample therefore consisted of 12,161 participants: 9,541 whites and 2,620 African Americans.
Assessment of kidney stones
As part of the Visit 3 annual follow up questionnaire, participants were asked whether a doctor had ever told them that they had kidney stones. Study personnel were instructed that a positive answer required diagnosis by a physician. Incident kidney stone disease was defined as the first kidney-stone related hospitalization through December 31, 2005 among individuals who at visit 3 neither had reported ever being diagnosed with kidney stones, nor had had a kidney stone related hospitalization. ICD-9 codes listed on hospital discharge summaries were used to define kidney stone-related hospitalizations: 592 (calculus of the kidney and ureter; n=1), 592.0 (calculus of kidney; n=47), 592.1 (calculus of ureter; n=43), 592.9 (urinary calculus, unspecified; n=3), and 274.11 (uric acid nephrolithiasis; n=0).
Exposures/covariates
Covariates were selected based on previously published research and on biological plausibility. Trained interviewers collected information on demographic factors such as age, race, and gender for all study participants at each clinical visit. Waist circumference (cm) was measured at the level of the umbilicus in a standing position. Body mass index (BMI) was calculated as weight (kg)/height (m2). Blood pressure measurements were based on 3 readings using a random zero sphygmomanometer, and analyzed as the average of the second and third reading. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or use of anti-hypertensive medication. Fasting blood samples were drawn, centrifuged, frozen and shipped to the ARIC study laboratory where serum high-density lipoprotein cholesterol (HDL), triglycerides, uric acid and glucose were measured following standard ARIC protocols. (NHLBI operations manual 7, 1988, NHLBI operations manual 8, 1987) Diabetes was defined as fasting serum glucose levels ≥126 mg/dl, non-fasting glucose levels ≥200 mg/dl, use of diabetes medication, or a reported physician-diagnosis of diabetes. As part of the Visit 3 annual follow up questionnaire, participants were also asked whether a doctor had ever told them that they had gallstone disease. Again, personnel were instructed that a positive answer required a physician diagnosis, and to record “no” if the participant was unsure. Gallstone disease was evaluated based on the a priori specified hypothesis that an observed clustering of gallstone and kidney stone disease may be indicative of a common contributing factor to both types of stones.
All covariates were assessed at visit 3, with exception of serum uric acid levels (measured at visits 1 and 2), and gout (self-reported at visit 4).
Data Analysis
Baseline characteristics of the study population by kidney stone disease status were investigated cross-sectionally using t- and chi-square tests as appropriate. Natural logarithmic transformation was applied to serum triglycerides because of its skewed distribution.
Prevalence ratios for kidney stone disease were calculated using multivariable adjusted Poisson regression with a robust standard error, since kidney stones are not a rare disease. Sensitivity analyses were conducted using logistic regression with similar results; the more conservative results from the Poisson regression are presented. Two different statistical models were explored: Model 1 included age, gender, race and region of residence only, and Model 2 additionally included waist circumference, hypertension, serum triglyceride levels, diabetes, uric acid levels (mean of visits 1 and 2 measurements), and gallstone disease as covariates. HDL, BMI, and gout were included in univariate but not multivariate regression models because of high correlations with triglycerides, waist circumference, and uric acid, respectively. We decided to retain correlated variables that were part of the metabolic syndrome (waist circumference, serum triglycerides) and uric acid. Waist circumference, triglycerides and uric acid were examined per standard deviation [SD] increase, and age per 10-year increase. Heterogeneity by race was assessed by including a multiplicative interaction term in the regression models, and testing the coefficient using a Wald test.
For prospective analyses, individuals were followed from visit 3 until the first date of a kidney-stone disease related hospitalization, December 31st, 2005, or loss to follow up, whichever occurred first. Kidney stone hospitalization incidence rates were calculated using Poisson regression, and risk factors associations were evaluated using Cox proportional hazard models. The proportional hazard assumption was tested using Schoenfeld residuals.
All analyses were conducted using Stata v10 software (College Station, TX).
Results
Of 12,161 study participants, 8.0%, 12.0% of men and 4.8% of women, reported a history of physician-diagnosed kidney stones. Individuals reporting kidney stones were more likely to be male, to have higher concentrations of serum triglycerides and uric acid, and to have lower HDL cholesterol compared to those not reporting kidney stones (Table 1). Co-morbidities such as diabetes, gout and gallstone disease were found more frequently in those reporting kidney stone disease. Mean BMI, waist circumference, and hypertension prevalence were similar across groups (Table 1). Of the overall study population, 21% reported African American race (36% men) and 79% reported white race (46% men). In race-stratified analyses more men reported kidney stones than women; this gender difference was more pronounced in whites compared to African Americans. Conversely, African Americans reporting a history of kidney stone disease had higher mean BMI and waist circumference than those without kidney stone disease, but this difference was not observed in whites.
Table 1.
Study sample characteristics of 12,161 Atherosclerosis Risk In Communities study participants at ARIC visit 3 (1993–95) overall and by race1
| Overall (n=12,161) | Whites (n=9,541) | African American (n=2,620) | ||||
|---|---|---|---|---|---|---|
| Characteristics | Kidney stone (n=974) | No kidney stone (n=11,187) | Kidney stone (n=871) | No kidney stone (n=8,670) | Kidney stone (n=103) | No kidney stone (n=2,517) |
| Age, yrs (SD) | 60.4 (5.8) | 60.0 (5.7) | 60.4 (5.8) | 60.3 (5.7) | 60.9 (5.8) | 59.0 (5.7) |
| Male gender, % (n) | 66.3 (646) | 42.3 (4,736) | 69.4 (604) | 44.2 (3,828) | 40.8 (42) | 36.1 (908) |
| ARIC center, % (n) | ||||||
| North Carolina | 40.7 (396) | 25.2 (2,818) | 43.0 (375) | 29.2 (2,533) | 20.4 (21) | 11.3 (285) |
| Jackson | 8.4 (82) | 20 (2,232) | NA | NA | 79.6 (82) | 88.7 (2,232) |
| Minnesota | 20.2 (197) | 28.5 (3,185) | 22.6 (197) | 36.7 (3,185) | NA | NA |
| Washington County | 30.7 (299) | 26.4 (2,952) | 34.3 (299) | 34.1 (2,952) | NA | NA |
| BMP2–3, kg/m2 (SD) | 28.3 (5.2) | 28.5 (5.5) | 28.1 (4.8) | 27.9 (5.2) | 30.8 (7.3) | 30.3 (6.3) |
| Waist circumference, cm (SD) | 101.6 (13.2) | 100.4 (14.4) | 101.3 (12.6) | 99.8 (14.0) | 104.1 (17.0) | 102.7 (15.6) |
| Diabetes, % (n) | 18.8 (183) | 14.6 (1,629) | 16.9 (147) | 11.9 (1,033) | 35.0 (36) | 23.7 (596) |
| Hypertension, % (n) | 40.0 (390) | 40.5 (4,531) | 37.0 (322) | 34.8 (3,015) | 66.0 (68) | 60.2 (1,516) |
| Triglycerides, mmol/l; median (IQR) | 1.5 (1.0–2.1) | 1.4 (1.0–1.9) | 1.5 (1.1–2.1) | 1.4 (1.0–2.0) | 1.3 (0.9–1.9) | 1.1 (0.8–1.5) |
| HDL cholesterol 2 , mmol/l (SD) | 1.2 (0.4) | 1.4 (0.5) | 1.2 (0.4) | 1.3 (0.5) | 1.4 (0.6) | 1.4 (0.5) |
| Uric acid, mg/dl (SD) | 6.5 (1.4) | 6.2 (1.5) | 6.5 (1.4) | 6.1 (1.4) | 6.5 (1.5) | 6.4 (1.6) |
| Gout 3 , % (n) | 9.2 (79) | 5.8 (570) | 8.6 (67) | 5.1 (400) | 15.4 (12) | 8.3 (170) |
| Gallstone, % (n) | 16.5 (161) | 12 (1,339) | 16.2 (141) | 13.4 (1,158) | 19.4 (20) | 7.2 (181) |
Data presented as percentage (n) for categorical variables and mean (SD) for continuous variables. Triglycerides are shown as median (25th and 75th percentile).
Abbreviations: BMI= body mass index, HDL= high-density lipoprotein, NA= Not applicable.
BMI was measured in 12,157 ARIC participants, 9,537 whites and 2,620 African Americans. Gout information was available for 10,689 participants, 8,564 whites and 2,125 African Americans.
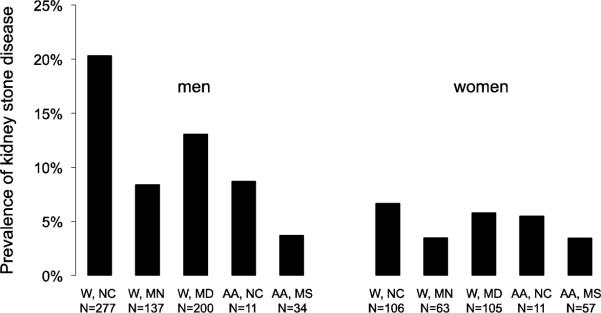
Figure 1 shows the prevalence of kidney stone disease separately by race and region of residence. The prevalence of kidney stone disease was highest among whites from North Carolina (13.0%), and lowest in African Americans from Jackson (3.6%). Analyses adjusting for age, gender, and a combined race–field center variable confirmed this association (Table 2a, Model 1).
Figure 1. Prevalence of kidney stone disease by sex, race and ARIC study center.
Differences in sample size to the data presented in the tables occur because individuals with missing covariates were excluded from data presentation in the tables. Abbreviations: W: white; AA: African American; NC: North Carolina; MN: Minnesota; MD: Maryland; MS: Mississippi. N is the number of individuals with kidney stone disease.
Table 2a.
Multivariate-adjusted prevalence ratios of kidney stone disease for selected variables in 12,161 Atherosclerosis Risk In Communities study participants.
| Model 11 | Model 22 | |
|---|---|---|
| PR (95% CI) | PR (95% CI) | |
| Age (10 yrs) | 1.03 (0.93, 1.14) | 0.99 (0.89, 1.11) |
| Male gender | 2.39 (2.10, 2.71) | 2.50 (2.17, 2.88) |
| Whites in North Carolina | 1.00 (ref) | 1.00 (ref) |
| Whites in Minnesota | 0.45 (0.38, 0.53) | 0.46 (0.39, 0.54) |
| Whites in Washington County | 0.72 (0.62, 0.82) | 0.71 (0.62, 0.82) |
| African Americans in North Carolina | 0.57 (0.38, 0.87) | 0.60 (0.39, 0.92) |
| African Americans in Jackson | 0.30 (0.24, 0.38) | 0.31 (0.25, 0.40) |
| Waist circumference (cm) 3 | 1.00 (0.93, 1.08) | |
| Hypertension | 1.02 (0.89, 1.16) | |
| (ln) Triglycerides (mmol/l) 3 | 1.07 (1.00, 1.14) | |
| Diabetes | 1.27 (1.08, 1.49) | |
| Uric acid (mg/dl) 3 | 0.99 (0.92, 1.07) | |
| Gallstone | 1.54 (1.31, 1.81) |
Model 1 includes adjustments for age, gender, race and region.
Model 2 includes adjustments for age, gender, race, region, waist circumference, triglycerides, hypertension, diabetes, uric acid and gallstone disease. Uric acid is defined as the mean uric acid of visit 1 and 2.
Modeled per standard deviation (SD) increase; SDs were 0.50 for (ln) Triglycerides, 14.40 for waist circumference (cm), and 1.47 for uric acid (mg/dl).,
The extended multivariable adjusted model showed additional significant associations of kidney stone disease with increased serum triglyceride levels, diabetes, and gallstone disease (Table 2a, Model 2). No significant overall relations to waist circumference, hypertension or uric acid levels and kidney stone disease were found. To address a potential role of BMI as a confounder in the association between kidney stone and gallstone disease, several sensitivity analyses were conducted. The inclusion of BMI in addition to waist circumference into Model 2, both continuous and as a squared term, as well as the inclusion of weight gain since age 25 as a covariate did not alter the risk estimate for gallstone disease.
In race-stratified analyses, older age was only related to kidney stone disease in African Americans (p-interaction=0.001). Additional statistically significant interactions were seen with race: increased triglycerides and gallstone disease showed stronger associations in African Americans compared to whites, whereas male gender showed a significantly stronger association in whites (Table 2b). No other interactions were detected.
Table 2b.
Multivariable adjusted prevalence ratios of kidney disease 9,541 white and 2,620 African American Atherosclerosis Risk In Communities study participants.1
| Whites (n=9,541) | African- Americans (n=2,620) | ||
|---|---|---|---|
| PR (95% CI) | PR (95% CI) | P-interaction | |
| Age (10 yrs) | 0.94 (0.84, 1.05) | 1.65 (1.18, 2.29) | 0.001 |
| Male gender | 2.70 (2.32, 3.15) | 1.55 (0.99, 2.44) | <0.001 |
| ARIC center | |||
| North Carolina | 1.00 (ref) | 1.00 (ref) | |
| Jackson | NA | 0.53 (0.34, 0.84) | |
| Minnesota | 0.45 (0.39, 0.54) | NA | |
| Washington County | 0.72 (0.62, 0.83) | NA | |
| Waist circumference (cm) 2 | 0.99 (0.91, 1.07) | 1.00 (0.81, 1.24) | 0.3 |
| Hypertension | 1.01 (0.88, 1.16) | 1.15 (0.74, 1.78) | 0.2 |
| (ln) Triglycerides (mmol/l) 2 | 1.05 (0.99, 1.13) | 1.28 (1.02, 1.61) | 0.03 |
| Diabetes | 1.24 (1.04, 1.48) | 1.35 (0.85, 2.16) | 0.2 |
| Uric acid (mg/dl) 2 | 1.01 (0.94, 1.10) | 0.86 (0.69, 1.06) | 0.06 |
| Gallstone | 1.46 (1.23, 1.73) | 3.01 (1.86, 4.88) | <0.001 |
Each variable was adjusted simultaneously for the other variables shown in the table.
Modeled per overall standard deviation (SD) increase. SDs were 0.50 for (ln) Triglycerides, 14.40 for waist circumference (cm), and 1.47 for uric acid (mg/dl).
Gallstone disease was one of the strongest and most consistent correlates of kidney stone disease overall and in both races. Therefore, the association with kidney stone disease was explored in more detail comparing participants with both gallstone and kidney stone disease with those with only kidney stone disease in order to answer whether the correlates for both conditions are the same as those for kidney stones only. Of 1,567 participants reporting gallstones, 10.8% (n=169) also reported a history of kidney stone disease. Of those reporting both gallstones and kidney stones, 82.1% reported surgical removal of their gallbladder, compared to 81.2% of participants with gallstones but without kidney stones. Results from multivariable adjusted Poisson regression are summarized in Table 3. Compared to participants with neither kidney stone nor gallstone disease, male gender was significantly associated with kidney stone disease only, while female gender was significantly associated with reporting both conditions.
Table 3.
Multivariable adjusted prevalence ratios of kidney disease in white and African American ARIC participants with both kidney and gallstone disease (n= 169) or only kidney stone disease (n=832) compared to those with neither kidney nor gallstone disease (reference group; n= 10,218).
| Both kidney and gallstone disease (n=169) | Only kidney stone disease (n=832) | |||
|---|---|---|---|---|
| PR (95% CI) | P | PR (95% CI) | P | |
| Age (10 yrs) | 1.50 (1.12, 2.01) | 0.007 | 1.00 (0.88, 1.12) | 0.95 |
| Male gender | 0.74 (0.51, 1.05) | 0.09 | 2.66 (2.27, 3.13) | <0.001 |
| White race | 2.45 (1.50, 4.02) | <0.001 | 2.29 (1.81, 2.89) | <0.001 |
| BMI (kg/m2) | ||||
| BMI < 25 | 1.00 (ref) | 1.00 (ref) | ||
| BMI 25 – 29 | 1.24 (0.80, 1.91) | 0.34 | 0.89 (0.76, 1.05) | 0.18 |
| BMI ≥ 30 | 1.43 (0.90, 2.26) | 0.13 | 0.88 (0.72, 1.06) | 0.18 |
| Diabetes | 2.01 (1.38, 2.93) | <0.001 | 1.24 (1.03, 1.49) | 0.02 |
| Hypertension | 1.20 (0.85, 1.71) | 0.30 | 1.02 (0.88, 1.18) | 0.77 |
| (ln) Triglycerides (mmol/l)1 | 1.24 (1.06, 1.44) | 0.006 | 1.08 (1.00, 1.16) | 0.045 |
| Uric acid (mg/dl)1 | 1.13 (0.95, 1.36) | 0.172 | 0.99 (0.91, 1.07) | 0.82 |
Modeled per standard deviation (SD) increase. SDs were 0.50 for (ln) Triglycerides and 1.47 for uric acid (mg/dl). Simultaneous adjustment for all covariates was performed. BMI was included instead of waist because of the known association between BMI and gallstone disease.
Incident kidney stone disease hospitalizations
Over a mean follow-up time of 10.8 years, 94 participants experienced a first kidney-stone disease related hospitalization. The incidence of kidney stone hospitalization was 0.8/1000 person-years. Due to the limited number of events (68 in whites, 26 in African Americans) race-stratified analyses were not conducted. In the multivariable adjusted model (Table 4), male gender, diabetes, and hypertension were significant predictors of incident kidney stone disease related hospitalizations. History of gallstone disease had a hazard ratio of 1.41 (95% CI 0.78–2.55) for incident kidney stone related hospitalization but was not significant.
Table 4.
Multivariate adjusted hazard ratios of incident kidney stone disease hospitalizations in 11,1731 Atherosclerosis Risk In Communities study participants.2
| HR (95% CI) | |
|---|---|
| Age (10 yrs) | 0.95 (0.66, 1.39) |
| Male gender | 1.70 (1.06, 2.73) |
| Whites in North Carolina | 1.00 (ref) |
| Whites in Minnesota | 1.16 (0.65, 2.08) |
| Whites in Washington County | 0.75 (0.39, 1.43) |
| African Americans in North Carolina | 0.83 (0.19, 3.65) |
| African Americans in Jackson | 1.22 (0.64, 2.35) |
| Waist circumference (cm) 3 | 1.11 (0.88, 1.40) |
| Hypertension | 1.69 (1.07, 2.67) |
| (ln) Triglycerides (mmol/l) 3 | 1.11 (0.90, 1.39) |
| Diabetes | 1.98 (1.20, 3.28) |
| Uric acid (mg/dl) 3 | 0.87 (0.68, 1.12) |
| Gallstone | 1.41 (0.78, 2.55) |
90 Incident kidney stone-related hospitalizations with information on all covariates.
Each variable was adjusted simultaneously for the other variables shown in the table.
Modeled per standard deviation (SD) increase. SDs were 0.50 for (ln) Triglycerides, 14.40 for waist circumference (cm), and 1.47 for uric acid (mg/dl).
Discussion
Our study in a large biracial population provides estimates of kidney stone disease prevalence and of the incidence of kidney stone disease-related hospitalizations. We identify novel correlates of prevalent kidney stone disease, serum triglycerides and gallstone disease, as well as confirm known correlates, male gender, white race, region of residence, and diabetes. Significant interactions with race were observed, with older age, higher triglycerides, and gallbladder disease showing stronger associations with kidney stones in African-Americans compared to whites, and male gender showing a stronger association with kidney stone disease in whites. Male gender, hypertension, and diabetes were identified as correlates of incident kidney stone disease-related hospitalizations.
The overall prevalence of kidney stone disease in our study population is in agreement with estimates from other studies.(Johnson, et al, 1979,Stamatelou, et al, 2003) Results from the nationally representative NHANES Surveys support the higher prevalence of self-reported kidney stone disease in whites compared to African Americans.(Stamatelou, et al, 2003) The highest prevalence of a history of kidney stone disease among white males is also consistent with previous findings.(Johnson, et al, 1979,Hiatt, et al, 1982)
Like other studies, we found diabetes to be associated with kidney stone disease, (Taylor, et al, 2005a) possibly due to an association with increased uric acid stone formation.(Daudon, et al, 2006) Several authors have also hypothesized that insulin resistance and the metabolic syndrome may represent a common ground for the development of both diabetes and kidney stones.(Sakhaee, 2008,Curhan, et al, 1993,Sakhaee and Maalouf, 2008,Curhan, et al, 1994) No study specifically addressed the association between diabetes and kidney stone disease in African Americans and whites; our results indicate that this association is of similar magnitude for both races.
In our study, hypertension was not significantly associated with prevalent kidney stone disease in either whites or African Americans. This in contrast to previous studies. (Ramey, et al, 2004,Hamano, et al, 2005,Cappuccio, et al, 1990) On the other hand, our data on incident kidney stone disease-related hospitalizations showed a positive association with hypertension, similar to some(Borghi, et al, 1999) but not all(Madore, et al, 1998b,Madore, et al, 1998a) prospective studies.
No study so far has focused on the simultaneous presence of kidney stone and gallstone diseases. A possible explanation for the observed association could be a shared biological mechanism or risk factor, such as older age. Essential components common to both types of stones such as calcium may also play a role.(Ljunghall, 1978,Rege, 2002) The comparable high rate of surgical gallbladder removal in individuals with gallstones irrespective of their kidney stone disease status as well as the differences in correlates between individuals with both types of stones compared with those with kidney stones only makes misclassification a less likely explanation for the association between kidney and gallstone disease.
Finally, it is noteworthy that we found a heterogeneous distribution of self-reported kidney stone disease among the various study centers. The higher rates with Southern latitude and Eastern longitude observed in whites have also been reported by previous studies.(Stamatelou, et al, 2003,Soucie, et al, 1996,Curhan, et al, 1994,Soucie, et al, 1994) Environmental components, such as weather conditions or heat exposure, may therefore be related to kidney stone disease.(Soucie, et al, 1996)
Several limitations of our study are worth mentioning. First, information on the onset of kidney stone disease was missing. Therefore, we were unable to address the question whether kidney stone or gallstone disease occurred first in participants with both conditions. We can also not exclude the possibility that the association between kidney and gallstone disease was due to an increased diagnosis of asymptomatic kidney stone disease in individuals during evaluation of gallbladder disease. Studies with information on both types of stones as well as on imaging could address this important limitation. Second, we did not investigate the association between dietary factors and kidney stone disease, as confounding by indication may be introduced due to changes in diet secondary to kidney stone disease. Third, self-report of kidney stones is subject to misclassification, but previous studies that used self-report of kidney stone disease found correlates that are largely consistent. Fourth, the number of individuals with incident kidney stone related hospitalizations was small, and hospitalizations may represent disease severity because kidney stone disease largely is an outpatient disease. We were however able to confirm positive associations with gender, hypertension and diabetes that have been reported in larger prospective studies. Community-based ascertainment of incident disease could address whether and to which degree our definition reflects the severity of kidney stone disease. Finally, we lacked data on kidney stone types, but most stones are likely to be calcium stones.(Asplin, et al,) Strengths of our study lie in the large biracial study population, the availability of both cross-sectional and prospective data, and the rigorous data collection and stringent quality control procedures of the ARIC Study.
In conclusion, male gender, white race, and region of residence were the strongest correlates of self-reported kidney stones. Risk factors for kidney stone disease were similar across races, but the strength of the association differed significantly for male gender, age, serum triglycerides and gallstone disease. Future studies should further investigate the observed association between kidney stone and gallstone disease.
Acknowledgements
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions. We thank Dr. Gary Curhan for helpful discussions and critical review of the manuscript. Some of the data in this abstract has been presented at the annual meeting of the American Society of Nephrology in November of 2009.
Support and Financial Disclosure Declaration The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure declaration The authors declare that there are no conflicts of interest.
References
- ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am.J.Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute . Operations Manual 7: Blood Collecting and Processing. Bethesda: 1988. National Heart, Lung, and Blood Institute Atherosclerosis Risk in Communities (ARIC) Study. [Google Scholar]
- National Heart, Lung, and Blood Institute . Operations Manual 8: Lipid and Lipoprotein Determinations. Bethesda: 1987. National Heart, Lung, and Blood Institute Atherosclerosis Risk in Communities (ARIC) Study. [Google Scholar]
- Asplin JR, Coe FL, Favus MJ, Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J. “Chapter 281. Nephrolithiasis” (Chapter) Harrison's Principles of Internal Medicine. 17e http://www.accessmedicine.com/content.aspx?aID=2874829.
- Borghi L, Meschi T, Guerra A, Briganti A, Schianchi T, Allegri F, et al. Essential arterial hypertension and stone disease. Kidney Int. 1999;55:2397–2406. doi: 10.1046/j.1523-1755.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Strazzullo P, Mancini M. Kidney stones and hypertension: population based study of an independent clinical association. BMJ. 1990;300:1234–1236. doi: 10.1136/bmj.300.6734.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe FL, Keck J, Norton ER. The natural history of calcium urolithiasis. JAMA. 1977;238:1519–1523. [PubMed] [Google Scholar]
- Curhan GC, Rimm EB, Willett WC, Stampfer MJ. Regional variation in nephrolithiasis incidence and prevalence among United States men. J.Urol. 1994;151:838–841. doi: 10.1016/s0022-5347(17)35101-7. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Rimm EB, Speizer FE, Stampfer MJ. Body size and risk of kidney stones. J.Am.Soc.Nephrol. 2005 1998 Sep;9(9):1645–52. doi: 10.1681/ASN.V991645. Kidney Int. 9, 1645-1652. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N.Engl.J.Med. 1993;328:833–838. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- Daudon M, Traxer O, Conort P, Lacour B, Jungers P. Type 2 diabetes increases the risk for uric acid stones. J.Am.Soc.Nephrol. 2006;17:2026–2033. doi: 10.1681/ASN.2006030262. [DOI] [PubMed] [Google Scholar]
- Gillen DL, Coe FL, Worcester EM. Nephrolithiasis and increased blood pressure among females with high body mass index. Am.J.Kidney Dis. 2005a;46:263–269. doi: 10.1053/j.ajkd.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Gillen DL, Worcester EM, Coe FL. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int. 2005b;67:685–690. doi: 10.1111/j.1523-1755.2005.67128.x. [DOI] [PubMed] [Google Scholar]
- Hamano S, Nakatsu H, Suzuki N, Tomioka S, Tanaka M, Murakami S. Kidney stone disease and risk factors for coronary heart disease. Int.J.Urol. 2005;12:859–863. doi: 10.1111/j.1442-2042.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- Hiatt RA, Dales LG, Friedman GD, Hunkeler EM. Frequency of urolithiasis in a prepaid medical care program. Am.J.Epidemiol. 1982;115:255–265. doi: 10.1093/oxfordjournals.aje.a113297. [DOI] [PubMed] [Google Scholar]
- Johnson CM, Wilson DM, O'Fallon WM, Malek RS, Kurland LT. Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int. 1979;16:624–631. doi: 10.1038/ki.1979.173. [DOI] [PubMed] [Google Scholar]
- Ljunghall S. Incidence and natural history of renal stone disease and its relationship to calcium metabolism. Eur.Urol. 1978;4:424–430. doi: 10.1159/000474013. [DOI] [PubMed] [Google Scholar]
- Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. Am.J.Hypertens. 1998a;11:46–53. doi: 10.1016/s0895-7061(97)00371-3. [DOI] [PubMed] [Google Scholar]
- Madore F, Stampfer MJ, Willett WC, Speizer FE, Curhan GC. Nephrolithiasis and risk of hypertension in women. Am.J.Kidney Dis. 1998b;32:802–807. doi: 10.1016/s0272-6386(98)70136-2. [DOI] [PubMed] [Google Scholar]
- Ramey SL, Franke WD, Shelley MC., 2nd Relationship among risk factors for nephrolithiasis, cardiovascular disease, and ethnicity: focus on a law enforcement cohort. AAOHN J. 2004;52:116–121. [PubMed] [Google Scholar]
- Rege RV. The role of biliary calcium in gallstone pathogenesis. Front.Biosci. 2002;7:e315–25. doi: 10.2741/A926. [DOI] [PubMed] [Google Scholar]
- Sakhaee K. Nephrolithiasis as a systemic disorder. Curr.Opin.Nephrol.Hypertens. 2008;17:304–309. doi: 10.1097/MNH.0b013e3282f8b34d. [DOI] [PubMed] [Google Scholar]
- Sakhaee K, Maalouf NM. Metabolic syndrome and uric acid nephrolithiasis. Semin.Nephrol. 2008;28:174–180. doi: 10.1016/j.semnephrol.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Siener R. Impact of dietary habits on stone incidence. Urol.Res. 2006;34:131–133. doi: 10.1007/s00240-005-0025-1. [DOI] [PubMed] [Google Scholar]
- Siener R, Glatz S, Nicolay C, Hesse A. The role of overweight and obesity in calcium oxalate stone formation. Obes.Res. 2004;12:106–113. doi: 10.1038/oby.2004.14. [DOI] [PubMed] [Google Scholar]
- Soucie JM, Coates RJ, McClellan W, Austin H, Thun M. Relation between geographic variability in kidney stones prevalence and risk factors for stones. Am.J.Epidemiol. 1996;143:487–495. doi: 10.1093/oxfordjournals.aje.a008769. [DOI] [PubMed] [Google Scholar]
- Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994;46:893–899. doi: 10.1038/ki.1994.347. [DOI] [PubMed] [Google Scholar]
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005a;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005b;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat.Genet. 2009 doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- West B, Luke A, Durazo-Arvizu RA, Cao G, Shoham D, Kramer H. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am.J.Kidney Dis. 2008;51:741–747. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]